Abstract
Background
Poor ovarian response (POR) to gonadotropin stimulation has led to a significant decline in success rate of fertility treatment. The immune system may play an important role in pathophysiology of POR by dysfunctions of cytokines and the growth factor network, and the presence of ovarian auto-antibodies. The aim of this study is to investigate the expression of toll-like receptors (TLR) 1, 2, 4, 5, 6 and cyclooxygenase (COX) 2 genes in follicular cells and concentration of interleukin (IL)-6, IL-8 and macrophage migration inhibitory factor (MIF), as major parts of innate immunity, in follicular fluid (FF) obtained from POR women in comparison with normal women.
Materials and Methods
In this case-control study, 20 infertile POR patients and 20 normal women took part in this study and underwent controlled ovarian stimulation. The FF was obtained from the largest follicle (>18 mm). The FF was centrifuged and cellular pellet was then used for evaluation of expression of TLRs and COX2 genes by real-time PCR. FF was used for quantitative analysis for IL-6, IL-8 and MIF by enzyme-linked immunosorbent assay (ELISA).
Results
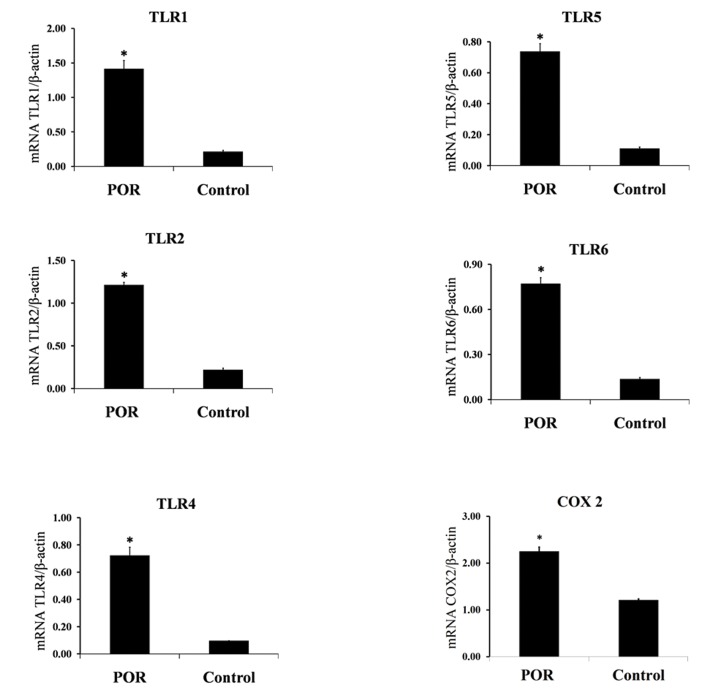
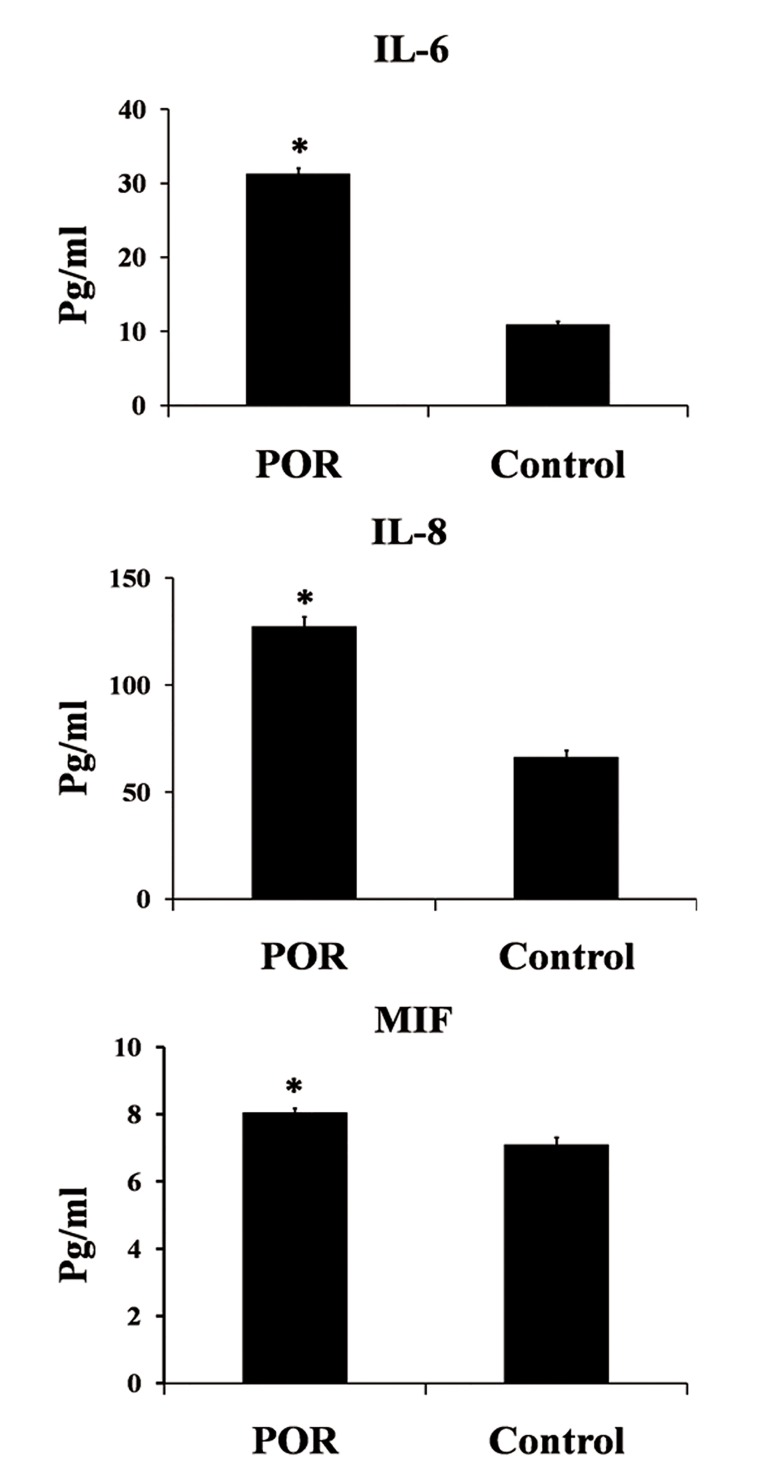
TLR1, 2, 4, 5, 6 and COX2 gene expression were significantly higher in POR (p<0.05). Concentration of IL-6, IL-8 and MIF proteins was significantly increased in POR compared with normal women (p<0.05).
Conclusion
These findings support the hypothesis that the immune system may be involved in pathophysiology of POR through TLRs.
Keywords: TLR, Ovary, Innate immunity, Infertility, MIF
Introduction
A considerable issue in assisted reproductive technology (ART) is poor ovarian response (POR) to gonadotropin stimulation. This condition affects approximately 9 to 24% of the in vitro fertilization (IVF) cycles (1). POR leads to cycle cancellation, significant decline in number of oocyte and embryo, and reduction in the success rate of fertility treatment (2). POR is challenging to define, however, investigators have defined POR in patients that have a peak of E2 