Abstract
This brief report provides an introduction to the topic of cognitive functioning in late-life depression (LLD). In addition to providing a review of the literature, we present a framework for understanding the heterogeneity of cognitive outcomes in this highly prevalent disorder. In addition, we discuss the relationship between LLD and dementia, and highlight the importance of regularly assessing cognitive functioning in older adults who present with depressive symptoms. If cognitive deficits are discovered during a neuropsychological assessment, we recommend referral to a geriatric psychiatrist or cognitive neurologist, for evaluation and treatment of the patient’s symptoms.
Keywords: Cognition, Depression, Elderly, Geriatric, Assessment, Treatment
INTRODUCTION
Late-life depression (LLD) is a significant public health concern that negatively impacts quality of life, global functioning, and physical health. In the clinical setting, patients with LLD frequently present with cognitive complaints, and 20–50% of individuals with LLD are estimated to have cognitive impairment greater than that of age- and education-matched comparators (Butters et al., 2004; Sheline et al., 2006). Cognitive deficits have been associated with increased rates of depression relapse, poorer antidepressant treatment response, and greater overall disability. With this clinical and public health relevance in mind, this brief review will provide a framework for understanding the presentation and heterogeneity of cognitive impairment in LLD, the likely relationship between LLD and dementia, and the importance of regularly assessing cognitive functioning in older adults who present with depressive symptoms. If deficits are discovered during a neuropsychological assessment, referral to a geriatric psychiatrist or cognitive neurologist is recommended, for medical evaluation and treatment of the depressive episode and related cognitive symptoms.
Late-Life Depression
LLD, defined as a major depressive episode occurring in an older adult (60 years or older), is a heterogeneous mood disorder that frequently presents with cognitive impairment. LLD encompasses both late-onset cases (i.e., first lifetime episode after age 60), as well as early-onset cases that recur or continue into later life. Epidemiological data suggest LLD prevalence of 1–4% among community-dwelling older adults, with higher prevalence (10–12%) among those hospitalized for medical or surgical reasons (Blazer, 2003). When sub-threshold (but still clinically significant) depressive symptoms are considered, these rates rise considerably, with prevalence estimates of 8–16% among community-dwelling elders, and greater than 30% among elders residing in hospital or long-term care facilities (Blazer, 2003). From a diagnostic perspective, LLD is often under-reported, under-recognized, misdiagnosed, and under-treated (Reynolds, Alexopoulos, Katz, & Lebowitz, 2001), due in part to characteristics that set it apart from mid-life depression. Compared to their younger counterparts, individuals with LLD are less likely to present with prominent sadness (Gallo, Rabins, Lyketsos, Tien, & Anthony, 1997) and are more likely to exhibit agitation or somatic symptoms such as gastrointestinal distress, insomnia, or fatigue (Hegeman, Kok, van der Mast, & Giltay, 2012). In addition, while midlife depression is more prevalent in women, such gender differences are greatly reduced in LLD (Steffens, Fisher, Langa, Potter, & Plassman, 2009). LLD is also often more chronic and difficult to treat to remission than mid-life depression, an observation that has been attributed in part to medical co-morbidity and cognitive dysfunction (Blazer, 2003). For these reasons and others, LLD is associated with significant mortality (including due to suicide), health care usage, physical disability, and functional decline (Beekman et al., 2002; Bruce et al., 2004; Steffens et al., 2006).
Cognitive Functioning in Late-Life Depression
Cognitive deficits that often co-occur with LLD include impairments of episodic memory, speed of information processing, executive functioning, and visuospatial ability (Baudic, Tzortzis, Barba, & Traykov, 2004; Butters et al., 2004; Elderkin-Thompson et al., 2003; Lockwood, Alexopoulos, & van Gorp, 2002; Nebes et al., 2000; Rapp et al., 2005; Sheline et al., 2006). Of these domains, information processing speed and executive functioning appear particularly vulnerable, and several studies have reported that cognitive impairment associated with LLD appears to be predominantly mediated by slowed information processing and/or working memory deficits. Nebes and colleagues (2000) found that depressed older adults performed significantly worse on measures of both processing speed and working memory, and while performance on these measures improved in patients whose LLD remitted, the extent of improvement was no greater than that seen in comparison subjects who underwent repeat assessment. In a similar vein, Butters and colleagues (2004) reported that relative to non-depressed comparison subjects, LLD patients performed poorer in all cognitive domains, with information processing speed and visuospatial and executive abilities being the most broadly and frequently impaired. More recently, Sheline and colleagues (2006) found that age, depression severity, education, race, and vascular risk factors all made significant and independent contributions to cognitive impairment in LLD, and that changes in information processing speed mediated the influence of predictor variables on other cognitive domains. In addition, patients with LLD who exhibit executive dysfunction may be at higher risk for poor outcomes, as deficits in executive functioning have been associated with poor or delayed antidepressant treatment response (Alexopoulos et al., 2005) as well as higher relapse and recurrence rates in some studies (Alexopoulos et al., 2000), although not in others (Butters et al., 2004).
