Intellectual disability, which is characterized by significant limitations in both intellectual functioning and adaptive behavior that begin before the age of 18 years,1 affects 1.5 to 2% of the population in Western countries.2 A diagnosis of intellectual disability is usually made when IQ testing reveals an IQ of less than 70, which means that often the diagnosis is not made until late childhood or early adulthood. However, most persons with intellectual disability are identified early in childhood on the basis of concern about developmental delays, which may include motor, cognitive, and speech delays. A genetic underpinning of this disorder has long been recognized in a subset of cases, with trisomy 21 (Down’s syndrome) detectable by chromosomal studies since 1959.3 Trisomy 21 remains the most important chromosomal cause of intellectual disability. Single-gene causes have also been identified for a number of intellectual disability syndromes and include both autosomal and X-linked genes, with the fragile X syndrome being the most common of inherited syndromes caused by a single-gene defect leading to this phenotype in male patients.
Autism spectrum disorders have been estimated to affect as many as 1 in 100 to 1 in 150 children.4,5 Disorders on the autism spectrum share features of impaired social relationships, impaired language and communication, and repetitive behaviors or a narrow range of interests. Many children with autism spectrum disorders also have intellectual disability, and approximately 75% have lifelong disability requiring substantial social and educational support. Thus, autism and intellectual disability together represent an important health burden in the population and are frequent reasons for referral to genetics and developmental pediatrics clinics for a diagnostic workup.
During the past decade, advances in genetic research have enabled genomewide discovery of chromosomal copy-number changes and single-nucleotide changes in patients with intellectual disability and autism as well as in those with other disorders. These technological advances — which include array comparative genomic hybridization (CGH), single-nucleotide-polymorphism (SNP) genotyping arrays, and massively parallel sequencing — have transformed the approach to the identification of etiologic genes and genomic rearrangements in the research laboratory and are now being applied in the clinical diagnostic arena. Here we review these techniques and how they have enabled the rapid discovery of chromosomal and single-gene causes of intellectual disability and autism.
COPY-NUMBER CHANGES
DELETIONS AND DUPLICATIONS
A copy-number change is defined as a deletion or duplication of a stretch of DNA as compared with the reference human genome. Copy-number changes may range in size from a kilobase (kb) to several megabases (Mb) or even an entire chromosome (trisomies and monosomies) and can involve one or more genes. Deletions may be heterozygous, in which one of the usual two copies is missing; homozygous, in which both copies are missing; or hemizygous (e.g., X-chromosome deletions in a male patient).
Duplications often result in three copies, as compared with the usual two copies, although some regions of the genome are present in more than three copies and the range of observed copy numbers is much greater. Multiple studies of large control cohorts have shown that some regions of the genome are tolerant of copy-number changes and that every person carries many copy-number changes that are, for the most part, benign.6–10 Two individual genomes may differ by several megabases of DNA content because of copy-number changes. In this article, we focus on copy-number changes that underlie intellectual disability and autism and are generally not found in control cohorts.
Changes in chromosomal copy number were first recognized as a cause of intellectual disability in 1959, when it was discovered that an extra copy of chromosome 21 is the cause of Down’s syndrome.3 Steady advances in chromosome-banding techniques (see the Glossary) facilitated the detection of unbalanced rearrangements, including translocations, large deletions or duplications, and supernumerary marker chromosomes. The minimum size of disrupted chromosome that can be detected by chromosome banding is approximately 5 to 10 Mb, and such cytogenetically visible rearrangements are responsible for 10 to 15% of cases of intellectual disability.11 It was soon recognized that some patients with syndromic forms of intellectual disability also had deletions in the same chromosomal region, a finding that resolved the molecular cause of microdeletion syndromes, including the Prader–Willi and Angelman syndromes (deletion of 15q11-q13),12 the Williams–Beuren syndrome (deletion of 7q11.23),13 and the Smith–Magenis syndrome (deletion of 17p12).14 It was also noted that 1 to 3% of patients with autism had a maternally inherited duplication involving 15q11-q13.15
Fluorescence in situ hybridization (FISH), which was developed in the 1980s, represented an important advance in the reliable detection of smaller chromosome rearrangements and allowed physicians to rapidly confirm the diagnosis of a suspected microdeletion or microduplication syndrome in a patient. Another assay that FISH permitted was the investigation of subtelomeric deletions and duplications, which were found to cause 2.5 to 5% of previously unexplained intellectual disability.16–18
The more recent introduction of genomewide techniques to identify submicroscopic copy-number changes has revolutionized both the approach used in the laboratory to identify chromosome abnormalities that are responsible for intellectual disability and the diagnostic approach used in the clinic for patients with developmental delays or intellectual disability. The two techniques that are routinely used for discovery of copy-number changes are array CGH and SNP genotyping arrays, collectively referred to as chromosome microarrays (see text box). Since their introduction, these techniques have been applied to large case series of patients with intellectual disability or developmental delays.19–24 Numerous studies have also investigated the role of rare copy-number changes in autism.25–30 Identification of specific copy-number changes in affected patients as compared with control subjects has led to a rapid increase in the discovery of novel microdeletion and microduplication syndromes associated with intellectual disability and autism.31 Many of these syndromes are listed in Table 1 and several are discussed below.
Table 1.
