Summary
SOX7 belongs to the SOX (SRY-related HMG-box) family of transcription factors that have been shown to regulate multiple biological processes, such as hematopoiesis, vasculogenesis and cardiogenesis during embryonic development. Recent studies indicate that several SOX family members play important roles in tumorigenesis. In this review, we introduce SOX7 gene and protein structures, and discuss its expression and functional role in cancer development and progression. SOX7 is frequently downregulated in many human cancers and its reduced expression correlates with poor prognoses of several cancers. Functional studies reveal many tumor suppressive properties of SOX7 in prostate, colon, lung, and breast cancers. To date, although a few target genes of SOX7 have been identified, SOX7-mediated gene expression has not been investigated in a cancer-relevant context. Our recent studies not only for the first time demonstrate a tumor suppressive role of SOX7 in a xenograft mouse model, but also unravel that many genes regulating cell death, growth and apoptosis are affected by SOX7, strongly supporting a pivotal role of SOX7 in tumorigenesis. Thus, currently available data clearly indicate a tumor suppressive role of SOX7, but the mechanisms underlying its gene expression and tumor suppressive activity remain undetermined. The research of SOX7 in cancers remains a fertile area to be explored.
Keywords: SOX7, Tumor suppressor, Gene expression, Transcription factor, Cancer
Introduction
The founding member of the Sex-determining region Y-box (SOX) family of transcription factors is Sry (sex-determining region Y), the principal determinant of male sex development (DiNapoli and Capel, 2008). Sry contains a high-mobility group (HMG) domain responsible for its DNA-binding at the specific sequence 5’ A/T A/T CAA A/T G 3’ (Sinclair et al., 1990; Harley et al., 1994). The HMG box domain is highly conserved among SOX proteins in the same subfamily, but the homology considerably decreases among more distantly related SOX members. The SOX-F subfamily is comprised of three SOX proteins: SOX7, SOX17, and SOX18. These genes have pivotal roles in cardiovascular development during embryogenesis (Francois et al., 2010), but SOX7 in particular has also been greatly implicated in a number of human cancers (Futaki et al., 2004; Guo et al., 2008; Zhang et al., 2009; Zhou et al., 2011; Chan et al., 2012; Fan et al., 2012; Li et al., 2012, Hayano et al., 2013; Stovall et al., 2013).
Cancer is a complex disease driven by both genetic and epigenetic alterations. Frequently, aberrant changes in oncogenes and tumor suppressors can initiate and promote tumorigenesis by altering signaling networks involved in cell proliferation, apoptosis, angiogenesis, invasion, and metastasis (Hanahan and Weinberg, 2011). Current literature suggests that SOX7 acts as a tumor suppressor in multiple cancers, including colon, prostate, lung, and breast cancers. Consistently, SOX7 downregulation has been shown in advanced tumors, and correlates with poor outcome in cancer patients (Guo et al., 2008; Zhang et al., 2009; Zhou et al., 2011; Chan et al., 2012; Fan et al., 2012; Li et al., 2012; Hayano et al., 2013; Stovall et al., 2013). Meanwhile, ectopic SOX7 expression antagonizes cell growth and promotes apoptosis in a number of cancer cell lines, whereas its knockdown leads to increased capability of nontumorigenic cells to form colonies in colony formation assays, indicative of neoplastic transformation (Guo et al., 2008; Zhang et al., 2009). While SOX7 has been demonstrated to negatively regulate the stability and function of β-catenin, the full mechanism underlying SOX7-mediated tumor suppression, especially the role of its transcriptional activity, has yet to be well understood (Takash et al., 2001; Guo et al., 2008; Zhang et al., 2009; Chan et al., 2012; Saegusa et al., 2012).
In this review, we discuss the structure and function of SOX7, its role in development and human cancers, and potential mechanisms underlying SOX7’s tumor suppressive activities.