level <300 pg/ml, fewer mature oocytes and lower pregnancy rates after standard stimulation by human menopausal gonadotropin (3). POR is associated with advanced age, previous ovarian surgery, pelvic adhesion and high body mass index (BMI). However, young women are also affected by unanticipated poor response (1). Numerous hypotheses, although controversial, have been suggested for POR including poor follicular blood flow (4), dysfunctions of cytokines and the growth factor network (5), and the presence of ovarian auto-antibodies (6).
The immune system may play an important role in pathophysiology of POR. A major part of immune system is innate immunity that generates more rapid and primary responses to pathogens than the adaptive immune system (7). Key mediators of the innate immune system are toll-like receptors (TLRs) (8). To date, ten functional TLRs have been identified in the human genome. They are classified based on their cellular location; cell membrane TLRs (TLR1, 2, 4, 5, 6, 10) and endosomal TLRs (3, 7, 8, 9). In the current study we investigate the expression of cell membrane TLRs except TLR10, because its specific ligand is not known. These TLRs identify various pathogen associated molecular patterns (PAMPs) and damage associated molecular pattern (DAMPs), which activate a variety of host responses (9). TLR2 can form heterodimers with TLRs 1 and 6 and recognizes peptidoglycan, lipoteichoic acid and lipoarabinomannan resultant from pathogens including mycobacteria and zymosan from fungi and yeast (10), and endogenous ligands such as heat shock protein (HSP) -60, 70 and 96 (11), and reactive oxygen species (ROS) (12). TLR4 recognizes the lipopolysaccharide (LPS) as a main constituent of the outer membrane of Gram-negative bacteria, (13), Hyaluronan (14), HSPs (15) and Fibronectin(16). Bacterial flagellin is recognized via TLR5 (17).
Previous studies have discussed the functional TLRs in the reproductive system. TLRs 1-10 are expressed in the female reproductive tract (18, 19) and their expression level varies in different phases of the menstrual cycle (20).
