Abstract
Constipation-predominant irritable bowel syndrome (IBS-C) is a commonly prevalent and clinically challenging disorder to treat. Until recently, most therapeutic agents had limited ability to address the complexity of symptoms inherent to the syndrome. The development of linaclotide provides a physiologically sound approach to treatment of the multiple symptoms of IBS-C. Clinical trials demonstrate the efficacy of linaclotide, and a platform to better understand the symptomatology of IBS-C. Based on recent clinical evidence, linaclotide should be considered for patients with IBS-C because it improves abdominal pain and bowel symptoms. In phase III trials, linaclotide met the US Food and Drug Administration responder endpoint with a number needed to treat (NNT) of 5.1–7.9, and European Medicines Agency coprimary endpoints at 12 weeks with a NNT of 4.39–7.69, and at 26 weeks with a NNT of 4.93–5.68. It is safe and effective, with diarrhea reported as the most common adverse effect, which leads to discontinuation of the medication in approximately 5% of patients.
Keywords: irritable bowel syndrome, linaclotide, constipation, IBS-C
Introduction
Irritable bowel syndrome (IBS) is a chronic illness in which disordered defecation or a change in bowel habits is associated with abdominal pain or discomfort over a period of at least 3 months [Longstreth et al. 2006]. Approximately 30 million people in North America meet the diagnostic criteria for IBS, with prevalence estimates ranging from 10% to 15%, for which IBS with constipation (IBS-C) accounts for approximately 5% [Saito et al. 2002]. Epidemiologic trends in many Asian, African, and European countries approximate or surpass this prevalence [Abdulmajeed et al. 2011; Chang et al. 2010; Krogsgaard et al. 2013; Okeke et al. 2009]. There is a 1.5 times higher female predominance for IBS, and it is more commonly diagnosed in patients less than 50 years of age. The course of illness is characterized by recurring symptoms, impaired quality of life (QOL), increased healthcare costs, and reduced work productivity [Brandt et al. 2009; Cash et al. 2005; Drossman et al. 2002; Pare et al. 2006].
IBS has been characterized as functional because its pathobiologic cause is not readily apparent. Peripheral, organ-specific pathophysiologic mechanisms in IBS may include sensitization of primary afferent pathways, infection, epithelial-immune activation, increased mast cells, epithelial permeability, and dysmotility [Mayer and Tillisch, 2011]. Emerging evidence suggests that alterations in intestinal microbiota and immune function impinge upon the brain–gut axis, and cause alterations in gastrointestinal function and clinical symptoms in patients with IBS [Ringel and Maharshak, 2013]. There is also increasing evidence showing that sensitizing proinflammatory and lipotoxic lipids, mast cells and their products, tryptases, enteroendocrine cells, and mononuclear phagocytes and their receptors are increased in tissues of patients with IBS. Changes in afferent excitability in colorectal afferent innervation in response to mechanical stimuli are consistent with modulation of discomfort and pain in this disorder, stressing the importance of afferent drive in this disorder [Feng et al. 2012]. Compared with controls, patients with IBS have a decreased ability to downregulate intestinal recruitment (CCR5) and activation phenotypes (CD28) in response to pathogen-associated molecular pattern stimulation [Rodriguez-Fandino et al. 2013]. This suggests that innate immunity and microbial triggers are involved in the development of IBS, and exacerbation of IBS symptoms.
Central pathophysiologic mechanisms in IBS may include enhanced stress responsiveness, central pain amplification, neuroimmune activation in the spinal cord, enhanced brain responses to visceral distention, enhanced brain responses to expectation of visceral pain, and structural brain changes. The brain receives interoceptive input from abdominal viscera and responds to these inputs in a reflexive way. In the healthy individual, this is not consciously perceived, however in IBS, interoceptive input and its perception can be altered by activity within stress and arousal circuits, which alter both the perception and feedback to these organs [Mayer and Tillisch, 2011]. In patients with IBS, a significant correlation has been found between specific psychological features and neuroendocrine markers, including plasma cortisol and neuropeptide Y [Stasi et al. 2013]. Possible mechanisms for visceral hyperalgesia include neurokinin 1, corticotropin-releasing factor 1, vasopressin 3, vanilloid (TRPV1), and endocannabinoid (CB1) receptors [Bradesi et al. 2009; Hong et al. 2009; Schwetz et al. 2004].