A complex relationship exists between the treatment of LLD and improvement (or resolution) of cognitive symptoms, complicated further by the widely varying temporal association between cognitive and depressive symptoms. Studies suggest that, following antidepressant treatment, a subset of individuals with LLD and cognitive dysfunction may exhibit improved cognition (Doraiswamy et al., 2003), although it is clear that a significant proportion will continue to experience cognitive impairment (Bhalla et al., 2006, 2009; Butters et al., 2000; Lee, Potter, Wagner, Welsh- Bohmer, & Steffens, 2007; Murphy & Alexopoulos, 2004; Nebes et al., 2003), even in individuals who achieve depression remission (Basso, Miller, Estevis, & Combs, 2013). In one intervention study, Bhalla and colleagues (2006) found that despite reaching remission, 94% of patients who were impaired at baseline when depressed remained impaired one year later. Of patients who were cognitively normal while depressed, 23% developed impairment one year later. In a subsequent cross-sectional study comparing individuals with remitted LLD and never-depressed comparison subjects, nearly twice as many remitted depressed subjects were diagnosed with MCI or dementia (48% vs. 28%), suggesting that despite adequate depression treatment, cognitive impairment persisted (Bhalla et al., 2009). Others have similarly noted persistent cognitive impairment following treatment and/or remission of depressive symptoms (Bruce et al., 2004; Butters et al., 2000; Doraiswamy et al., 2003; Lee et al., 2007; Reynolds et al., 2011), with certain risk factors, including lower baseline cognitive function, older age, later age of depression onset, and greater vascular burden associated with less improvement in cognitive function (Barch et al., 2012). With regard to age, Arve and colleagues reported a doubling of co-morbid depressive symptoms and cognitive impairment for every 5-year increase in age (after 70), an observation that extrapolated to a prevalence of roughly 25% in individuals 85 and older presenting with both low mood and impaired cognition (Arve, Tilvis, Lehtonen, Valvanne, & Sairanen, 1999).
Characterizing differences between older adults with recurrent early-onset depression (EOD) versus those with late-onset depression (LOD) has been of great interest in recent years (Herrmann, Goodwin, & Ebmeier, 2007; Janssen et al., 2007; Rapp et al., 2005), partly due to hypothesized differences in their neurobiological etiologies. Janssen and colleagues, for example, suggested that in EOD, stress-related neurotoxicity associated with repeated depressive episodes may result in hippocampal atrophy, while in LOD, large subcortical white matter lesions may serve as an intermediary between cerebrovascular disease and depression. Schneider and colleagues, however, have demonstrated that among community-dwelling older adults, individuals with dementia most often have mixed brain pathologies (Schneider, Arvanitakis, Bang, & Bennett, 2007), suggesting that subtypes related to age of depression onset (EOD vs. LOD) may not correlate well with observed neuropathological etiologies in a real-world clinical population. Regardless of the underlying neurobiology—of which a detailed discussion is beyond the scope of this short review—LOD is generally defined as depressive illness that develops for the first time after age 60, while EOD indicates depression that first presented before this age. From a cognitive perspective, the literature has been inconsistent as to whether EOD and LOD are associated with worse or different cognitive profiles. Although both may present with cognitive impairment, most studies suggest that LOD may be associated with more executive and attentional deficits (Rapp et al., 2005), as opposed to more primary episodic memory deficits in EOD (Salloway et al., 1996). A recent meta-analysis found that patients with LOD exhibit slower processing speed and poorer executive functioning than patients with EOD and non-depressed comparison subjects, although both depressed groups exhibit reduced functioning in all cognitive domains as compared to comparison subjects (Herrmann et al., 2007). While this suggests that pronounced executive deficits may be typical of LOD patients, the more robust finding is that older depressed patients demonstrate reduced cognitive functioning compared with non-depressed controls. Taken together, it is clear that LLD is a complicated, heterogeneous disorder, and although studying EOD and LOD phenotypes is appealing, the literature to date remains unclear as to whether consistent distinguishing characteristics exist.