TLRs are activated by specific ligands and can create intra-cellular signals via MYD88-dependent and independent pathways (9). These signals lead to induce gene expression of some inflammation related enzymes such as cyclo-ocygenase (COX) 2 which is a key enzyme in the conversion of arachidonic acid to prostaglandins (21). COX2 transcript has been identified in granulose cells and protein is present in pre-ovulatory follicular fluid (22, 23). Previous study has shown that COX2 negative follicles were anovulatory follicles (24). It is therefore concluded that COX2 has a central role in ovulation (24).
Moreover activation of TLRs leads to the stimulation of chemokine and cytokine expression including interleukin (IL) - 6, IL-8 and macrophage migration inhibitory factor (MIF) that activate a variety of host responses (9). These cytokines modulates systemic and local inflammatory and immune responses (25). Aboussahoud et al. demonstrated that TLRs expressed in endometrial epithelial cell lines and their stimulation led to IL-6 and IL-8 production (26).
Follicular cells play an important role in folliculogenesis, steroidogenesis and oocyte maturation (27). Previous studies have shown that ovarian follicular cells have innate immune capability (28) and express TLRs; Shimida et al. reported that mouse granulosa cells express TLR2, 4, 8 and 9 and are involved in cytokine and chemokine production (29). Zhou et al. (30) also suggested that the surface epithelium of human ovaries has high expression of TLR2-5.
Therefore, regarding the involvement of the immune system in POR pathogenesis (6, 31), the aim of the present study was to investigate the expression changes of TLRs and COX2 genes in follicular cells as well as the concentration of MIF, IL-6 and IL-8 proteins in follicular fluid obtained from POR patients.
Materials and Methods
Patient characteristics
This case-control study was approved by Iran University of Medical Sciences and Royan Institute Ethics Committees. Forty participants (20 infertile poor ovarian responder patients and 20 normal women with male factor infertility as control) attending the Reproductive Medicine Unit in Royan Infertility clinic, Tehran, Iran, for intracytoplasmic sperm injection (ICSI) treatment were invited to participate in the study during 2012. An information sheet was offered to all women, and informed written consent was obtained.
Inclusion criteria were infertile women aged 20-35 undergoing ICSI treatment, receiving the same standard long protocol. The exclusion criteria were endometriosis, polycystic ovarian syndrome, endocrine disorder like hyperprolactinemia, history of ovarian surgery and female reproductive tract infection.
POR were defined as any patient who had abnormal ovarian reserve test [antral follicle count (AFC) <5-6 follicle or anti-Mullerian hormone (AMH) <0.5-1.1ng/ml] and a previous poor response (<3 oocyte retrieved) in control ovarian hyperstimulation (COH).
Anthropometric measurements were taken, including BMI [BMI, calculated as weight/(height)2 (kg/m2)]. Luteinizing hormone (LH), follicle stimulating hormone (FSH), anti mullerian hormone (AMH) and testosterone levels were determined. All laboratory parameters were determined in the early follicular phase of the menstrual cycle.
Protocol for controlled ovarian stimulation
Patients underwent a standard long protocol using GnRH-a (Superfact, Aventis, Frankfurt, Germany) at a daily dose of 0.5 mg subcutaneous start on the day 17-19 of the natural menstrual cycle as a pre-treatment. Once pituitary desensitization was confirmed (endometrial thickness <5 mm and serum estradiol level <50 pg/ml), the GnRH-a dose was reduced by one-half and ovarian stimulation was initiated.
In all patients, ovarian stimulation started with a dose of 150-225 IU r-FSH (Gonal-F, Merck Serono, Switzerland) depending on the age of the patient. It was continued until the day of ovulatory human chorionic gonadotropin (hCG) administration according to the ovarian response. When at least two follicles were greater than 18 mm, 10,000 IU urinary hCG (Choriomon, IBSA, Lugano, Switzerland) was administered intramuscularly for ovulation induction and oocyte retrieval was performed 34-36 hours later (32).
Sample collection
Follicular fluid aspiration was carried out with transvaginal ultrasound guidance using an aspiration needle from the largest follicle (>18mm) without flushing medium and blood contamination. The follicular fluid was transferred to a sterile Petri dish, and after the oocytes were removed, the fluid was located into a 15-mL conical tube and centrifuged at 300 g for 5 minutes. Supernatant was then removed.