Linaclotide (MD-1100 acetate) is a novel, orally active, 14-amino acid peptide of the guanylin family of cyclic guanosine monophosphate (cGMP)-regulating guanylate cyclase-C (GC-C) agonists that is approved by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for the treatment of moderate to severe IBS-C in adults. It acts by increasing fluid secretion, thereby accelerating gastrointestinal (GI) transit, and has GC-C-mediated analgesic effects. In this review, we examine the rationale for linaclotide in the treatment of IBS-C, provide an overview of the clinical evidence and experience to date, and examine its role in the management of IBS-C.
Rationale for linaclotide
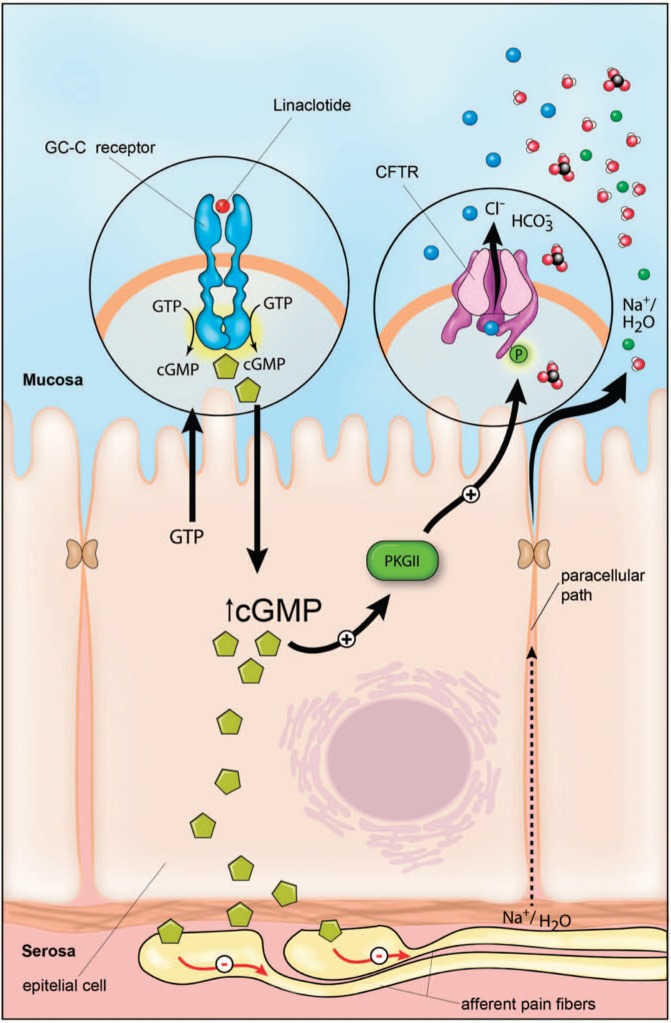
GC-C is located on the luminal/apical surface of intestinal enterocytes, and its two endogenous ligands in mammals are guanylin and uroguanylin, 2-disulfide paracrine hormones, primarily produced by intestinal goblet and enteroendocrine cells. It is a soluble and single-spanning enzyme that is part of a larger family of GC enzymes that serve as receptors for A-type, B-type, and C-type natriuretic peptides. The other two guanylin peptides are lymphoguanylin and renoguanylin [Bharucha and Linden, 2010; Potter, 2011]. Guanylin is a 15-amino acid peptide primarily synthesized in the distal ileum and proximal colon, while uroguanylin is a 19-amino acid peptide synthesized primarily in the duodenum. Both are evolutionarily conserved across species, strongly indicating their unique physiological roles [Brierley, 2012; Whitaker et al. 1997]. Additionally, the guanylin peptides may prevent postprandial hypernatremia and hypervolemia by increasing intestinal and renal secretion [Bharucha and Linden, 2010], and may also have an anorexigenic effect postprandially by activating GC-C on hypothalamic neurons [Brierley, 2012]. GC-C null mice have been shown to retain a natriuretic response in response to guanylin, uroguanylin, and heat-stable enterotoxins (STa), pointing to a cGMP-dependent, GC-C independent renal tubular process, making fluid-ion hemostasis an unlikely contributor to altered visceral hypersensitivity [Carrithers et al. 2004].