Late-Life Depression as a Risk Factor for Cognitive Decline and Dementia
Although the temporal association between cognitive and depressive symptoms in LLD varies widely, increasing evidence suggests that depression contributes to the development of persistent cognitive dysfunction in a subset of individuals. Clinical, case-control, and epidemiologic studies have reported an association between LLD and progressive cognitive deficits, and it is clear that cognitive deficits accompanying LLD often persist following treatment of depressive symptoms (Bhalla et al., 2006; Butters et al., 2000; Diniz, Butters, Albert, Dew, & Reynolds, 2013; Jorm, 2001; Murphy & Alexopoulos, 2004; Nebes et al., 2000; Ownby, Crocco, Acevedo, John, & Loewenstein, 2006; Paradiso, Lamberty, Garvey, & Robinson, 1997). Findings from a recent longitudinal observational study of women who were cognitively healthy at baseline, for example, showed that baseline scores on the Geriatric Depression Scale (Yesavage et al., 1982) were highly associated with subsequent impairment on all cognitive tests (immediate recall, delayed recall, psychomotor speed, executive functioning), suggesting that depression may be a risk factor for cognitive decline, and thus a potential target for diagnostic and therapeutic intervention (Rosenberg, Mielke, Xue, & Carlson, 2010). Other studies have found LLD to be associated with an approximately 50% increased likelihood of developing all-cause dementia, including Alzheimer’s disease and, in particular, vascular dementia (Diniz et al., 2013; Jorm 2001; Ownby et al., 2006). Some reports, however, have failed to find this association (Becker et al., 2009; Ganguli, Du, Dodge, Ratcliff, & Chang, 2006; Lindsay et al., 2002). Furthermore, it is likely that the relationship between vascular disease and LLD (and related cognitive impairment) is, to a certain extent, bi-directional (Thomas, Kalaria, & O’Brien, 2004). However, the details of this complex issue are beyond the scope of the current review, and are discussed elsewhere (e.g., Naismith, Norrie, Mowszowski, & Hickie, 2012; Taylor, Aizenstein, & Alexopoulos, 2013).
Evidence from several studies suggests that risk for MCI or dementia is proportional to an individual’s cumulative depression burden, as measured by lifetime duration of depression or number of depressive episodes. A large Danish case-registry study, for example, found that the rate of a subsequent diagnosis of dementia was significantly correlated with the number of prior depressive episodes. On average, the rate of dementia increased by 13% with every depressive episode that led to an inpatient admission (Kessing & Andersen, 2004). Several studies (Geerlings, den Heijer, Koudstaal, Hofman, & Breteler, 2008; Palsson, Aevarsson, & Skoog, 1999; Speck et al., 1995) have found that a longer interval between onset of first depressive episode and diagnosis of cognitive dysfunction increases the risk for developing dementia—supporting the aforementioned hypothesis regarding cumulative depressive burden and risk for cognitive dysfunction—while others have suggested that a shorter interval between the two leads to greater dementia risk (Green et al., 2003; Steffens et al., 1997; Wetherell, Gatz, Johansson, & Pedersen, 1999). Others, still, have found no relationship between the two (Ganguli et al., 2006; Godin et al., 2007; Lindsay et al., 2002; Mendez et al., 1992). Overall, these mixed findings emphasize the heterogeneity of LLD and its relationship to cognitive comorbidity and sequelae, and further raise the question of whether prior depression is a true etiologic risk factor for dementia, or instead a prodromal clinical manifestation of underlying dementia neuropathology. Complexities of depression as a syndrome, and variability in the measures, methodologies, and treatments used in these studies, likewise contribute to inconsistent findings.