RNA isolation, cDNA production and RT-PCR
One milliliter of TRI reagent (Sigma, Pool, UK) was added on the cellular pellet and homogenized for total RNA extraction following a standard protocol according to manufacturer’s instructions. Obtained total RNA in both groups was treated three times with DNase I (fermentase, sanktleon-rot, Germany) to remove genomic DNA contamination from the samples. First strand cDNA synthesis was performed using oligodT primers and reverse transcription by Super-Script II (Fermentas). Negative controls were prepared without addition of the enzyme (non-reverse transcribed controls, RT controls). The RT-PCR was performed using cDNA of each patient, Platinum Blue PCR Super Mix (Invitrogen, Pairsley, UK) and the forward and reverse primers for TLR1, 2, 4, 5, 6, and COX2 (Metabion, martinsried, Germany). The forward and reverse primer sequences used are given in table 1. High cycle PCR allow us to identify the genes with low level of expression. The amplification was persistent for 40 cycles under the following setting: 95˚ for 30 seconds, 60-63˚ (Table 1) for 30 seconds, and 72˚ for 30 seconds. All experiments included RT controls as negative controls (no cDNA) and water control. PCR products were separated on 1.2% agarose gel. The amplified PCR products were sequenced to confirm the identity of amplified products. β-actin was used as a housekeeping control and its expression was checked between the two groups. Its expression was not different in POR and control groups (Fig 1).
Table 1.
list of primers were used for regular PCR and real time –PCR
| Variables | Forward primer(5-3) | Reverse primer(3-5) | Annealing temperature(C) | Product size(bp) |
|---|---|---|---|---|
| TLR1 | GGGTCAGCTGGACTTCAGA | AAAATCCAAATGCAGGAACG | 63 | 250 |
| TLR2 | TCGGAGTTCTCCCAGTTCTCT | TCCAGTGCTTCAACCCACAA | 60 | 175 |
| TLR4 | TGATGTCTGCCTCGCGCCTG | AACCACCTCCACGCAGGGCT | 60 | 98 |
| TLR5 | CACCAAACCAGGGATGCTAT | CCTGTGTATTGATGGGCAAA | 60 | 111 |
| TLR6 | GCCACCATGCTGGTGTTGGCT | CGCCGAGTCTGGGTCCACTG | 60 | 101 |
| COX2 | CAGCCATACAGCAAATCCT | TCTCCATAGAATCCTGTCCG | 60 | 113 |
| β-actin | CAAGATCATTGCTCCTCCTG | ATCCACATCTGCTGGAAGG | 60 | 90 |
Sequence of TLRs, COX2 and β-actin primers used in the current investigation in RT-PCR. TLR; Toll-like receptor and COX; Cyclooxygenase.
Fig 1.
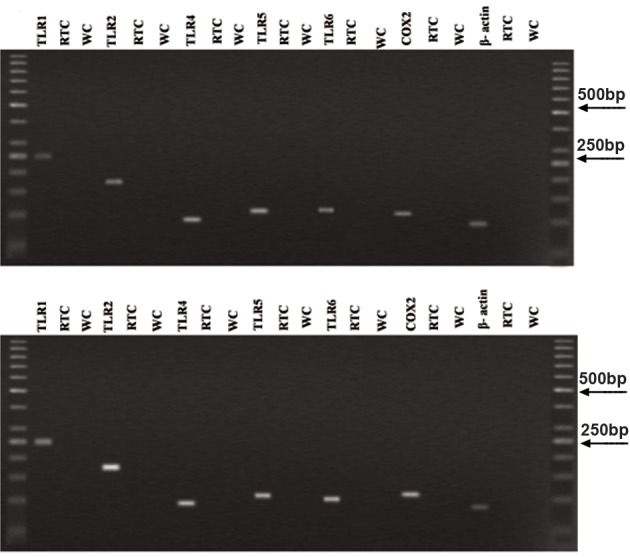
Result of RT-PCR for TLR1, 2, 4, 5, 6, COX2 and β-actin mRNA expression in control (A) and POR (B) groups. RTC; RT control, WC; Water control and COX; Cyclooxygenase.
Quantitative real time PCR (QPCR)
QPCR was performed with the cDNA prepared from follicular cell pellet. QPCR reactions were carried out in triplicates using an ABI Prism 7300 Sequence Detector (Applied Biosystems, foster, USA) in a total volume of 20 μl containing 250 ng cDNA, 5 pmol gene specific primers and SYBR Green reagent (Applied Biosystems) with ROX dye as passive control for signal intensity. The thermal cycle profile followed 50 cycles at 95˚for 30 seconds, 60-63˚ (Table 1) for 30 seconds, and 72˚ for 30 seconds. Samples were run in triplicates. Melting curve analysis permitted determination of the specificity of the PCR fragments. All melting curves yielded one peak per PCR product. Standard curves were obtained using the logarithmic dilution series of total RNA.