The guanylin peptides are released in an autocrine or paracrine fashion into the intestinal lumen. Levels of cGMP, an intracellular second messenger, are then increased and activate its three downstream effectors: cGMP-dependent protein kinases (PKGs), phosphodiesterases, and cyclic nucleotide-gated channels. This subsequently increases chloride (Cl−), bicarbonate, and fluid secretion into the intestinal lumen through the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (see Figure 1). PKGII displays a rostral-caudal gradient of expression, with the highest levels found in the small intestine and the lowest levels found in the distal colon. Expression of PKGII is highest in the villi, and lowest in the crypts of the small intestine. PKGII is colocalized at the apical surface of enterocytes with, and activates, the CFTR Cl− channel. GC-C is also the principal receptor that mediates secretion in response to STa, the major cause of Escherichia coli induced secretory diarrhea, exemplifying molecular mimicry and convergent evolution, whereby bacteria have coopted a normal mammalian physiologic function [Lin et al. 2010; Vaandrager et al. 1998].
Figure 1.
Proposed mechanism of action of linaclotide. CFTR, cystic fibrosis transmembrane conductance regulator; cGMP, cyclic guanosine monophosphate; GC-C, guanylate cyclase type C; GTP, guanosine triphosphate; PKG, cGMP-dependent protein kinases. From Lacy et al. [2012].
Eutamene and colleagues studied the effect of linaclotide in different rodent models. In a trinitrobenzene sulfonic acid induced colitis model of visceral hypersensitivity, linaclotide decreased the number of abdominal contractions in response to colorectal distention without affecting wall elasticity change in response to distending pressures in wild type Wistar rats, but not GC-C null mice. Albeit nonlinearly, linaclotide also decreased colonic hypersensitivity in rodents exposed to water avoidance stress and restraint stress [Eutamene et al. 2010]. Like the endogenous agonists, linaclotide activates GC-C and subsequently enhances Cl− and bicarbonate (HCO3−) secretion through the CFTR Cl− channel, inhibits luminal sodium (Na+) absorption through a blockade of Na+/H+ exchange increasing water excretion, and activates other CFTR-independent mechanisms. Blocking the transport of cGMP from isolated murine colorectal epithelia into the basolateral extracellular space has been shown to abolish the inhibitory effect of uroguanylin on colorectal afferents on directly modulated responses as well as sensitization of mechanosensitive colorectal primary afferents. Therefore GC-C agonists, like linaclotide, alleviate colorectal pain and hypersensitivity by dampening stretch-sensitive afferent mechanosensitivity and normalizing afferent sensitization [Feng et al. 2013].
Linaclotide does not contain the two N-terminal residues present in guanylin and uroguanylin, which keep it from being pH restricted, supporting its ability to modulate intestinal fluid homeostasis along the whole longitudinal axis of the GI tract. It also has very low oral bioavailability (0.1%), which supports the hypothesis that its effects on GI function are mediated through local activation of GC-C receptors [Bryant et al. 2010; Busby et al. 2010]. Pharmacologic study has shown that linaclotide is stable in the acidic environment of the stomach and in the small intestine its disulfide bonds are reduced, proteolyzed, and degraded. There it is converted to MM-419447, the predominant 13-amino acid active linaclotide metabolite in rats and humans, which exhibits dose-dependent increases in cGMP levels and increased intestinal transit [Busby et al. 2013].