To shed light on this topic, a recent review examined sixteen clinical studies that investigated the association between dementia and depression, and concluded that earlier-life depression was a consistent risk factor (and unlikely prodrome) for dementia (Byers & Yaffe, 2011). In addition, while current research findings suggest an association between LLD and risk of dementia, several inconsistencies across individual studies exist, perhaps due to methodological, cultural, or sub-population differences across the studies (Byers & Yaffe, 2011). Furthermore, the designs of many of these studies make it difficult (if not impossible) to determine whether depressive episodes were a symptom of prodromal dementia, or instead the psychological consequence of an already present cognitive deficit.
Neurobiological Pathways Linking Late-Life Depression to Cognitive Impairment and Dementia
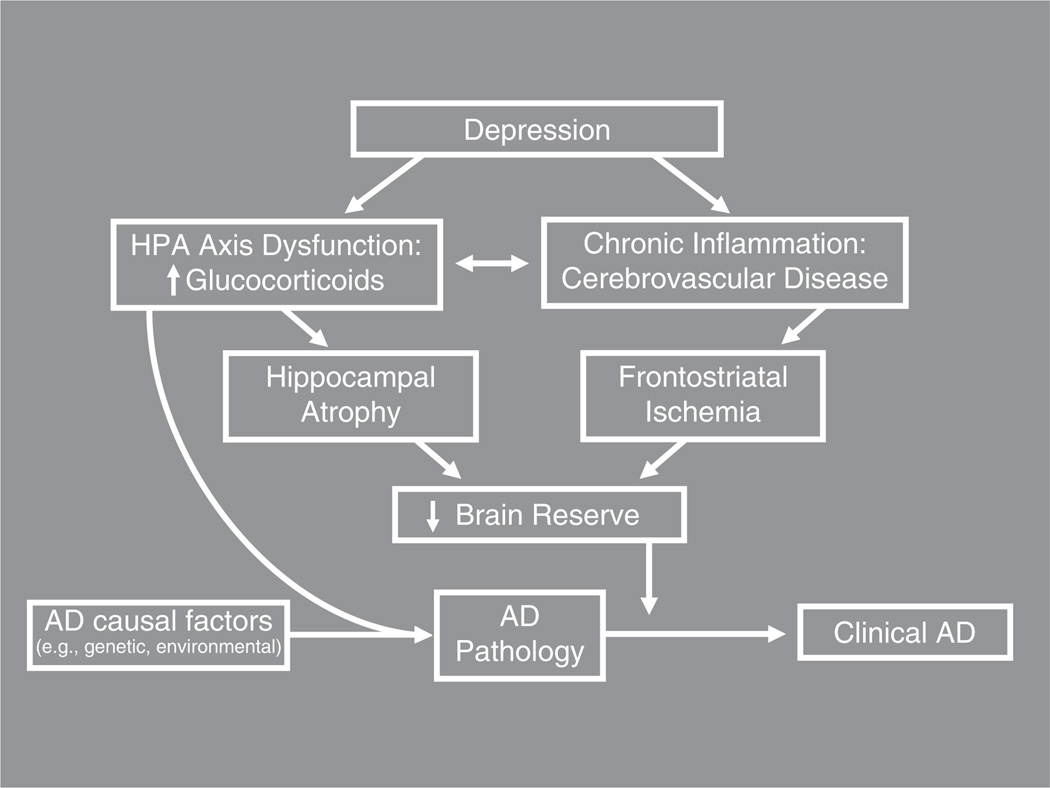
Despite ongoing efforts to elucidate their underpinnings, the neurobiological mechanisms linking depression to increased risk for cognitive decline and dementia remain poorly understood. While a comprehensive discussion of this topic is beyond the scope of this review, some of these mechanisms are highlighted in Figure 1, and are described elsewhere in greater detail (e.g., Butters et al., 2008, Byers & Yaffe, 2011). The current literature strongly suggests that from diagnostic (Diniz et al., 2013) and neuropathological (Sweet et al., 2004) perspectives, LLD is most commonly associated with increased risk for clinical Alzheimer’s disease (AD). However, there is also evidence that non-AD neuropathological changes (such as Lewy bodies, vascular disease, and hippocampal atrophy related to hypercortisolemia) occur in individuals with LLD at greater rates than in non-depressed individuals (Schneider et al., 2007; Sweet et al., 2004). Figure 1 depicts mechanisms by which depression may contribute both directly to AD pathology and indirectly, by reducing brain reserve, to the earlier appearance of clinical dementia symptoms. The figure does not, however, present a comprehensive model of all neurobiological mechanisms involved (including AD pathogenesis and various clinical outcomes), and does not depict all interrelationships among constituent parts. For example, it does not depict the possibility that in some individuals, depression may interact directly with AD causal risk factors to cause clinical manifestations of AD; in this scenario, depression may only be a risk factor in the presence of another established risk factor (Geda et al., 2006).