Novel Recurrent Copy-Number Changes Associated with Intellectual Disability and Related Disorders.*
| Chromosome Region | Coordinates in Mb† | Deletion or Duplication Associated with Disorder | Selected References |
|---|---|---|---|
| 1q21.1 | Chromosome 1: 145.0–146.35 | Deletion: intellectual disability, schizophrenia, multiple congenital anomalies Duplication: intellectual disability, autism |
Brunetti-Pierri et al.,32 Mefford et al.,33 International Schizophrenia Consortium,34 Stefansson et al.,35 Greenway et al.,36 Haldeman-Englert and Jewett37 |
| 3q29 | Chromosome 3: 197.4–198.9 | Deletion: intellectual disability, schizophrenia Duplication: intellectual disability |
Ballif et al.,38 Lisi et al.,39 Willatt et al.40 |
| 10q22-q23 | Chromosome 10: 81.12–89.07 | Deletion: intellectual disability | Balciuniene et al.,41 van Bon et al.42 |
| 15q11.2 | Chromosome 15: 20.3–20.7 | Deletion: intellectual disability, schizophrenia, epilepsy | Stefansson et al.,35 de Kovel et al.,43 Mefford et al.,44 Burnside et al.,45 Doornbos et al.,46 Murthy et al.,47 von der Lippe et al.48 |
| 15q13.3 | Chromosome 15: 28.7–30.2 | Deletion: intellectual disability, epilepsy, schizophrenia, autism | Stefansson et al.,35 Helbig et al.,49 Sharp et al.,50 van Bon et al.,51 Ben-Shachar et al.,52 Pagnamenta et al.,53 Miller et al.54 |
| 15q24 | Chromosome 15: 72.2–73.8 | Deletion: intellectual disability, autism | Andrieux et al.,55 Sharp et al.,56 Mefford et al.,57 El-Hattab et al.58 |
| 16p11.2 (a) | Chromosome 16: 29.5–30.1 | Deletion: intellectual disability, autism, obesity Duplication: schizophrenia |
Weiss et al.,29 Battaglia et al.,59 Bijlsma et al.,60 Hempel et al.,61 Shinawi et al.,62 Jacquemont et al.,63 Walters et al.,64 McCarthy et al.65 |
| 16p11.2 (b) | Chromosome 16: 28.7–29.0 | Deletion: intellectual disability, obesity | Bachmann-Gagescu et al.,66 Bochukova et al.67 |
| 16p12 | Chromosome 16: 21.8–22.4 | Deletion: intellectual disability | Girirajan et al.68 |
| 16p13.11 | Chromosome 16: 15.4–16.4 | Deletion: intellectual disability, epilepsy, autism, schizophrenia Duplication: intellectual disability, ADHD, autism |
de Kovel et al.,43 Mefford et al.,44 Heinzen et al.,69 Williams et al.,70 Ullmann et al.,71 Kirov et al.72 |
| 17q12 | Chromosome 17: 31.8–33.3 | Deletion: intellectual disability, autism, schizophrenia | Moreno-De-Luca et al.,73 Loirat et al.74 |
| 17q21.3 | Chromosome 17: 41.0–41.7 | Deletion: intellectual disability | Koolen et al.,20 Sharp et al.,23 Shaw-Smith et al.,24 Koolen et al.75 |
The listed recurrent deletions and duplications are those that have been reported since 2006. ADHD denotes attention deficit–hyperactivity disorder.
The coordinates are based on the National Center for Biotechnology Information (NCBI) build 36.
CHROMOSOME MICROARRAYS.
Array comparative genomic hybridization (CGH)
Array CGH is a comparative assay in which DNA from the patient is fluorescently labeled with one fluorescent dye and DNA from a healthy control subject (reference DNA) is labeled with a second fluorescent dye. The samples are cohybridized to an array containing known DNA sequences called probes. The fluorescence intensity of each dye at each spot is measured. Differences in relative fluorescence intensities at a given spot on the array reflect differences in copy number between the genome of the patient and that of the reference DNA. The size of the copy-number change that can be identified by this method varies according to the number and spacing of probes on the array.
Single-nucleotide-polymorphism (SNP) genotyping array
A SNP is a site in the genome at which two different alleles are present in the general population, often referred to as the A allele and the B allele. SNP genotyping arrays are fluorescence-based assays in which the A allele is tagged with one fluorescent dye and the B allele is tagged with another. Analysis of SNP array data includes measurement of the total fluorescence intensity for a site and calculation of the ratio of the fluorescence intensities for the two dyes. At each site, most subjects will have one of three genotypes, or combinations of alleles: AA, AB, or BB. If there is a deletion, the total fluorescence intensity will be lower and the subject will have only one allele (e.g., A–) at all SNP sites within the deleted region. Duplications are represented by an increased total fluorescence intensity and altered ratio of alleles: AAA, AAB, ABB, or BBB. Because SNP arrays provide genotype information, they can also be used to identify large stretches of homozygosity in the genome, which can represent consanguinity or uniparental disomy, neither of which is detectable by means of array CGH.
ROLE IN INTELLECTUAL DISABILITY SYNDROMES
Several novel microdeletions have been identified in patients who have a similar clinical picture. Heterozygous deletions of 17q21.31, which were described by three groups simultaneously,20,23,24 are associated with moderate-to-severe intellectual disability, hypotonia, facial dysmorphic features, occasional cardiac and renal abnormalities, and seizures. The deletion is 500 to 650 kb in size and is not detectable by routine karyotyping. All 17q21.31 deletions that have been identified are de novo, and the deletion has never been seen in healthy control subjects. Its prevalence is estimated to be approximately 1 in 16,000 persons.75 Deletions of 15q24 are much rarer, but patients with 15q24 microdeletions also have an intellectual disability syndrome with recognizable features.55–57,76,77 Common features include developmental delay and intellectual disability that is usually moderate to severe; prolonged speech delay or the absence of speech; dysmorphic features, including a high anterior hairline, prominent forehead, and downslanting palpebral fissures; joint laxity; and hypotonia. Many patients also have some features of autism spectrum disorders. The 15q24 deletions that have been described vary with respect to breakpoints and size, but most include the 1.1-Mb region that is thought to be critical for the phenotype.