The QPCR data were analyzed using the comparative CT method (33). In brief, the difference in cycle time (ΔCT) was determined as the difference between the number of cycles required for amplification of the test gene and the reference housekeeping gene, human β-actin. We then obtained ΔΔCT by finding the difference between groups. The fold change (FC) was calculated as -2-ΔΔCT.
Immunoassay
Obtained FF from each patient was centrifuged at 300 g for 5 minutes, then supernatant was used to determine the concentration of IL-6, IL-8 and MIF by commercially Enzyme-Linked Immunosorbent Assay (ELISA) kits for IL-6 (eBioscience, Vienna, Austria), IL-8 (eBioscience, Vienna, Austria) and MIF (glory science, TX, USA).
Briefly, this technique uses a microwell plate coated with monoclonal antibody to human IL-6 (eBioscience), human IL-8/NAP-1 (eBioscience), human MIF (glory science), biotin-conjugate anti human IL-6 monoclonal antibody (eBioscience), biotin-conjugate anti human IL-8/NAP-1 monoclonal antibody (eBioscience), biotin-conjugate anti human MIF monoclonal antibody (glory science), streptavidine- HRP and tetramethyl-benzidine as a substrate.
Color change is measured spectrophotometrically at a wavelength of 450 nm. The concentration of these cytokines in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Cytokine concentration were considered zero, if the detected cytokine concentration was equal to or less than the lower limit of kits (IL-6: 0.92 pg/ml, IL-8: 2.0 pg/ml and MIF: 0.003 pg/ml).
Statistics
The results were expressed as mean ± SEM. Statistical analysis was performed by using t test in SPSS 18 software. P<0.05 was considered significant.
Results
Clinical characteristics of the patients are presented in table 2.There is a significant difference in AMH, number of mature oocyte and total rFSH dose (IU) between POR and control groups.
Table 2.
Clinical characteristics
| P value | Control Group (N=20) | POR Group (N=20) | Variable |
|---|---|---|---|
| Age (Y) | 30.47 ± 4.62 | 30.75 ± 3.89 | 0.727 |
| BMI (kg/m2), | 25.87 ± 2.92 | 25.20 ± 4.19 | 0.55 |
| Duration of infertility (Y) | 9.76 ± 6.04 | 7.70 ± 5.41 | 0.25 |
| Menstrual type, n (%) | |||
| Regular | 20 (100) | 20 (100) | |
| Irregular | 0 | 0 | |
| LH (mU/ml) | 4.23 ± 2.24 | 5.02 ± 3.39 | 0.38 |
| FSH (mU/ml) | 10.99 ± 3.24 | 8.55 ± 5.37 | 0.08 |
| LH/FSH ratio | 0.56 ± 0.92 | 0.64 ± 0.33 | 0.71 |
| Testosterone (ng/ml) | 1.21 ± 0.31 | 1.31 ± 0.54 | 0.33 |
| AMH (ng/ml) | 0.175 ± 0.03 | 0.63 ± 0.18 | 0.02 |
| No. of mature oocyte | 1.5 ± 0.44 | 10.1 ± 1.59 | 0.00 |
| Total rFSH dose (IU) | 3321 ± 243.65 | 2390.3 ± 190.39 | 0.005 |
Presented as mean ± SD and compared by t test.
BMI; Body mass index, AMH; Anti-mullerian hormone, LH; Luteinizing hormone, FHS; Follicle stimulating hormone and *; P<0.05.
Clinical characteristics of the patients. There is significant difference in AMH, No. of mature oocyte and total rFSH dose (IU) between POR and control groups. Data were analyzed by t test.
Figure 1 shows the results of RT-PCR for mRNA expression of TLRs and COX2 genes in human follicular cells in both groups. All amplified products had the expected size for that particular gene. There was no product amplified innegative control indicative of the lack of genomic DNA contamination.
The quantitative expression profiles of TLR genes in follicular cells in both groups are shown in figure 2. TLR1, 2, 4, 5, 6 and COX2 showed a significantly higher expression in POR patients compared to the control (p<0.05).
The quantitative analysis of IL-6, IL-8 and MIF concentrations in FF by ELISA are shown in figure 3. IL-6, IL-8 and MIF were significantly increased in POR compared with control (p<0.05).
Fig 2.