Fig. 1.
Potential pathways linking late-life depression to clinical onset of dementia. Caption: Adapted from Butters, M.A. et al. (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10(3), 345–357. Reproduced with permission of the publisher (Les Laboratoires Servier: Suresnes, France).
The model in Figure 1 depicts three interacting pathways, based on well-established findings that LLD is associated with hypothalamic-pituitary-adrenal (HPA) -axis dysfunction leading to elevated adrenal glucocorticoid production, as well as chronic inflammation (Butters et al., 2008). There are two potentially independent pathways—each involving HPA-axis dysfunction—through which depression may lead to increased risk for, or incidence of, AD. In the first, HPA-axis dysfunction may directly contribute to AD pathology, based on evidence from rodent studies (Dong & Csernansky, 2009; Rothman & Mattson, 2010). In the second, HPA-axis dysfunction in the form of hypercortisolemia may, over repeated episodes, lead to apoptosis of hippocampal cortisol receptors, resulting in hippocampal atrophy. The third pathway in the model involves depression-related chronic inflammation leading to ischemia, especially in frontostriatal pathways. The combination of hippocampal atrophy, generalized ischemia, and frontostriatal dysfunction likely lowers brain reserve, which in the context of underlying AD neuropathology hastens the clinical presentation of dementia. In this model, it is the depression-related increase in AD pathology, together with the reduction in brain reserve caused by depression-associated neurotoxocity, which reduces time to expression of clinical dementia (thereby accounting for the frequently reported increased risk of cognitive decline and dementia among individuals with a history of depression).
A detailed discussion of the literature summarizing the above-mentioned pathways is beyond the scope of this brief review. For recent work on the relationship between stress, HPA-axis dysfunction, and AD pathology in rodents, the interested reader is referred to reviews by Dong and Csernansky (2009) and Rothman & Mattson (2010). For detailed discussions of the relationship between hippocampal volume and depression, the reader is referred to publications by Videbech & Ravnkilde (2004), Janssen and colleagues (2007), and Sheline, Gado, and Kraemer (2003). Lastly, for a review of important work on the relationship between frontostriatal lesions and depression, the reader is referred to studies by Herrmann, Le Masurier, and Ebmeier (2008) and Krishnan and colleagues (2006).
CONCLUSIONS
Late-life depression is a heterogeneous disorder that “co-travels” with cognitive impairment but also often precedes cognitive decline. While cognitive dysfunction may improve in a subset of individuals whose depression is successfully treated, cognitive deficits are likely to persist in a significant proportion. At least some of the heterogeneity observed in the aforementioned studies could be related to differences in clinical samples, adding additional complexity to our understanding of the likelihood for cognitive improvement and highlighting the need for further study.
From a clinical perspective, regular assessment of cognitive functioning in older adults with depression is recommended during, but also following, treatment of affective symptoms. Potential adverse outcomes associated with untreated cognitive dysfunction include worsening of global functioning, diminished quality of life, and exacerbation of co-morbid medical illnesses. From a treatment standpoint, it is critical to ensure that an individual’s depressive symptoms have been treated to remission, as some cognitive deficits—such as deficits in working memory—may improve as depressive symptoms remit. Further studies are required, however, to determine the impact of depression treatment on dementia progression rates among individuals with MCI.
Given the complicated nature of the disorder, for patients with LLD and cognitive dysfunction, we recommend treatment under the supervision of a geriatric psychiatrist or cognitive neurologist. In addition to evaluating the patient’s medical history, the physician may choose to prescribe a course of antidepressant pharmacotherapy (Wiese, 2011), perhaps enhanced by an evidence-based psychotherapy such as Problem Solving Therapy (Alexopoulos, Raue, Kanellopoulos, Mackin, & Arean, 2008). With respect to specific treatment of cognitive dysfunction, cognitive augmentation or training strategies may be helpful for some patients (Fava, Ruini, & Sonino, 2003), and can be explored in combination with treatment of the depressive episode. While the introduction of a cholinesterase inhibitor (e.g., donepezil) may be considered, the potential benefit (modest improvement in cognition and functioning) must be weighed against an increased risk for worsening or recurrent depression (Reynolds et al., 2011). Finally, follow-up with a primary care physician for management of co-morbid medical illnesses is crucial to facilitate guideline-based management of conditions such as diabetes, hypertension, and heart disease, which are known to have a deleterious effect on both mood and cognition (Vance, Larsen, Eagerton, & Wright, 2011).