Structure and function of SOX7
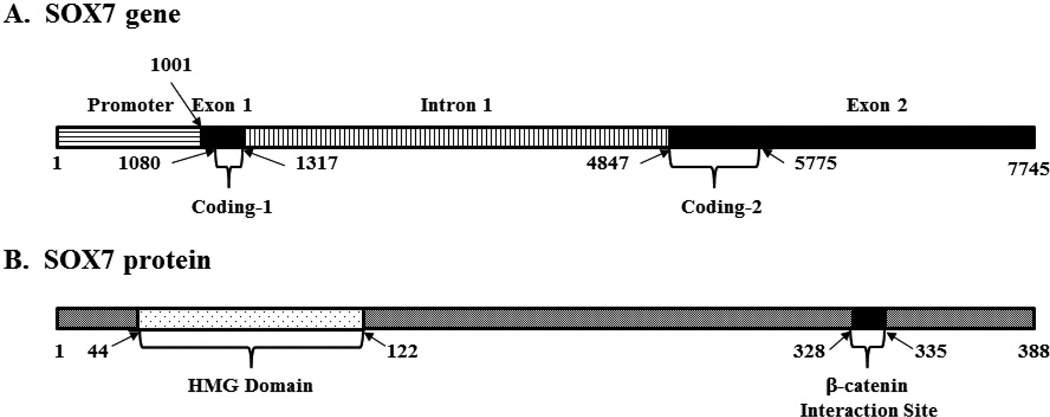
The human SOX7 gene is located in a region of chromosome 8p23.1 and is approximately 7.7 kilo base pairs (bps) in length (Fig. 1A). Two exons comprise the mRNA product with a coding region of 1,165 bps and a nearly 2 kilo bps long 3’ untranslated region (UTR). The promoter region of the SOX7 gene contains a frequently methylated CpG island that plays a role in regulating SOX7 gene expression. The human SOX7 protein is 388 amino acids in length, characterized by a C-terminal transactivation domain and an N-terminal HMG domain (amino acids 44 – 122) (Takash et al., 2001) (Fig. 1B). Previous studies suggested that phosphorylation within the HMG domain markedly reduces the DNA binding affinity of HMG domain-containing proteins (Siino et al., 1995; Stros et al., 2007). Within the HMG domain of SOX7, there are four putative phosphorylation sites (S76, S82, S89 and T87) that may contribute to SOX7 binding to DNA. The SOX7 protein bears 70% and 51% homology to SOX17 and SOX18, respectively. Although SOX7 shares its DNA binding element with many other SOX proteins, it has little homology with them outside the HMG box region, with the highest homology (58%) to SOX17. This suggests that SOX7 may have interacting partners and biological functions distinct from other SOX proteins. Many SOX proteins have been shown to interact with β-catenin, and either inhibit or promote its transcriptional activity (Bernard and Harley, 2010). SOX7 directly binds β-catenin and negatively regulates its activity. Guo et al. mapped a specific β-catenin binding site to the residues D328RNEFDQY335 of SOX7 and suggested that E331 and F332 are essential to β-catenin binding (Guo et al., 2008). Interestingly, both SOX7 and SOX17 antagonize SOX4-mediated activation of β-catenin’s transcriptional activity and inhibit Wnt signaling in endometrial carcinoma cells, likely through competitive binding to β-catenin (Sinner et al., 2007; Saegusa et al., 2012).
Fig. 1.
Structure of the SOX7 gene and protein. A. The SOX7 gene is illustrated, beginning 1kilo bp upstream of the transcription start site (1,001). Two exons (filled sections) flank a single intron (vertical bars). The two coding regions (1080 – 1317 and 4847 – 5775) are indicated in brackets. B. The SOX7 protein is depicted, with its N-terminal HMG domain (amino acids 44 – 122) and C-terminal β-catenin interaction site (328 – 335) indicated.
SOX7 possesses various functions in development, such as the initiation and maintenance of arterial identity (Herpers et al., 2008). Additionally, the transcriptional activity of SOX7 plays a role in parietal endoderm differentiation through inducing the expression of the fibroblast growth factor 3 (Fgf-3), Gata-4, Gata-6, vascular endothelial (VE)-cadherin, and laminin-·1 (Lama1) genes (Futaki et al., 2004; Murakami et al., 2004; Niimi et al., 2004; Costa et al., 2012). SOX7 modulates the balance between proliferation and differentiation in mesodermal precursors and its knockdown inhibits hematopoietic differentiation (Gandillet et al., 2009). Seguin et al demonstrated that ectopic SOX7 expression can transform human embryonic stem cells into stable endoderm progenitors, suggesting a critical role for SOX7 in lineage determination (Seguin et al., 2008). SOX7 is integral to cardiogenesis, and mice with homozygous deletion of the SOX7 exon 2 (SOX7Δex2/Δex2) showed embryonic lethality with features indicative of cardiovascular failure (Herpers et al., 2008; Wat et al., 2012). SOX7 also regulates angiogenesis and vasculogenesis through mechanisms that are redundant with those of SOX18, implicating its relevance to cancer (Zhang et al., 2005; Herpers et al., 2008; Chung et al., 2011). Consistent with its important role in normal development, SOX7 dysregulation has been implicated in different diseases. Wat et al reported that mice with the homozygous deletion of SOX7’s exon 2, comprising 79% of its coding region, are embryonically lethal and those with the heterozygous mutation develop congenital diaphragmatic hernia (Wat et al., 2012). These data suggest that SOX7 haploinsufficiency may contribute to development of congenital diaphragmatic hernia in humans.