QPCR was used to quantify the expression of TLR1, 2, 4, 5, 6 and COX2 mRNA in POR and control groups. Data are presented as mean ± SEM of normalized expression values against internal controls (β-actin mRNA) in POR and control. TLR1, 2, 4, 5, 6 and COX2 showed a significantly higher expression in POR patients compared with the normal women. Data were analyzed by t test. *; P<0.05, POR; Poor ovarian response, MIF; Migration inhibitory factor, TLR; Toll-like receptor and COX; Cyclooxygenase.
Fig 3.
IL-6, IL-8 and MIF protein concentration obtained by ELISA in FF of POR and control groups. IL-6, IL-8 and MIF showed a significantly higher expression in POR compared with the control. Data were analyzed by t test. *; P<0.05, POR; Poor ovarian response, MIF; Migration inhibitory factor and IL; Interleukin.
Discussion
We found higher expression of TLR1, 2, 4, 5 and 6 in POR compare to normal participants. Our data suggest that elevated expression of TLRs is correlated with the POR to standard ovarian stimulation protocol.
Numerous factors affect ovarian response to gonadotropin stimulation. It is suggested that the main reason of POR is diminished ovarian reserve (34). However, some young women with normal ovarian reserve present a poor response to ovarian stimulation (1). A hypothesis has been proposed for these patients: dysfunction of cytokines and the growth factor network (5) as a product of TLR signaling (35). Therefore, in the present study we investigated the expression of cell membrane TLRs in follicular cells of POR patients.
Our findings show that TLR 1, 2, 4, 5, 6 were expressed in follicular cells of the control group, consistent with a recent study which showed COV434 human granulosa cell line expresses TLR4-10 (36).
However, in relation to the TLR overexpression in POR patients, several hypotheses are discussed; i. TLR overexpression is a consequence of the presence of their endogenous or exogenous ligands in FF. Keay et al. (37) have reported a significantly higher prevalence of serum IgG antibodies to C. trachomatis in poor responders. Darville et al. (38) showed that C. trachomatis engages TLR2. ii. This expression pattern may be due to a susceptible genetic background in these POR patients where excessive TLR activation could be involved in the pathogenesis of POR through several mechanisms.
TLR activation leads to apoptosis through the Fas associated death domain (FADD) (39). Therefore increased follicular cell apoptosis leads to folliculogenesis impairment. Also, TLR activation results in excessive expression of COX-2 as a key mediator in ovulation (40). For instance Fukata et al. (41) demonstrated that TLR4 activation leads to COX-2 induction. Consistent with this study, our findings have shown that COX-2 expression is significantly higher in POR subsequent to TLR4 overexpression. COX-2 is a key enzyme in the conversion of arachidonic acid to prostaglandins (40). COX2 is upregulated in response to cytokines, growth factors, and estradiol stimuli. Likewise, it has been suggested that this enzyme is mostly associated with the inflammatory response (21).
TLRs have important role in cytokines production and autoimmunity (42). In agreement with this, our findings suggest that the overexpression of TLRs in follicular cells and excessive production of IL-6, IL-8 and MIF in follicular fluid simultaneously occur in the POR group. These cytokines affect the ovarian function and oocyte development through several mechanisms.
IL-6 diminishes aromatase activity within follicles which result in decreased intrafollicular E2 level, fertility and fertilizing capacity (43). IL-8 is a chemotactic activating cytokine for leukocytes and macrophages (44) with which their activation leads to increased ROS production (45). ROS bind to TLR 2 and 6 and consequently activate them (12).
Our study showed significant higher MIF protein production, consistent with significant TLR4 over expression. This finding is in agreement with a recent study that showed TLR4 ligation leads to increased MIF level in epithelial ovarian cancer cells (46). MIF binding to CD74 stimulates pro-inflammatory cytokines, including IL-8, which leads to altered inflammatory and immune responses, cell proliferation and angiogenesis (47, 48).
Moreover, TLR signaling results in elevated levels of IL-10 in FF (49). It prevents p27 down-regulation in developing granulosa cells. Subsequently, G0 arrest of granulosa cell cycle leads to folliculogenesis impairment (50). Also, IL-10 is an anti-inflammatory cytokine and controls inflammation response via inhibiting TLR signaling pathways (51).
As previously stated, overstimulation of TLRs contribute to autoimmune response and tissue injury (42). Besides, a high correlation between POR and the presence of ovarian auto-antibody was seen (52, 53). These autoimmune responses primarily targets theca and granulosa cells (54), yielding dramatically reduced FSH receptor (FSHR) in POR. In a previous study, it has been shown that relative quantity of FSHR is positively correlated with two markers of ovarian response including number of mature oocytes and the peak level of serum E2 (3). Moreover, it has been stated that serum anti-FSH antibodies are increased in POR (55). FSH-FSHR interaction elicits intracellular signaling pathways responsible for proliferation and differentiation of granulosa cells. Subsequently, the FSH-stimulated granulosa cells produce E2 and sufficient E2 within the developing follicles further sustains oocyte development and maturation (56). Therefore, activation of TLRs may disturb the FSH-FSHR interaction which leads to poor proliferation and differentiation of granulosa cells and also reduced production of E2.