ACKNOWLEDGMENTS
In the past year, Dr. Koenig has received financial support from the APIRE/Janssen Resident Psychiatric Research Scholars program. In the past year, Dr. Butters has received grant support from NIMH (R01s 072947 & 080240) and has received remuneration from GlaxoSmithKline for neuropsychological services.
Footnotes
Drs. Koenig, Bhalla, and Butters report no conflicts of interest specifically pertaining to this project.
REFERENCES
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Hull J. Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Kanellopoulos D, Mackin S, Arean PA. Problem solving therapy for the depression-executive dysfunction syndrome of late life. International Journal of Geriatric Psychiatry. 2008;23(8):782–788. doi: 10.1002/gps.1988. [DOI] [PubMed] [Google Scholar]
- Arve S, Tilvis RS, Lehtonen A, Valvanne J, Sairanen S. Coexistence of lowered mood and cognitive impairment of elderly people in five birth cohorts. Aging (Milano) 1999;11(2):90–95. [PubMed] [Google Scholar]
- Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, Sheline YI. Cognitive improvement following treatment in late-life depression: Relationship to vascular risk and age of onset. American Journal of Geriatric Psychiatry. 2012;20(8):682–690. doi: 10.1097/JGP.0b013e318246b6cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso M, Miller A, Estevis E, Combs D. Neuropsychological deficits in major depressive disorder: Correlates and conundrums. In: Arnett P, editor. Secondary influences on neuropsychological test performance. New York: Oxford University Press; 2013. pp. 41–46. [Google Scholar]
- Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. Journal of Geriatric Psychiatry and Neurology. 2004;17(4):195–201. doi: 10.1177/0891988704269823. [DOI] [PubMed] [Google Scholar]
- Becker JT, Chang YF, Lopez OL, Dew MA, Sweet RA, Barnes D, Reynolds CF., III Depressed mood is not a risk factor for incident dementia in a community-based cohort. American Journal of Geriatric Psychiatry. 2009;17(8):653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman AT, Penninx BW, Deeg DJ, de Beurs E, Geerling SW, van Tilburg W. The impact of depression on the well-being, disability and use of services in older adults: A longitudinal perspective. American Journal of Geriatric Psychiatry. 2002;17(4):308–316. doi: 10.1034/j.1600-0447.2002.10078.x. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. American Journal of Geriatric Psychiatry. 2006;14(5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, Reynolds CF., 3rd Patterns of mild cognitive impairment after treatment of depression in the elderly. American Journal of Geriatric Psychiatry. 2009;17(4):308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: Review and commentary. The Journal of Gerontology Series A. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Ten Have TR, Reynolds CF, III, Katz II, Schulberg HC, Mulsant BH, Alexopoulos GS. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: A randomized controlled trial. Journal of the American Medical Association. 2004;291(9):1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF., III Changes in cognitive functioning following treatment of late-life depression. The American Journal of Psychiatry. 2000;157(12):1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, III, Becker JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nature Reviews Neurology. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., III Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry. 2013;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. Journal of Alzheimer’s Disease. 2009;18(2):459–469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, Clary CM. Does antidepressant therapy improve cognition in elderly depressed patients? The Journal of Gerontology Series A. 2003;58(12):M1137–M1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, Gur RE. Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology. 2003;18(5):529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Fava GA, Ruini C, Sonino N. Treatment of recurrent depression: A sequential psychotherapeutic and psychopharmacological approach. CNS Drugs. 2003;17(15):1109–1117. doi: 10.2165/00023210-200317150-00005. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. Depression without sadness: Functional outcomes of nondysphoric depression in later life. Journal of the American Geriatrics Society. 1997;45(5):570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Archives of General Psychiatry. 2006;63(2):153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: A prospective cohort study. Archives of Neurology. 2006;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- Godin O, Dufouil C, Ritchie K, Dartigues JF, Tzourio C, Peres K, Alperovitch A. Depressive symptoms, major depressive episode and cognition in the elderly: The three-city study. Neuroepidemiology. 2007;28(2):101–108. doi: 10.1159/000101508. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Farrer L. Depression as a risk factor for Alzheimer disease: The MIRAGE Study. Archives of Neurology. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: Meta-analysis. British Journal of Psychiatry. 2012;200(4):275–281. doi: 10.1192/bjp.bp.111.095950. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychological Medicine. 