The role of SOX7 in human cancers
As many well-characterized tumor suppressors, SOX7 expression is reduced in human cancers compared with normal tissues. Most studies demonstrated frequent downregulation of SOX7 in a variety of human cancers, including breast, lung, colon, and prostate (Katoh, 2002; Guo et al., 2008; Zhang et al., 2009; Chan et al., 2012; Zhong et al., 2012; Hayano et al., 2013; Stovall et al., 2013), while only one report suggested increased SOX7 transcript levels in several pancreatic, gastric, and esophageal cancer cell lines, and four cases of primary gastric cancer (Katoh, 2002). Multiple mechanisms contribute to the suppression of gene expression, including allele deletion, promoter methylation, histone modifications, mRNA translation or stability, and protein turnover rates (Suva et al., 2013). SOX7 downregulation has predominantly been attributed to the hypermethylation of its promoter that correlates with poor prognosis in myelodysplastic syndrome (MDS) and lung cancer patients (Fan et al., 2012; Li et al., 2012). Recently, we demonstrated that shRNA-mediated silencing of DNA methyltransferase 1 (DNMT1), the key enzyme that maintains DNA methylation patterns during cell division, results in elevated SOX7 mRNA levels in MCF-7 and MDA-MB-231 breast cancer cells (Stovall et al., 2013). Consistently, SOX7 mRNA expression is restored upon pharmacological inhibition of DNMTs by 5-aza-2’-deoxycytidine in multiple cancer cell lines (Guo et al., 2008; Zhang et al., 2009; Stovall et al., 2013), indicating that SOX7 is a common target of aberrant tumor epigenetics. However, while SOX7 protein levels are consistently increased in prostate and colon cancer cells upon DNMT inhibition, we were unable to detect concomitant protein increase in breast cancer cell lines, suggesting that additional mechanisms are involved in downregulating SOX7 expression (Stovall et al., 2013).
The SOX7 gene is deleted (both homozygously and hemizygously) in multiple non-small cell lung cancer (NSCLC) cell lines (Hayano et al., 2013). Additionally, loss of heterozygosity (LOH) at the 8p22–23 locus that houses the SOX7 gene was found in 36% of ductal carcinoma in situ breast cancer patients (Anbazhagan et al., 1998). Collectively, these reports indicate that several human cancers preferentially downregulate SOX7 through epigenetic and genetic mechanisms.
The effects of overexpressing SOX7 on cancer cells in vitro have been extensively studied by several groups. In prostate and colon cancer cells, forced SOX7 expression inhibits proliferation and colony formation (Guo et al., 2008; Zhang et al., 2009), and induces apoptosis in NSCLC, colon, endometrial, and prostate cancer cells (Zhang et al., 2009; Chan et al., 2012; Hayano et al., 2013). We demonstrated that ectopic SOX7 expression through a Doxycycline (Dox)-inducible system significantly reduces MDA-MB-231 breast cancer cell proliferation and their xenograft tumor growth in athymic nude mice (Stovall et al., 2013). Importantly, SOX7 silencing conferred a growth advantage to HEK293 cells (Zhang et al., 2009) and significantly increased proliferation, migration and invasion of nontumorigenic breast cells (Stovall et al., 2013). The proliferative effects of SOX7 depletion provide strong support to its tumor suppressive role, because these studies can truly recapitulate the scenario of SOX7 downregulation during tumorigenesis.
Despite the growing body of evidence for SOX7’s tumor suppressive role in many cancers, the precise molecular mechanism(s) by which it achieves these effects remains unclear. Currently, SOX7 has been demonstrated to activate the expression of FGF3, GATA4, GATA6, LAMA1, VE-Cadherin and SOX4 (Futaki et al., 2004; Murakami et al., 2004; Niimi et al., 2004; Costa et al., 2012; Saegusa et al., 2012). Thus, the target genes of the transcription factor SOX7 are highly understudied. To date, only one report is relevant to SOX7-mediated gene expression in a cancer-relevant setting (Saegusa et al., 2012); most SOX7-target genes were identified through developmental studies. SOX7 activates expression of the basement membrane component Lama1, a large glycoprotein that participates in regulating cell migration and other processes through its interactions with various receptors, such as integrins (Beck et al., 1990; Engel, 1992; Timpl and Brown, 1994). SOX7 activates Lama1 through binding its enhancer, and acts synergistically with other transcription actors (SP1/SP3, NF-Y, and SOX17) to mediate Lama1 expression (Niimi et al., 2004). Coexpression of SOX7 with the Fgf-3 promoter-driven luciferase reporter led to increased luciferase activity, and mutation of the SOX7 binding site significantly reduced this activation, suggesting an activating role for SOX7 in Fgf-3 transcription (Murakami et al., 2004). Fgf-3 is critical to development (Wilkinson et al., 1989; Mahmood et al., 1995; McKay et al., 1996) and suggested to act as an oncogene in mouse mammary tumors (Dickson et al., 1984); thus, its activation by SOX7 in tumors would contradict the widely supported tumor suppressive function of SOX7. These functional discrepancies are likely due to the dependency of SOX7-regulated gene expression on the cellular and molecular contexts of a particular cell type. Consistent with this prediction, SOX7 activates Lama1 in undifferentiated mouse F9 embryonal cells, but not HeLa cells (Niimi et al., 2004).