VARIABLE PHENOTYPES
In contrast to the syndromic microdeletions described above, several recurrent microdeletions and duplications have been associated with a wide range of phenotypic features and severity. Deletions of 1q21.1 have been associated with variable degrees of intellectual disability, and some patients have one or more congenital anomalies, including cataracts and congenital heart disease.32,33,78 The deletion is quite often inherited from one of the patient’s parents, who may be only mildly affected or unaffected. Deletions of this region have also been associated with schizophrenia.34,35 Duplications in the same region are also associated with mild-to-moderate intellectual disability and autistic features in some patients.32,33 Although dysmorphic features have been reported in many patients, there is no characteristic constellation of features in the majority of patients. A study involving patients with congenital heart disease suggests an increased frequency of the 1q21.1 duplication in this population as well.36
Another example of a copy-number change with highly variable outcomes is the 16p11.2 deletion. Deletions of 16p11.2 were first identified in patients with autism29,79 and are present in up to 1% of those with autism spectrum disorders, but it is now clear that such deletions are also associated with intellectual disability without autistic features.59–62,80 Deletions of the same region are also associated with early-onset obesity in subjects with and those without developmental delays.63,64 The 16p11.2 deletion is associated with dysmorphic features, but like the 1q21.1 rearrangement, it is not associated with a recognizable constellation of clinical features.
DIAGNOSTIC YIELD AND RECOMMENDATIONS
Several large studies have addressed the overall importance of copy-number changes in the diagnostic workup for intellectual disability, autism, and developmental delays,21,22,81,82 and it is clear that the use of CGH has a higher diagnostic yield than the standard karyotype. The International Standards for Cytogenomic Arrays consortium81 reviewed 33 published studies involving 21,698 patients with developmental delays, congenital anomalies, or autism who were tested for copy-number variants with the use of a chromosome microarray. The diagnostic yield (i.e., the rate of a positive genetic diagnosis) was approximately 12% across the studies. Recently, Cooper and colleagues82 looked at data from 15,767 patients who had undergone array CGH analysis as part of the diagnostic workup. Overall, the authors concluded that about 14% of cases of developmental delay can be explained by a detectable copy-number variation; their study provides a genetic morbidity map of developmental delays resulting from copy-number variations. The current recommendation is to perform chromosome microarray analysis instead of standard karyotype analysis early in the diagnostic workup of children with developmental delays, congenital anomalies, intellectual disability, or autism (Fig. 1).81,83
Figure 1. A Diagnostic Algorithm for the Evaluation of a Patient with Intellectual Disability of Unknown Cause.
Evaluation for copy-number changes with the use of array comparative genomic hybridization (CGH) should be performed early in the diagnostic workup. Indications for magnetic resonance imaging (MRI) include macrocephaly or microcephaly, asymmetric neurologic findings, intractable epilepsy or focal seizures, abnormal movements (e.g., dystonia, chorea, or other extrapyramidal findings), hypotonia or long tract signs, facial stigmata associated with developmental brain abnormalities, and a history of a progressive neurologic disorder.
THE GENETICS OF RELATED DISORDERS
Array CGH studies have also been applied to other disorders, many of which are related to and often coexist with intellectual disability and autism. Copy-number changes have been identified that are risk factors for schizophrenia,34,35 epilepsy,43,49,69,84 and attention deficit–hyperactivity disorder (ADHD).70,85,86 There is substantial overlap among the copy-number variations that have been identified in each of these disorders and in cases of intellectual disability and autism. For example, microdeletions of 15q13.3 have been associated with intellectual disability,50,51 autism,52–54 and schizophrenia34,35 and occur with increased frequency in patients with generalized epilepsy43,49,84,87 (Table 1).
Similarly, microdeletions of 1q21 are associated with autism, schizophrenia, and epilepsy and, most commonly, with intellectual disability. Deletions of 16p13.11 were first described in patients with autism and intellectual disability,44,71,88 but studies of epilepsy have shown that the frequency of this deletion is also significantly increased in patients with both generalized and focal forms of epilepsy.43,69,84 Duplications of 16p13.11 have also been associated with an increased risk of a range of neuropsychiatric disorders, including intellectual disability, autism, ADHD, and perhaps schizophrenia.44,71,72,86,89 The range of conditions that have been associated with these and other copy-number changes highlights the fact that these disorders are related and that common genetic factors have a causal role. Therefore, it is likely that etiologic sequence changes will be identified in some of the genes and gene networks that have been implicated in these disorders as well.
SINGLE-GENE CAUSES OF INTELLECTUAL DISABILITY
The advent of family-based genetic linkage studies and DNA sequencing in the 1990s led to the identification of increasing numbers of single genes causing intellectual disability. Many of these studies have been focused on identifying genes on the X chromosome, in part because X-linked forms of intellectual disability can be transmitted through unaffected females in families, allowing pedigree analysis. The most well-known example is the fragile X syndrome, which is caused by dynamic triplet-repeat-expansion mutations in the gene FMR1 and is the most common genetic cause of intellectual disability. Clinical trials are under way to test new therapies for the fragile X syndrome on the basis of the known function of FMR1. Another important X-linked cause of syndromic intellectual disability is mutation in MECP2, encoding methyl-CpG–binding protein 2, in Rett’s syndrome (affecting girls). In a recent study, Tarpey and colleagues90 sequenced the exons of 718 genes on the X chromosome in 208 families and identified 9 genes associated with X-linked intellectual disability. Their study, which used standard sequencing methods, provided a foreshadowing of the type of data that are now being generated with higher-throughput methods.
Mutations in more than 90 X-linked genes are now known to cause intellectual disability and account for about 10% of cases.91 Autosomal genes have been more difficult to identify, because there are few familial forms of intellectual disability. Many genetic syndromes for which the causative genes are known are characterized by variable intellectual disability. Some examples include neurofibromatosis, myotonic dystrophy, Duchenne’s muscular dystrophy, Noonan-spectrum disorders, and tuberous sclerosis. Many autosomal recessive metabolic disorders are also associated with poor developmental outcomes. However, it is thought that the majority of cases of moderate-to-severe intellectual disability are due to de novo mutations, which cannot be detected by means of linkage mapping. Similarly, single-gene causes of autism have been identified. Most notably, mutations in PTEN are associated with autism and macro-cephaly in some patients,92 and mutations in SHANK3 have also been identified.93 As described below, new sequencing approaches are facilitating gene discovery in this previously intractable form of inheritance.