Despite the study being well-designed, the present study possesses the following limitations:
i. The number of included subjects was small and ii. It is a fact that POR group received increased volume of gonadotropins; therefore this increased dosage may have affected the immunological mechanisms.
Conclusion
The association between increased TLR expression in follicular cells in POR suggest that TLRs may play important roles in the pathophysiology of POR. Further studies should be performed in future to confirm these findings and to determine the extent TLRs or other components of the TLR signaling pathway contribute to POR.
Acknowledgments
We thank the staffs of female infertility research lab and embryology lab in Royan institute. This study was supported by Iran University of Medical Sciences and Royan institute. There is no conflict of interest in this study.
References
- 1.Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997;104(5):521–527. doi: 10.1111/j.1471-0528.1997.tb11525.x. [DOI] [PubMed] [Google Scholar]
- 2.Veleva Z, Jarvela IY, Nuojua-Huttunen S, Martikainen H, Tapanainen JS. An initial low response predicts poor outcome in in vitro fertilization/intracytoplasmic sperm injection despite improved ovarian response in consecutive cycles. Fertil Steril. 2005;83(5):1384–1390. doi: 10.1016/j.fertnstert.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Lou HY, Dong MY, Lu XE, Zhu YM, Gao HJ, et al. Poor ovarian response to gonadotropin stimulation is associated with low expression of follicle-stimulating hormone receptor in granulosa cells. Fertil Steril. 2007;87(6):1350–1356. doi: 10.1016/j.fertnstert.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Voutilainen R, Franks S, Mason HD, Martikainen H. Expression of insulin-like growth factor (IGF), IGF-binding protein, and IGF receptor messenger ribonucleic acids in normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81(3):1003–1008. doi: 10.1210/jcem.81.3.8772565. [DOI] [PubMed] [Google Scholar]
- 5.Salmassi A, Schmutzler AG, Schaefer S, Koch K, Hedderich J, Jonat W, et al. Is granulocyte colony-stimulating factor level predictive for human IVF outcome? Hum Reprod. 2005;20(9):2434–2440. doi: 10.1093/humrep/dei071. [DOI] [PubMed] [Google Scholar]
- 6.Hovav Y, Almagor M, Benbenishti D, Margalioth EJ, Kafka I, Yaffe H. Immunity to zona pellucida in women with low response to ovarian stimulation, in unexplained infertility and after multiple IVF attempts. Hum Reprod. 1994;9(4):643–645. doi: 10.1093/oxfordjournals.humrep.a138563. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 8.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 9.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168(2):554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 11.Watters TM, Kenny EF, O'Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85(6):411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276(7):5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 13.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 15.Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103(5):1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 16.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 17.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167(4):1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 18.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20(5):1372–1378. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 19.Aflatoonian R, Fazeli A. Toll-like receptors in female reproductive tract and their menstrual cycle dependent expression. J Reprod Immunol. 2008;77(1):7–13. doi: 10.1016/j.jri.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Aflatoonian R, Tuckerman E, Elliott SL, Bruce C, Aflatoonian A, Li TC, et al. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22(2):586–593. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- 21.Williams CS, Dubois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270(3 Pt 1):G393–400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 22.Narko K, Ritvos O, Ristimaki A. Induction of cyclooxygenase-2 and prostaglandin F2alpha receptor expression by interleukin-1beta in cultured human granulosa-luteal cells. Endocrinology. 1997;138(9):3638–3644. doi: 10.1210/endo.138.9.5388. [DOI] [PubMed] [Google Scholar]
- 23.Tokuyama O, Nakamura Y, Muso A, Honda K, Ishiko O, Ogita S. Expression and distribution of cyclooxygenase-2 in human periovulatory ovary. Int J Mol Med. 2001;8(6):603–606. doi: 10.3892/ijmm.8.6.603. [DOI] [PubMed] [Google Scholar]
- 24.Laurincik J, Oberfranc M, Hyttel P, Grafenau P, Tomanek M, Pivko J. Characterization of the periovulatory period in superovulated heifers. Theriogenology. 1993;39(2):537–544. doi: 10.1016/0093-691x(93)90395-l. [DOI] [PubMed] [Google Scholar]