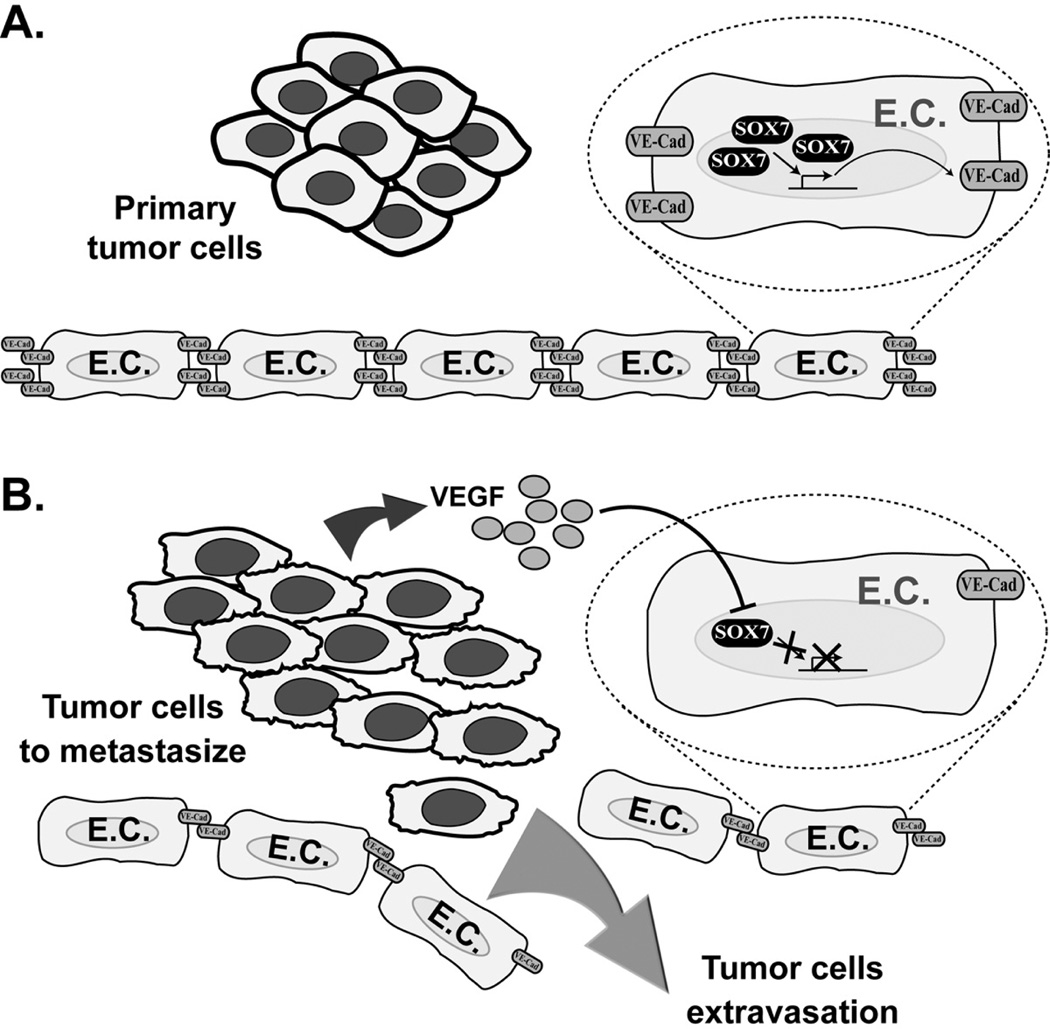
SOX7 also targets vascular endothelial (VE)-cadherin (Costa et al., 2012), an adhesion protein essential for maintaining endothelial cell contacts (Vestweber, 2008). SOX7 binding to the VE-cadherin promoter led to its activation in a reporter assay in HEK293 cells, and the integrity of the SOX7 binding site proximal to the transcription start site is necessary for complete promoter activation in bEnd.3 endothelial cells (Costa et al., 2012). Interestingly, stimulation by vascular endothelial growth factor (VEGF) that is frequently overexpressed in human cancers downregulates SOX7 (Costa et al., 2012), while the same treatment also increases the permeability of endothelial cell monolayers (Esser et al., 1998). Thus, it is reasonable to hypothesize a feedback loop in human tumors with upregulated VEGF signaling and downregulated SOX7, in which VEGF signaling inhibits SOX7-mediated VE-cadherin expression, resulting in reduced endothelial cell contacts and increased intravasation of tumor cells into the bloodstream (Fig. 2). This predicted mechanism may explain the positive correlation between SOX7 expression and distant metastasis-free survival in prostate and breast cancers (Zhong et al., 2012; Stovall et al., 2013). Notably, the effects of SOX7 on its currently discovered target genes are all activating (Futaki et al., 2004; Murakami et al., 2004; Niimi et al., 2004; Costa et al., 2012). This is consistent with a previous study indicating that SOX7 possesses a transactivation domain but lacks any well-defined transrepression domain (Lefebvre et al., 2007).