MASSIVELY PARALLEL SEQUENCING
USE IN GENE DISCOVERY
Sanger sequencing was introduced in the 1970s94 and has been the mainstay of gene sequence analysis for nearly three decades. The technology is robust and reliable but subject to relatively low throughput. It was used to produce the first complete human genome sequence. In the past several years, the development of next-generation sequencing has revolutionized the field and is likely to deliver the so-called $1,000 genome (on the basis of the anticipated cost). The emerging techniques that are enabling whole-genome sequencing have been reviewed in the Journal95 and elsewhere.96 Briefly, the method that is now widely used is referred to as massively parallel sequencing, which involves highly parallelized sequence analysis of millions of short DNA fragments from the genome.
Whereas sequence analysis of the first human genome required $3 billion and took more than 10 years, whole-genome sequencing with the use of massively parallel sequencing can be completed in a matter of weeks at a cost of $50,000 or less, and the cost is rapidly decreasing. However, sequencing an entire genome with the use of massively parallel sequencing remains a relatively expensive and time-consuming task, both for humans and for computers. A more tractable approach that is making rapid inroads into the practice of medicine is sequencing of the protein-coding parts of the genome, called exome sequencing. The exome refers to the exons, or coding units, of genes, which comprise approximately 30 million base pairs, or 1% of the entire genome. Exome sequencing is accomplished by selectively capturing the exons with the use of one of several array-based or solution-based methods that are now commercially available. The captured DNA is then sequenced by massively parallel sequencing, and SNPs are identified by comparison with the reference genome.
This approach is attractive for several reasons. First, the majority of disease-causing sequence mutations that have been identified occur in exons. Therefore, it is likely that sequence analysis of the exome will continue to be a successful approach to identifying novel disease genes. Second, it is easier to assign functional and therefore clinical significance to changes in coding sequences (exons) than to changes in noncoding DNA, the function of which is largely unknown. In addition, the human and computer requirements for sequencing and analyzing a patient’s exome are currently much more tractable than those for an entire genome, with a cost of approximately $1,000. It must be acknowledged that noncoding mutations (i.e., those that occur in promoters, introns, or other nonexonic sequences) will certainly be found to be important for some disorders, and these mutations will not be detected by exome sequencing.
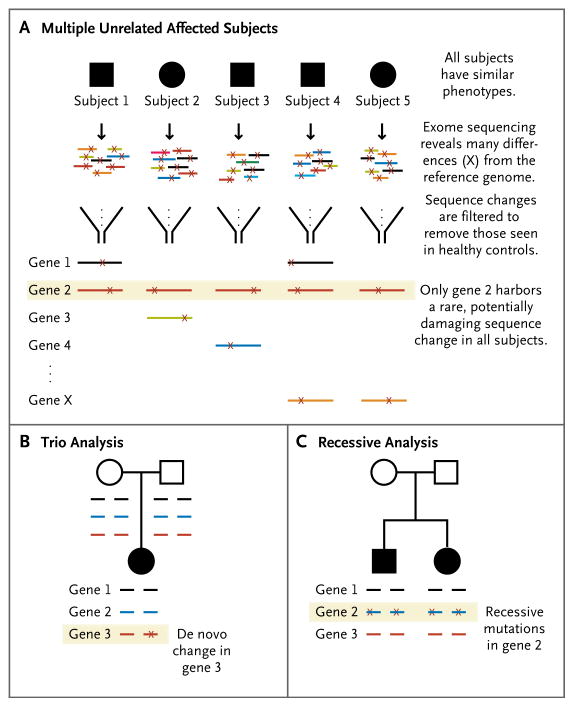
Several experimental approaches have been successfully used for disease identification by means of exome sequencing (Table 2 and Fig. 2). The first approach involves sequencing in several unrelated affected subjects with the same phenotype. The sequence data are then analyzed to identify genes in which all or most affected subjects have a potentially deleterious sequence variant. This approach assumes that the phenotype in all (or most) of the subjects being analyzed is a result of mutations in the same gene. Therefore, this approach has been most successful in subjects with recognizable or fairly homogeneous disorders. The first proof-of-principle experiment was successful on the basis of studies in only four subjects with the Freeman–Sheldon syndrome (also known as the whistling-face syndrome and already known to be caused by mutations in MYH3).103 Subsequently, this strategy has been used to identify the causative gene for the Kabuki syndrome (intellectual disability, facial dysmorphisms, and congenital heart disease caused by de novo mutations in MLL2)97 and the Schinzel–Giedion syndrome (severe intellectual disability, facial dysmorphisms, and multiple congenital anomalies caused by de novo mutations in SETBP1).98
Table 2.
Studies Using Massively Parallel Sequencing to Identify Genes Associated with Intellectual Disability and Autism.
| Study | Disorder | Presumed Inheritance | Type of Analysis | Genes |
|---|---|---|---|---|
| Ng et al.97 | Kabuki syndrome | De novo dominant | Multiple affected | MLL2 |
| Hoischen et al.98 | Schinzel–Giedion syndrome | De novo dominant | Multiple affected | SETBP1 |
| Vissers et al.99 | Nonsyndromic sporadic intellectual disability | De novo dominant | Trio | Multiple |
| Najmabadi et al.100 | Recessive intellectual disability | Autosomal recessive, consanguineous families | Targeted recessive | Multiple |
| Calişkan et al.101 | Recessive intellectual disability | Autosomal recessive, consanguineous family | Recessive | TECR |
| O’Roak et al.102 | Autism | De novo dominant | Trio | FOXP1, GRIN2B, SCN1A, LAMC3 |
Figure 2. Three Strategies for Exome Sequencing in Gene Discovery.
Panel A shows the sequencing of DNA samples from multiple, unrelated, similarly affected subjects to identify genes in which some or all of the subjects carry a mutation. Panel B shows trio analysis, in which samples from the affected child and both unaffected parents are analyzed to identify de novo changes in the child. Panel C shows recessive analysis, in which samples from one or more affected children are sequenced to identify the genes that harbor two mutations (one on each allele). Open circles and squares represent unaffected female and male subjects, respectively; solid symbols indicate affected status. In all the panels, horizontal lines represent exonic sequences, and X represents a sequence change as compared with the reference human genome.