- 25.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 26.Aboussahoud W, Aflatoonian R, Bruce C, Elliott S, Ward J, Newton S, et al. Expression and function of Toll-like receptors in human endometrial epithelial cell lines. J Reprod Immunol. 2010;84(1):41–51. doi: 10.1016/j.jri.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 28.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–693. doi: 10.1530/REP-07-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;20(12):3228–3239. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- 30.Zhou M, McFarland-Mancini MM, Funk HM, Husseinzadeh N, Mounajjed T, Drew AF. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol Immunother. 2009;58(9):1375–1385. doi: 10.1007/s00262-008-0650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer WR, Lavy G, DeCherney AH, Visintin I, Economy K, Luborsky JL. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response to exogenous gonadotropin. Obstet Gynecol. 1990;75(5):795–799. [PubMed] [Google Scholar]
- 32.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26(6):347–354. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9(1):61–76. doi: 10.1093/humupd/dmg007. [DOI] [PubMed] [Google Scholar]
- 35.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117(5):979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Price JC, Cronin J, Sheldon IM. Toll-like receptor expression and function in the COV434 granulosa cell line. Am J Reprod Immunol. 2012;68(3):205–217. doi: 10.1111/j.1600-0897.2011.01103.x. [DOI] [PubMed] [Google Scholar]
- 37.Keay SD, Barlow R, Eley A, Masson GM, Anthony FW, Jenkins JM. The relation between immunoglobulin G antibodies to Chlamydia trachomatis and poor ovarian response to gonadotropin stimulation before in vitro fertilization. Fertil Steril. 1998;70(2):214–218. doi: 10.1016/s0015-0282(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 38.Darville T, O'Neill JM, Andrews CW Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171(11):6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 39.Salaun B, Romero P, Lebecque S. Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37(12):3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 40.Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Doré M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10(5):373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 41.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131(3):862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadimitraki ED, Bertsias GK, Boumpas DT. Toll like receptors and autoimmunity: a critical appraisal. J Autoimmun. 2007;29(4):310–318. doi: 10.1016/j.jaut.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi J, Simon C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70(3):425–431. doi: 10.1016/s0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 44.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeller JM, Henig I, Radwanska E, Dmowski WP. Enhancement of human monocyte and peritoneal macrophage chemiluminescence activities in women with endometriosis. Am J Reprod Immunol Microbiol. 1987;13(3):78–82. doi: 10.1111/j.1600-0897.1987.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal R, Whang DH, Alvero AB, Visintin I, Lai Y, Segal EA, et al. Macrophage migration inhibitory factor expression in ovarian cancer. Am J Obstet Gynecol. 2007;196(4):348. e1-5. doi: 10.1016/j.ajog.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261(2):147–157. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 48.Beswick EJ, Reyes VE. Macrophage migration inhibitory factor and interleukin-8 produced by gastric epithelial cells during Helicobacter pylori exposure induce expression and activation of the epidermal growth factor receptor. Infect Immun. 2008;76(7):3233–3240. doi: 10.1128/IAI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagawa Y, Onoe K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178(10):6173–6180. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- 50.Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174(5):1522–1526. doi: 10.1016/s0002-9378(96)70600-2. [DOI] [PubMed] [Google Scholar]
- 51.Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation--a continuing puzzle. Immunology. 2004;113(3):281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luborsky JL, Thiruppathi P, Rivnay B, Roussev R, Coulam C, Radwanska E. Evidence for different aetiologies of low estradiol response to FSH: age-related accelerated luteinization of follicles or presence of ovarian autoantibodies. Hum Reprod. 2002;17(10):2641–2649. doi: 10.1093/humrep/17.10.2641. [DOI] [PubMed] [Google Scholar]
- 53.Pires ES, Parikh FR, Mande PV, Uttamchandani SA, Savkar S, Khole VV. Can anti-ovarian antibody testing be useful in an IVF-ET clinic? J Assist Reprod Genet. 2011;28(1):55–64. doi: 10.1007/s10815-010-9488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Marca A, Brozzetti A, Sighinolfi G, Marzotti S, Volpe A, Falorni A. Primary ovarian insufficiency: autoimmune causes. Curr Opin Obstet Gynecol. 2010;22(4):277–282. doi: 10.1097/GCO.0b013e32833b6c70. [DOI] [PubMed] [Google Scholar]
- 55.Haller K, Salumets A, Uibo R. Anti-FSH antibodies associate with poor outcome of ovarian stimulation in IVF. Reprod Biomed Online. 2008;16(3):350–355. doi: 10.1016/s1472-6483(10)60595-0. [DOI] [PubMed] [Google Scholar]
- 56.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18(6):739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]