Fig. 2.
Proposed role of SOX7 in early metastasis. A. Overexpression of VEGF by the primary tumor inhibits SOX7 expression in endothelial cells (ECs), decreasing VE-cadherin gene expression and reducing endothelial cell contacts. B. Elevated VEGF levels contribute to SOX7 downregulation in tumor cells, promoting their proliferation, migration and invasion through the more permeable EC wall.
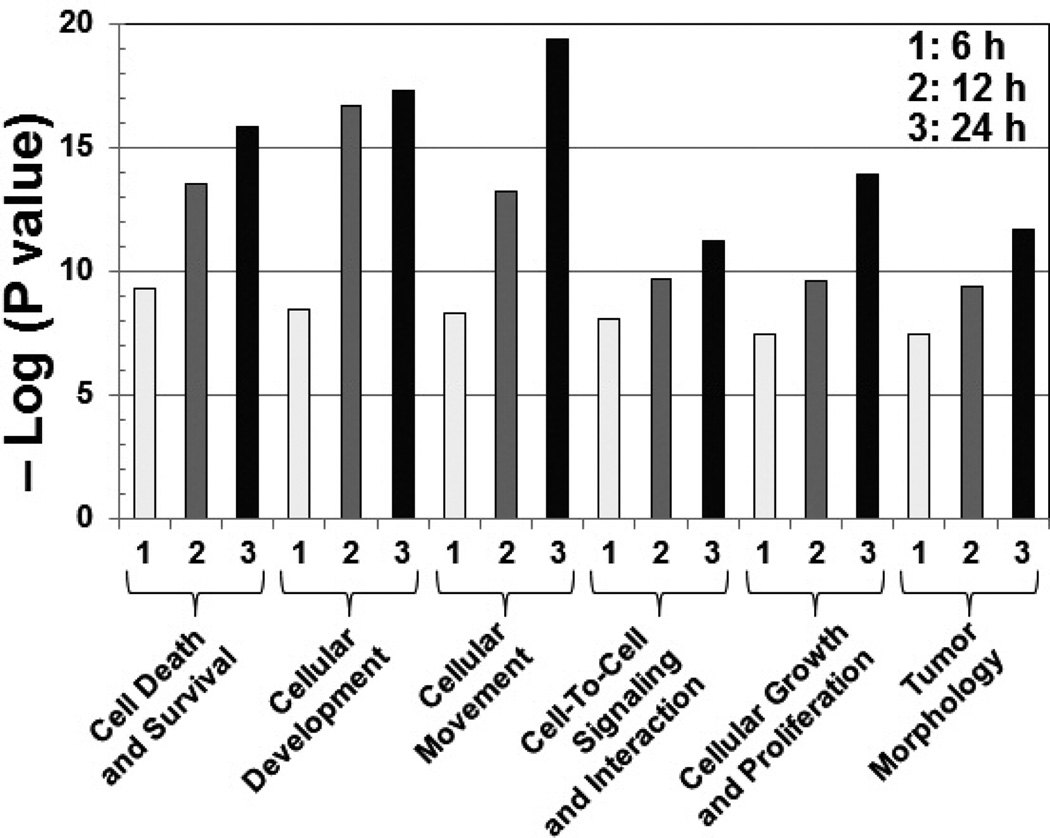
To better understand the role of SOX7 as a transcription factor in cancer, we performed a microarray study to analyze changes in gene expression in breast cancer cells upon ectopic SOX7 expression for 6, 12, or 24 hours (h). Interestingly, we observed altered levels of multiple cancer-relevant genes. We grouped genes affected by SOX7 expression into ontological categories based on their biological functions, and observed that genes involved in cell death and survival, movement, and growth and proliferation were highly and significantly altered (Fig. 3). These data support a regulatory role of SOX7 as a transcription factor in mammary oncogenesis.
Fig. 3.
Ectopic SOX7 leads to changes in genes of multiple functional groups. SOX7 was overexpressed in breast cancer cells for 6, 12, or 24 h, and changes in gene expression were analyzed by a microarray. P values are given as “-log” values and tend to increase from left to right.