2007;37(12):1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: A systematic review. Journal of Neurology, Neurosurgery, & Psychiatry. 2008;79(6):619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Janssen J, Hulshoff Pol HE, de Leeuw FE, Schnack HG, Lampe IK, Kok RM, Heeren TJ. Hippocampal volume and subcortical white matter lesions in late life depression: Comparison of early and late onset depression. Journal of Neurology, Neurosurgery, & Psychiatry. 2007;78(6):638–640. doi: 10.1136/jnnp.2006.098087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: An updated review. Australian & New Zealand Journal of Psychiatry. 2001;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? Journal of Neurology, Neurosurgery, & Psychiatry. 2004;75(12):1662–1666. doi: 10.1136/jnnp.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MS, O’Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T, Inzitari D. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. International Journal of Geriatric Psychiatry. 2006;21(10):983–989. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. International Psychogeriatrics. 2007;19(1):125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. American Journal of Psychiatry. 2002;159(7):1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Underwood KL, Zander BA, Mastri AR, Sung JH, Frey WH., Jr Risk factors in Alzheimer’s disease: A clinicopathologic study. Neurology. 1992;42(4):770–775. doi: 10.1212/wnl.42.4.770. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. American Journal of Geriatric Psychiatry. 2004;12(1):50–56. [PubMed] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology. 2012;98(1):99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF., III Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30(3):679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., III Persistence of cognitive impairment in geriatric patients following antidepressant treatment: A randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37(2):99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia. Population study of 85-year-olds. British Journal of Psychiatry. 1999;174:249–253. doi: 10.1192/bjp.174.3.249. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG. Cognitive impairment in the euthymic phase of chronic unipolar depression. Journal of Nervous and Mental Disease. 1997;185(12):748–754. doi: 10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. American Journal of Psychiatry. 2005;162(4):691–698. doi: 10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, Alexopoulos GS, Katz IR, Lebowitz BD. Chronic depression in the elderly: Approaches for prevention. Drugs & Aging. 2001;18(7):507–514. doi: 10.2165/00002512-200118070-00004. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, DeKosky ST. Maintenance treatment of depression in old age: A randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Archives of General Psychiatry. 2011;68(1):51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Xue QL, Carlson MC. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. American Journal of Geriatric Psychiatry. 2010;18(3):204–211. doi: 10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. NeuroMolecular Medicine. 2010;12(1):56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Westlake R. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46(6):1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Doraiswamy PM. Cognitive function in late life depression: Relationships to depression severity, cerebrovascular risk factors and processing speed. Biological Psychiatry. 2006;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Speck CE, Kukull WA, Brenner DE, Bowen JD, McCormick WC, Teri L, Larson EB. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6(4):366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: The Aging, Demographics and Memory Study. International Psychogeriatrics. 2009;21(5):879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, Yesavage J. Perspectives on depression, mild cognitive impairment, and cognitive decline. Archives of General Psychiatry. 2006;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JC. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer’s disease. Biological Psychiatry. 1997;41(8):851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Reynolds CF., III Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29(12):2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Molecular Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: What is the relationship? Journal of Affective Disorders. 2004;79(1–3):81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Vance D, Larsen KI, Eagerton G, Wright MA. Comorbidities and cognitive functioning: Implications for nursing research and practice. Journal of Neuroscience in Nursing. 2011;43(4):215–224. doi: 10.1097/JNN.0b013e3182212a04. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Johansson B, Pedersen NL. History of depression and other psychiatric illness as risk factors for Alzheimer disease in a twin sample. Alzheimer Disease and Associated Disorders. 1999;13(1):47–52. doi: 10.1097/00002093-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Wiese B. Geriatric depression: The use of antidepressants in the elderly. British Columbia Medical Journal. 2011;53(7):341–347. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]