In both the Kabuki and Schinzel–Giedion syndromes, the mutation in the child was not seen in either of the parents, and the de novo occurrence of mutations in clinically similar children is strong evidence of causality. The analysis of trios (i.e., genes from the affected patient and his or her parents) has been a particularly successful approach in interpreting the large volumes of exome sequencing data (Fig. 2B). This strategy is used when the patient is expected to have a de novo mutation that is unlikely to be found in either parent’s exome. It is predicted that the average newborn will harbor no more than one de novo sequence change that alters an amino acid.104 Therefore, the sequencing of the exomes of an affected child and his or her unaffected parents seems to be an efficient method for identifying de novo disease-causing mutations.
Trio analysis is proving to be an effective means of identifying underlying genetic causes in non-syndromic intellectual disability as well. Vissers and colleagues99 applied this strategy to 10 cases of nonsyndromic intellectual disability without a family history in order to identify de novo changes. In 6 cases, they identified 9 true de novo variants (in 9 different genes). Two patients each had a de novo mutation in a gene with a known association with intellectual disability. In 4 other cases, patients had a de novo variant in a plausible candidate gene. Although each of the candidate genes that were identified in this study requires further study to confirm its role in intellectual disability, the results indicate that trio analysis is an efficient method of detecting de novo mutations and novel candidate genes. O’Roak and colleagues102 used the trio approach to analyze the exome sequence in 20 children with autism and their unaffected parents. In 4 of the 20 children, the authors found arguably compelling de novo mutations in genes that are known to be involved in brain development (FOXP1,105 GRIN2B,106 SCN1A,107 and LAMC3108).
Exome sequencing has also been used to identify genes associated with recessive diseases (Fig. 2C). The first examples were the diagnosis of congenital chloride diarrhea in a child suspected of having another disorder109 and the identification of the gene causing the Miller syndrome, a craniofacial disorder.110 Several studies have used massively parallel sequencing to investigate autosomal recessive intellectual disability. In a large consanguineous family with multiple affected children, Calişkan and colleagues101 sequenced the exomes of the parents to look for heterozygous deleterious mutations within a 2-Mb linkage region. They identified a mutation in TECR that was homozygous in all affected children. Recently, Najmabadi and colleagues100 investigated autosomal recessive intellectual disability in 136 consanguineous families. Because they had linkage data for the families that narrowed the genomic regions of interest, they captured the subset of exons within linkage regions for each family instead of sequencing the entire exome. They found mutations in 23 known intellectual-disability genes in 26 families, providing a definitive diagnosis. In the remaining families, they identified 50 novel candidate genes, each with a homozygous mutation in a single family. Clearly, these candidate genes need to be validated in additional samples, but the study provides a framework for evaluation of recessive forms of intellectual disability.
The value of exome sequencing in the identification of novel gene mutations has been endorsed by the National Institutes of Health, which announced in December 2011 that it will provide $48 million during the next 4 years to three centers for the sequencing of exomes and genomes of persons who have rare disorders with causes that are still unknown (http://mendelian.org).
USE IN CLINICAL DIAGNOSTICS
Next-generation sequencing has already moved into clinical diagnostic laboratories. Several laboratories now offer gene panels in which a set of known disease genes (rather than the whole exome) is captured and subjected to massively parallel sequencing. This approach provides simultaneous evaluation of multiple genes rather than the current gene-by-gene analysis that is often required in the clinic. For example, it is now possible to order an X-linked intellectual-disability panel that includes 30, 60, or 90 genes. Exome sequencing is moving very quickly into the clinical arena and is now offered by at least two clinical laboratories at a cost of approximately $10,000 for data generation and interpretation of results.
Although clinical exomes are likely to yield answers in some cases, it will be important to proceed cautiously with careful selection of patients. The studies described above and listed in Table 2 represent the success stories. However, there are challenges in interpreting exome data, and in the studies published to date, not every case has been solved. Each individual exome harbors approximately 20,000 sequence variants as compared with the human reference genome, including some 5000 variants that will affect protein sequence and could be considered potentially deleterious. The variants can be further filtered to exclude those reported in SNP databases or in control exome studies. Once these criteria are applied, each person generally carries 100 to 200 heterozygous private sequence variants that are potentially deleterious, as well as several genes that have potentially damaging recessive mutations. Careful follow-up of individuals and families and studies in additional patients will be necessary to interpret the clinical significance of many of the variants identified by exome sequencing.
SUMMARY
Chromosome microarrays and next-generation sequencing have revolutionized gene discovery in intellectual disability, autism, and other disorders. Chromosome microarray analysis, which is recommended as a first-line test in the genetic work-up of children with intellectual disability, developmental delays, autism, or congenital anomalies, provides a molecular diagnosis in 15 to 20% of cases. Exome sequencing has proved to be successful in the research laboratory and is moving rapidly into the diagnostic laboratory. As the data continue to accumulate, our understanding of genes, pathways, and molecular mechanisms will continue to evolve and translate into better diagnosis, prognosis, and therapies for these severe disorders.