Multiple studies indicate SOX7 antagonism of Wnt/β-catenin signaling (Takash et al., 2001; Guo et al., 2008; Zhang et al., 2009; Chan et al., 2012; Saegusa et al., 2012), a commonly upregulated pathway in human cancers involved in the regulation of cell proliferation, survival, and migration (Logan and Nusse, 2004). SOX7 was first found to inhibit TCF/LEF-β-catenin activation of the TCF/LEF-dependent TOPFLASH reporter in HEK293 cells (Takash et al., 2001), and this observation was later recapitulated in colon and prostate cancer cell lines (Guo et al., 2008; Zhang et al., 2009). Further, SOX7 colocalizes and physically interacts with β-catenin in endometrial cancer cells (Chan et al., 2012). As discussed above, the SOX7-β-catenin interaction is mediated by D328RNEFDQY335 of SOX7 (Guo et al., 2008); however, the SOX7-binding region in β-catenin has yet to be determined. Functionally, SOX7 overexpression reduces mRNA and protein levels of the Wnt-signaling targets cyclin D1 and survivin in colon cancer cells (Zhang et al., 2009), but whether this regulation is through SOX7-mediated expression of these two genes is unclear. Interestingly, a SOX7-related protein, SOX4, may function as either an oncogene or tumor suppressor, depending on cellular contexts (Vervoort et al., 2012). It acts as a positive regulator of β-catenin signaling through its upregulation of TCF4 transcription (Saegusa et al., 2012). SOX7 transcriptionally activates SOX4 expression in endometrial cancers, but also inhibits SOX4-mediated β-catenin/TCF4-driven transcription (Saegusa et al., 2012), indicating that a complex regulatory network likely exists in the tumor scenario among different SOX members. The well-characterized SOX7 inhibition of Wnt/β-catenin signaling suggests that SOX7 may have a tumor suppressive role that is independent of its DNA-binding ability, in addition to its activity in regulating gene transcription.
Based on markedly decreased SOX7 expression in multiple human tumors, and its tumor suppressive effects on cancer cell lines both in vitro and in vivo, SOX7 can potentially serve as a prognostic marker for a number of human cancers. In prostate cancer, high serum levels of prostate-specific antigen (PSA) and metastasis suggest poor clinical outcomes of the patients; SOX7 protein levels were markedly reduced in patients with these poor prognostic indicators (Zhong et al., 2012). Consistently, SOX7 downregulation was an independent predictor of decreased recurrence-free survival in prostate cancer patients (Zhong et al., 2012). In breast cancer, we observed that SOX7 expression was reduced in relatively aggressive breast cancer subtypes (e.g. HER2 enriched, Luminal B, and basal-like) compared to those subtypes with favorable prognosis (normal-like) (Stovall et al., 2013). We also found that reduced SOX7 transcript levels correlated with significantly decreased distant metastasis-free survival in an international breast cancer metacohort consisting of 674 patients (Stovall et al., 2013), strongly supporting SOX7 downregulation as a prognostic marker for metastatic outcome of breast cancer patients. Similarly, reduced SOX7 expression in lung adenocarcinomas correlated with multiple poor prognostic indicators and both overall and disease-free survival, and was further validated as an independent prognostic factor (Li et al., 2012). Finally, SOX7 promoter methylation, the most studied mechanism of its downregulation in human cancers, is strongly correlated with shorter overall and cumulative survival in MDS patients (Fan et al., 2012). Taken together, these data strongly suggest that SOX7 serves as a prognostic marker for disease progression in a number of human cancers, and warrant its study in additional tumor types.
Conclusions and future directions
SOX7 is a member of the SOX family of transcription factors and has critical roles in multiple developmental processes. Many SOX proteins, such as SOX2, SOX4, and SOX9, have been demonstrated to have oncogenic activities in human tumors (Castillo and Sanchez-Cespedes, 2012). However, an increasing body of evidence supports a tumor suppressive role of SOX7 in human cancers. First, its downregulation has been reported in several cancers, including prostate, colon, breast and lung cancers. Second, SOX7 overexpression in multiple cancer cell lines inhibits their proliferation, invasion and colony formation while promoting apoptosis; consistently, SOX7 knockdown confers a growth advantage to nontumorigenic cells (Guo et al., 2008; Zhang et al., 2009; Stovall et al., 2013). Third, frequent SOX7 promoter hypermethylation was detected in human tumor tissues and cell lines (Guo et al., 2008; Zhang et al., 2009; Chan et al., 2012; Li et al., 2012; Zhong et al., 2012; Hayano et al., 2013; Stovall et al., 2013). Finally, decreased SOX7 expression frequently correlates with poor disease outcomes of cancer patients, indicating its potential as a valuable prognostic marker (Fan et al., 2012; Li et al., 2012; Zhong et al., 2012; Stovall et al., 2013).
While SOX7 appears to execute a tumor suppressive function in a broad range of cancers, the precise molecular mechanism behind these effects remains unclear. As a transcription factor, SOX7 target genes in the context of cancer have not been extensively studied. However, multiple reports indicate that SOX7 inhibits proliferative β-catenin signaling through direct protein interaction, suggesting that it antagonizes tumor growth, at least in part, by its activities independent of DNA binding (Takash et al., 2001; Guo et al., 2008; Zhang et al., 2009; Chan et al., 2012; Saegusa et al., 2012). It is likely that additional, complex regulatory networks exist between SOX7 and other SOX factors, and their disruption contributes to cancer progression. Future studies should be aimed at identifying SOX7 transcriptional targets and protein interaction partners in cancers. This is necessary to fully understand the mechanisms underlying SOX7-mediated tumor suppression.
Acknowledgements
DBS and PC were supported by NCI training grant 5T32CA079448. GS was supported by the American Cancer Society (RSG-09-082-01-MGO) and the National Institutes of Health (R01 CA106314).
References
- Anbazhagan R, Fujii H, Gabrielson E. Allelic loss of chromosomal arm 8p in breast cancer progression. Am. J. Pathol. 1998;152:815–819. [PMC free article] [PubMed] [Google Scholar]
- Beck K, Hunter I, Engel J. Structure and function of laminin: Anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bernard P, Harley VR. Acquisition of sox transcription factor specificity through protein-protein interaction, modulation of wnt signalling and post-translational modification. Int. J. Biochem. Cell Biol. 2010;42:400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Castillo SD, Sanchez-Cespedes M. The sox family of genes in cancer development: Biological relevance and opportunities for therapy. Expert Opin. Ther. Targets. 2012;16:903–919. doi: 10.1517/14728222.2012.709239. [DOI] [PubMed] [Google Scholar]
- Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of sox7 is associated with aberrant activation of wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–1556. doi: 10.18632/oncotarget.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MI, Ma AC, Fung TK, Leung AY. Characterization of sry-related hmg box group f genes in zebrafish hematopoiesis. Exp. Hematol. 2011;39:986–998. doi: 10.1016/j.exphem.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Costa G, Mazan A, Gandillet A, Pearson S, Lacaud G, Kouskoff V. Sox7 regulates the expression of ve-cadherin in the haemogenic endothelium at the onset of haematopoietic development. Development. 2012;139:1587–1598. doi: 10.1242/dev.071282. [DOI] [PubMed] [Google Scholar]
- Dickson C, Smith R, Brookes S, Peters G. Tumorigenesis by mouse mammary tumor virus: Proviral activation of a cellular gene in the common integration region int-2. Cell. 1984;37:529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- DiNapoli L, Capel B. Sry and the standoff in sex determination. Mol. Endocrinol. 2008;22:1–9. doi: 10.1210/me.2007-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Laminins and other strange proteins. Biochemistry. 1992;31:10643–10651. doi: 10.1021/bi00159a001. [DOI] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces ve-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998;111(Pt 13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Fan R, Zhang LY, Wang H, Yang B, Han T, Zhao XL, Wang W, Wang XQ, Lin GW. Methylation of the cpg island near sox7 gene promoter is correlated with the poor prognosis of patients with myelodysplastic syndrome. Tohoku J. Exp. Med. 2012;227:119–128. doi: 10.1620/tjem.227.119. [DOI] [PubMed] [Google Scholar]
- Francois M, Koopman P, Beltrame M. Soxf genes: Key players in the development of the cardio-vascular system. Int. J. Biochem. Cell Biol. 2010;42:445–448. doi: 10.1016/j.biocel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Futaki S, Hayashi Y, Emoto T, Weber CN, Sekiguchi K. Sox7 plays crucial roles in parietal endoderm differentiation in f9 embryonal carcinoma cells through regulating gata-4 and gata-6 expression. Mol. Cell Biol. 2004;24:10492–10503. doi: 10.1128/MCB.24.23.10492-10503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandillet A, Serrano AG, Pearson S, Lie ALM, Lacaud G, Kouskoff V. Sox7-sustained expression alters the balance between proliferation and differentiation of hematopoietic progenitors at the onset of blood specification. Blood. 2009;114:4813–4822. doi: 10.1182/blood-2009-06-226290. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhong D, Lau S, Liu X, Dong XY, Sun X, Yang VW, Vertino PM, Moreno CS, Varma V, Dong JT, Zhou W. Sox7 is an independent checkpoint for beta-catenin function in prostate and colon epithelial cells. Mol. Cancer Res. 2008;6:1421–1430. doi: 10.1158/1541-7786.MCR-07-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harley VR, Lovell-Badge R, Goodfellow PN. Definition of a consensus DNA binding site for sry. Nucleic Acids Res. 1994;22:1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano T, Garg M, Yin D, Sudo M, Kawamata N, Shi S, Chien W, Ding LW, Leong G, Mori S, Xie D, Tan P, Koeffler HP. Sox7 is down-regulated in lung cancer. J. Exp. Clin. Cancer Res. 2013;32:17. doi: 10.1186/1756-9966-32-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ. Res. 2008;102:12–15. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]