Glossary
- Candidate gene
A gene that has been selected on the basis of a perceived match between the known or presumed function of the gene and the biologic characteristics of the disease in question
- Chromosome banding
The treatment of chromosomes to reveal characteristic patterns of horizontal bands
- Chromosome microarray
An assay that can identify multiple deletions and duplications across the genome simultaneously; the term encompasses both array comparative genomic hybridization (CGH) and single-nucleotide-polymorphism (SNP) arrays
- De novo mutation
Any DNA sequence change that occurs during replication, such as a heritable gene alteration occurring in a family for the first time as a result of a DNA sequence change in a germ cell or fertilized egg
- Fluorescence in situ hybridization (FISH)
A laboratory technique for detecting and locating a specific DNA sequence on a chromosome. The technique relies on exposing chromosomes to a small DNA sequence, called a probe, that has a tag (usually a fluorescent molecule) attached to it. The probe sequence binds to its corresponding sequence on the chromosome
- Massively parallel (or next-generation) sequencing
DNA sequencing that harnesses advances in miniaturization technology to simultaneously sequence multiple areas of the genome rapidly and at low cost
- Microdeletion syndrome
A syndrome caused by a chromosomal deletion spanning several genes that is too small to be detected under the microscope with the use of conventional cytogenetic methods
- Supernumerary marker chromosome
A small chromosome containing a centromere occasionally seen in tissue culture, often in a mosaic state (i.e., present in some cells but not in others). A marker chromosome may be of little clinical significance, or if it contains material from one or both arms of another chromosome, it may create an imbalance for whatever genes are present; assessment to establish clinical significance, particularly for a marker chromosome found in a fetal karyotype, is often difficult
- Triplet (trinucleotide) repeat
Sequences of three nucleotides that are repeated in tandem on the same chromosome a number of times. A normal, polymorphic variation in repeat number with no clinical significance commonly occurs between persons; however, repeat numbers over a certain threshold can, in some cases, lead to adverse effects on the function of the gene, resulting in genetic disease
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Diagnostic criteria from DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev. 2002;8:117–34. doi: 10.1002/mrdd.10031. [DOI] [PubMed] [Google Scholar]
- 3.Lejeune J, Gautier M, Turpin R. Etudes des chromosomes somatique de neuf enfants mongoliens. CR Hebd Seances Acad Sci. 1959;248:1721–2. [PubMed] [Google Scholar]
- 4.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–9. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- 5.Idem. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–41. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 6.Iafrate AJ, Feuk L, Rivera MN, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–51. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 7.Itsara A, Cooper GM, Baker C, et al. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet. 2009;84:148–61. doi: 10.1016/j.ajhg.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke DP, Sharp AJ, McCarroll SA, et al. Linkage disequilibrium and heritability of copy-number polymorphisms within duplicated regions of the human genome. Am J Hum Genet. 2006;79:275–90. doi: 10.1086/505653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–54. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebat J, Lakshmi B, Troge J, et al. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–8. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 11.Ropers HH. Genetics of intellectual disability. Curr Opin Genet Dev. 2008;18:241–50. doi: 10.1016/j.gde.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez Jurado LA, Peoples R, Kaplan P, Hamel BC, Francke U. Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am J Hum Genet. 1996;59:781–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AC, McGavran L, Robinson J, et al. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986;24:393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- 15.Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2010;38:181–91. doi: 10.1016/j.nbd.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballif BC, Sulpizio SG, Lloyd RM, et al. The clinical utility of enhanced sub-telomeric coverage in array CGH. Am J Med Genet A. 2007;143A:1850–7. doi: 10.1002/ajmg.a.31842. [DOI] [PubMed] [Google Scholar]
- 17.Knight SJ, Regan R, Nicod A, et al. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet. 1999;354:1676–81. doi: 10.1016/S0140-6736(99)03070-6. [DOI] [PubMed] [Google Scholar]
- 18.Ravnan JB, Tepperberg JH, Papenhausen P, et al. Subtelomere FISH analysis of 11 688 cases: an evaluation of the frequency and pattern of subtelomere rearrangements in individuals with developmental disabilities. J Med Genet. 2006;43:478–89. doi: 10.1136/jmg.2005.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries BB, Pfundt R, Leisink M, et al. Diagnostic genome profiling in mental retardation. Am J Hum Genet. 2005;77:606–16. doi: 10.1086/491719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koolen DA, Vissers LE, Pfundt R, et al. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet. 2006;38:999–1001. doi: 10.1038/ng1853. [DOI] [PubMed] [Google Scholar]
- 21.Sagoo GS, Butterworth AS, Sanderson S, Shaw-Smith C, Higgins JP, Burton H. Array CGH in patients with learning disability (mental retardation) and congenital anomalies: updated systematic review and meta-analysis of 19 studies and 13,926 subjects. Genet Med. 2009;11:139–46. doi: 10.1097/GIM.0b013e318194ee8f. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer LG, Kashork CD, Saleki R, et al. Targeted genomic microarray analysis for identification of chromosome abnormalities in 1500 consecutive clinical cases. J Pediatr. 2006;149:98–102. doi: 10.1016/j.jpeds.2006.02.006. Erratum, J Pediatr 2006;149:585. [DOI] [PubMed] [Google Scholar]
- 23.Sharp AJ, Hansen S, Selzer RR, et al. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet. 2006;38:1038–42. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- 24.Shaw-Smith C, Pittman AM, Willatt L, et al. Microdeletion encompassing MAPT at chromosome 17q21. 3 is associated with developmental delay and learning disability. Nat Genet. 2006;38:1032–7. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 25.Christian SL, Brune CW, Sudi J, et al. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol Psychiatry. 2008;63:1111–7. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–88. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szatmari P, Paterson AD, Zwaigenbaum L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–28. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11. 2 and autism. N Engl J Med. 2008;358:667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 30.Pinto D, Pagnamenta AT, Klei L, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–72. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mefford HC, Eichler EE. Duplication hotspots, rare genomic disorders, and common disease. Curr Opin Genet Dev. 2009;19:196–204. doi: 10.1016/j.gde.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunetti-Pierri N, Berg JS, Scaglia F, et al. Recurrent reciprocal 1q21. 1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40:1466–71. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mefford HC, Sharp AJ, Baker C, et al. Recurrent rearrangements of chromosome 1q21. 1 and variable pediatric phenotypes. N Engl J Med. 2008;359:1685–99. doi: 10.1056/NEJMoa0805384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefansson H, Rujescu D, Cichon S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenway SC, Pereira AC, Lin JC, et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat Genet. 2009;41:931–5. doi: 10.1038/ng.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haldeman-Englert C, Jewett T. 1q21.1 Microdeletion. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. Seattle: University of Washington; 1993. [Google Scholar]