- Katoh M. Expression of human sox7 in normal tissues and tumors. Int. J. Mol. Med. 2002;9:363–368. [PubMed] [Google Scholar]
- Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by sry-related high-mobility-group box (sox) transcription factors. Int. J. Biochem. Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ge Z, Song S, Zhang S, Yan H, Huang B, Zhang Y. Decreased expression of sox7 is correlated with poor prognosis in lung adenocarcinoma patients. Pathol. Oncol. Res. 2012;18:1039–1045. doi: 10.1007/s12253-012-9542-8. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for fgf-3 during cranial neural development in the chicken. Development. 1995;121:1399–1410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- McKay IJ, Lewis J, Lumsden A. The role of fgf-3 in early inner ear development: An analysis in normal and kreisler mutant mice. Dev. Biol. 1996;174:370–378. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- Murakami A, Shen H, Ishida S, Dickson C. Sox7 and gata-4 are competitive activators of fgf-3 transcription. J. Biol. Chem. 2004;279:28564–28573. doi: 10.1074/jbc.M313814200. [DOI] [PubMed] [Google Scholar]
- Niimi T, Hayashi Y, Futaki S, Sekiguchi K. Sox7 and sox17 regulate the parietal endoderm-specific enhancer activity of mouse laminin alpha1 gene. J. Biol. Chem. 2004;279:38055–38061. doi: 10.1074/jbc.M403724200. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Kuwata T. Sox4 functions as a positive regulator of beta-catenin signaling through upregulation of tcf4 during morular differentiation of endometrial carcinomas. Lab. Invest. 2012;92:511–521. doi: 10.1038/labinvest.2011.196. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by sox transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Siino JS, Nissen MS, Reeves R. Replacement of conserved threonines by alanine residues in high mobility group protein hmg-i(y): Effect on DNA binding affinity. Biochem. Biophys. Res. Commun. 1995;207:497–507. doi: 10.1006/bbrc.1995.1216. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and sox4 differentially regulate beta-catenin/t-cell factor activity and proliferation of colon carcinoma cells. Mol. Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall DB, Wan M, Miller LD, Cao P, Maglic D, Zhang Q, Stampfer MR, Liu W, Xu J, Sui G. The regulation of sox7 and its tumor suppressive role in breast cancer. Am. J. Pathol. 2013;183:1645–1653. doi: 10.1016/j.ajpath.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M, Launholt D, Grasser KD. The hmg-box: A versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 2007;64:2590–2606. doi: 10.1007/s00018-007-7162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takash W, Canizares J, Bonneaud N, Poulat F, Mattei MG, Jay P, Berta P. Sox7 transcription factor: Sequence, chromosomal localisation, expression, transactivation and interference with wnt signalling. Nucleic Acids Res. 2001;29:4274–4283. doi: 10.1093/nar/29.21.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Vervoort SJ, van Boxtel R, Coffer PJ. The role of sry-related hmg box transcription factor 4 (sox4) in tumorigenesis and metastasis: Friend or foe? Oncogene. 2012;32:3397–3409. doi: 10.1038/onc.2012.506. [DOI] [PubMed] [Google Scholar]
- Vestweber D. Ve-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- Wat MJ, Beck TF, Hernandez-Garcia A, Yu Z, Veenma D, Garcia M, Holder AM, Wat JJ, Chen Y, Mohila CA, Lally KP, Dickinson M, Tibboel D, de Klein A, Lee B, Scott DA. Mouse model reveals the role of sox7 in the development of congenital diaphragmatic hernia associated with recurrent deletions of 8p23.1. Hum. Mol. Genet. 2012;21:4115–4125. doi: 10.1093/hmg/dds241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, McMahon AP. Expression pattern of the fgf-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989;105:131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Klymkowsky MW. Sox7 and sox18 are essential for cardiogenesis in xenopus. Dev. Dyn. 2005;234:878–891. doi: 10.1002/dvdy.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang S, Dong W, Li L, Feng Y, Pan L, Han Z, Wang X, Ren G, Su D, Huang B, Lu J. Sox7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2009;277:29–37. doi: 10.1016/j.canlet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Zhong WD, Qin GQ, Dai QS, Han ZD, Chen SM, Ling XH, Fu X, Cai C, Chen JH, Chen XB, Lin ZY, Deng YH, Wu SL, He HC, Wu CL. Soxs in human prostate cancer: Implication as progression and prognosis factors. BMC Cancer. 2012;12:248. doi: 10.1186/1471-2407-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Huang SY, Feng JX, Gao YY, Zhao L, Lu J, Huang BQ, Zhang Y. Sox7 is involved in aspirin-mediated growth inhibition of human colorectal cancer cells. World J. Gastroenterol. 2011;17:4922–4927. doi: 10.3748/wjg.v17.i44.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]