- 38.Ballif BC, Theisen A, Coppinger J, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal micro-duplication. Mol Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisi EC, Hamosh A, Doheny KF, et al. 3q29 Interstitial microduplication: a new syndrome in a three-generation family. Am J Med Genet A. 2008;146A:601–9. doi: 10.1002/ajmg.a.32190. [DOI] [PubMed] [Google Scholar]
- 40.Willatt L, Cox J, Barber J, et al. 3q29 Microdeletion syndrome: clinical and molecular characterization of a new syndrome. Am J Hum Genet. 2005;77:154–60. doi: 10.1086/431653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balciuniene J, Feng N, Iyadurai K, et al. Recurrent 10q22-q23 deletions: a genomic disorder on 10q associated with cognitive and behavioral abnormalities. Am J Hum Genet. 2007;80:938–47. doi: 10.1086/513607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Bon BW, Balciuniene J, Fruhman G, et al. The phenotype of recurrent 10q22q23 deletions and duplications. Eur J Hum Genet. 2011;19:400–8. doi: 10.1038/ejhg.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Kovel CG, Trucks H, Helbig I, et al. Recurrent microdeletions at 15q11.2 and 16p13. 11 predispose to idiopathic generalized epilepsies. Brain. 2010;133:23–32. doi: 10.1093/brain/awp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mefford HC, Cooper GM, Zerr T, et al. A method for rapid, targeted CNV genotyping identifies rare variants associated with neurocognitive disease. Genome Res. 2009;19:1579–85. doi: 10.1101/gr.094987.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnside RD, Pasion R, Mikhail FM, et al. Microdeletion/microduplication of proximal 15q11. 2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay. Hum Genet. 2011;130:517–28. doi: 10.1007/s00439-011-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doornbos M, Sikkema-Raddatz B, Ruijvenkamp CA, et al. Nine patients with a microdeletion 15q11. 2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbances. Eur J Med Genet. 2009;52:108–15. doi: 10.1016/j.ejmg.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Murthy SK, Nygren AO, El Shakankiry HM, et al. Detection of a novel familial deletion of four genes between BP1 and BP2 of the Prader-Willi/Angelman syndrome critical region by oligo-array CGH in a child with neurological disorder and speech impairment. Cytogenet Genome Res. 2007;116:135–40. doi: 10.1159/000097433. [DOI] [PubMed] [Google Scholar]
- 48.von der Lippe C, Rustad C, Heimdal K, Rodningen OK. 15q11. 2 Microdeletion — seven new patients with delayed development and/or behavioural problems. Eur J Med Genet. 2011;54:357–60. doi: 10.1016/j.ejmg.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Helbig I, Mefford HC, Sharp AJ, et al. 15q13. 3 Microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–2. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharp AJ, Mefford HC, Li K, et al. A recurrent 15q13. 3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet. 2008;40:322–8. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Bon BW, Mefford HC, Menten B, et al. Further delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet. 2009;46:511–23. doi: 10.1136/jmg.2008.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ben-Shachar S, Lanpher B, German JR, et al. Microdeletion 15q13. 3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet. 2009;46:382–8. doi: 10.1136/jmg.2008.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pagnamenta AT, Wing K, Akha ES, et al. A 15q13. 3 microdeletion segregating with autism. Eur J Hum Genet. 2009;17:687–92. doi: 10.1038/ejhg.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller DT, Shen Y, Weiss LA, et al. Microdeletion/duplication at 15q13.2q13. 3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet. 2009;46:242–8. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrieux J, Dubourg C, Rio M, et al. Genotype-phenotype correlation in four 15q24 deleted patients identified by array-CGH. Am J Med Genet A. 2009;149A:2813–9. doi: 10.1002/ajmg.a.33097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp AJ, Selzer RR, Veltman JA, et al. Characterization of a recurrent 15q24 microdeletion syndrome. Hum Mol Genet. 2007;16:567–72. doi: 10.1093/hmg/ddm016. [DOI] [PubMed] [Google Scholar]
- 57.Mefford HC, Rosenfeld JA, Shur N, et al. Further clinical and molecular delineation of the 15q24 microdeletion syndrome. J Med Genet. 2012;49:110–8. doi: 10.1136/jmedgenet-2011-100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Hattab AW, Zhang F, Maxim R, et al. Deletion and duplication of 15q24: molecular mechanisms and potential modification by additional copy number variants. Genet Med. 2010;12:573–86. doi: 10.1097/GIM.0b013e3181eb9b4a. [DOI] [PubMed] [Google Scholar]
- 59.Battaglia A, Novelli A, Bernardini L, Igliozzi R, Parrini B. Further characterization of the new microdeletion syndrome of 16p11.2-p12. 2. Am J Med Genet A. 2009;149A:1200–4. doi: 10.1002/ajmg.a.32847. [DOI] [PubMed] [Google Scholar]
- 60.Bijlsma EK, Gijsbers AC, Schuurs-Hoeijmakers JH, et al. Extending the phenotype of recurrent rearrangements of 16p11. 2: deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet. 2009;52:77–87. doi: 10.1016/j.ejmg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Hempel M, Rivera Brugués N, Wagenstaller J, et al. Microdeletion syndrome 16p11.2-p12. 2: clinical and molecular characterization. Am J Med Genet A. 2009;149A:2106–12. doi: 10.1002/ajmg.a.33042. [DOI] [PubMed] [Google Scholar]
- 62.Shinawi M, Liu P, Kang SH, et al. Recurrent reciprocal 16p11. 2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47:332–41. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquemont S, Reymond A, Zufferey F, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11. 2 locus. Nature. 2011;478:97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walters RG, Jacquemont S, Valsesia A, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11. 2. Nature. 2010;463:671–5. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy SE, Makarov V, Kirov G, et al. Microduplications of 16p11. 2 are associated with schizophrenia. Nat Genet. 2009;41:1223–7. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bachmann-Gagescu R, Mefford HC, Cowan C, et al. Recurrent 200-kb deletions of 16p11. 2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–7. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- 67.Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girirajan S, Rosenfeld JA, Cooper GM, et al. A recurrent 16p12. 1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–9. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinzen EL, Radtke RA, Urban TJ, et al. Rare deletions at 16p13. 11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet. 2010;86:707–18. doi: 10.1016/j.ajhg.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams NM, Zaharieva I, Martin A, et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376:1401–8. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ullmann R, Turner G, Kirchhoff M, et al. Array CGH identifies reciprocal 16p13. 1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28:674–82. doi: 10.1002/humu.20546. [DOI] [PubMed] [Google Scholar]
- 72.Kirov G, Grozeva D, Norton N, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18:1497–503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreno-De-Luca D, Mulle JG, Kaminsky EB, et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87:618–30. doi: 10.1016/j.ajhg.2010.10.004. Erratum, Am J Hum Genet 2011;88:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loirat C, Bellanné-Chantelot C, Husson I, Deschênes G, Guigonis V, Chabane N. Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrol Dial Transplant. 2010;25:3430–3. doi: 10.1093/ndt/gfq380. [DOI] [PubMed] [Google Scholar]
- 75.Koolen DA, Sharp AJ, Hurst JA, et al. Clinical and molecular delineation of the 17q21. 31 microdeletion syndrome. J Med Genet. 2008;45:710–20. doi: 10.1136/jmg.2008.058701. Erratum, J Med Genet 2009;46:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Hattab AW, Smolarek TA, Walker ME, et al. Redefined genomic architecture in 15q24 directed by patient deletion/duplication breakpoint mapping. Hum Genet. 2009;126:589–602. doi: 10.1007/s00439-009-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klopocki E, Graul-Neumann LM, Grieben U, et al. A further case of the recurrent 15q24 microdeletion syndrome, detected by array CGH. Eur J Pediatr. 2008;167:903–8. doi: 10.1007/s00431-007-0616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christiansen J, Dyck JD, Elyas BG, et al. Chromosome 1q21. 1 contiguous gene deletion is associated with congenital heart disease. Circ Res. 2004;94:1429–35. doi: 10.1161/01.RES.0000130528.72330.5c. [DOI] [PubMed] [Google Scholar]
- 79.Kumar RA, KaraMohamed S, Sudi J, et al. Recurrent 16p11. 2 microdeletions in autism. Hum Mol Genet. 2008;17:628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 80.Rosenfeld JA, Coppinger J, Bejjani BA, et al. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11. 2 microdeletions and microduplications. J Neurodev Disord. 2009;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–64. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper GM, Coe BP, Girirajan S, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–46. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manning M, Hudgins L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12:742–5. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mefford HC, Muhle H, Ostertag P, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6(5):e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lionel AC, Crosbie J, Barbosa N, et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. 2011;3:95ra75. doi: 10.1126/scitranslmed.3002464. [DOI] [PubMed] [Google Scholar]
- 86.Ramalingam A, Zhou XG, Fiedler SD, et al. 16p13. 11 Duplication is a risk factor for a wide spectrum of neuropsychiatric disorders. J Hum Genet. 2011;56:541–4. doi: 10.1038/jhg.2011.42. [DOI] [PubMed] [Google Scholar]
- 87.Dibbens LM, Mullen S, Helbig I, et al. Familial and sporadic 15q13. 3 microdeletions in idiopathic generalized epilepsy: precedent for disorders with complex inheritance. Hum Mol Genet. 2009;18:3626–31. doi: 10.1093/hmg/ddp311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hannes FD, Sharp AJ, Mefford HC, et al. Recurrent reciprocal deletions and duplications of 16p13. 11: the deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet. 2009;46:223–32. doi: 10.1136/jmg.2007.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grozeva D, Conrad DF, Barnes CP, et al. Independent estimation of the frequency of rare CNVs in the UK population confirms their role in schizophrenia. Schizophr Res. 2011 Nov 28; doi: 10.1016/j.schres.2011.11.004. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tarpey PS, Smith R, Pleasance E, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–43. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ropers HH. Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet. 2010;11:161–87. doi: 10.1146/annurev-genom-082509-141640. [DOI] [PubMed] [Google Scholar]
- 92.Butler MG, Dasouki MJ, Zhou XP, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanger F, Air GM, Barrell BG, et al. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977;265:687–95. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- 95.Feero WG, Guttmacher AE, Collins FS. Genomic medicine — an updated primer. N Engl J Med. 2010;362:2001–11. doi: 10.1056/NEJMra0907175. [DOI] [PubMed] [Google Scholar]
- 96.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–45. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 97.Ng SB, Bigham AW, Buckingham KJ, et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010;42:790–3. doi: 10.1038/ng.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoischen A, van Bon BW, Gilissen C, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42:483–5. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 99.Vissers LE, de Ligt J, Gilissen C, et al. A de novo paradigm for mental retardation. Nat Genet. 2010;42:1109–12. doi: 10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
- 100.Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 101.Calişkan M, Chong JX, Uricchio L, et al. Exome sequencing reveals a novel mutation for autosomal recessive non-syndromic mental retardation in the TECR gene on chromosome 19p13. Hum Mol Genet. 2011;20:1285–9. doi: 10.1093/hmg/ddq569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ng SB, Turner EH, Robertson PD, et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272–6. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lynch M. Rate, molecular spectrum, and consequences of human mutation. Proc Natl Acad Sci U S A. 2010;107:961–8. doi: 10.1073/pnas.0912629107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamdan FF, Daoud H, Rochefort D, et al. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010;87:671–8. doi: 10.1016/j.ajhg.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Endele S, Rosenberger G, Geider K, et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 2010;42:1021–6. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 107.Claes L, Ceulemans B, Audenaert D, et al. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat. 2003;21:615–21. doi: 10.1002/humu.10217. [DOI] [PubMed] [Google Scholar]
- 108.Barak T, Kwan KY, Louvi A, et al. Recessive LAMC3 mutations cause malformations of occipital cortical development. Nat Genet. 2011;43:590–4. doi: 10.1038/ng.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi M, Scholl UI, Ji W, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106:19096–101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ng SB, Buckingham KJ, Lee C, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30–5. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]