When given exogenously, cGMP has been shown to demonstrate the same analgesic effects guanylin peptides demonstrated in several models of colonic hypersensitivity. This pathway is the principal regulator of intestinal fluid homeostasis, and plays important roles in the restoration of mucosal barrier function in intestinal disorders and homeostatic control of the intestinal crypt–villus axis [Silos-Santiago et al. 2013]. In a murine model, intracolonic administration of linaclotide reduced signal processing of noxious colorectal distention to the thoracolumbar spinal cord in vivo, correlating with prior in vitro observations. Colonic mucosa, but not neurons, expressed linaclotide’s target GC-C, emphasizing the importance of cGMP as a downstream effector of GC-C [Castro et al. 2013].
Experience with linaclotide for IBS-C: clinical trials
Physiological studies of linaclotide showed safety in animals with a greater than a 1000-fold therapeutic index, and subsequent phase I studies showed safety and efficacy of linaclotide at single doses ranging from 30 to 3000 µg with increases in stool consistency and stool weight, and with 7-day treatment regimens between 30 and 1000 µg in healthy volunteers, with dose-dependent increases in stool frequency and stool weight from baseline [Currie et al. 2005; Kurtz et al. 2006]. No evidence of systemic exposure was found to linaclotide or its metabolite MM-419447 after oral administration, and the medication was shown to be safe and well tolerated.
In 2007, Andresen and colleagues evaluated the effect of 5 days of linaclotide on transit and bowel function in patients with IBS-C by Rome II criteria in a phase IIa randomized, double-blind, placebo-controlled trial. In this study, 36 women with IBS-C were randomized in a 1:1:1 fashion to placebo, linaclotide 100 µg, and linaclotide 1000 µg. Five-day baseline and 5-day treatment periods were studied, and in order to be enrolled in the treatment period, patients had to have slow colonic transit, or slower transit than the mean for healthy controls. Information was collected regarding gastric, small bowel, and colonic transit by scintigraphy, and bowel function using stool diaries, which included Bristol Stool Form Scale (BSFS) scores for stool consistency, ease of stool passage scores, and completeness of evacuation. No treatment effects were seen for gastric emptying or colonic filling with linaclotide. Significant treatment effects were found for ascending colon emptying t½ times (p = 0.015) and overall total colonic transit times at 48 h (p = 0.02), for the 1000 µg dose (p = 0.004) but not the 100 µg dose, as well as overall treatment effects on increased stool frequency, decreased stool consistency, improved ease of passage, and acceleration of time to first bowel movement (p < 0.001) [Andresen et al. 2007] (Table 1).
Table 1.
Phase II clinical trials of linaclotide in constipation-predominant irritable bowel syndrome.
| Study/phase/design | Number of patients/sex/duration/doses studied | Primary endpoints | Efficacy results | Secondary endpoints | Efficacy results |
|---|---|---|---|---|---|
| Andresen et al. [2007] | N = 36 | Gastric transit | No treatment effect | NA | NA |
| Phase IIa | Female patients = 100% | Small bowel transit | Increased ascending colon emptying t½ times (p = 0.015) and increased total colonic transit times at 48 h (p = 0.02) (for 1000 µg dose; p = 0.004) | ||
| Randomized, double-blind, placebo-controlled trial | 5-day baseline and 5-day treatment periods | Colonic transit (by scintigraphy) | Increased stool frequency, decreased stool consistency, improved ease of passage, and time to first bowel movement (p < 0.001) | ||
| Placebo, 100 and 1000 µg linaclotide | Bowel function (using stool diaries) | ||||
| Johnston et al. [2010] | N = 420 | Change in the number of CSBMs | For the 75, 150, 300, 600 µg linaclotide doses: mean change in CSBMs/week were 2.90, 2.49, 3.61 and 2.68 respectively (p < 0.01) | Effect on individual symptoms | Improved frequency of SBMs (p ≤ 0.001), CSBMs (p ≤ 0.01), severity of straining (p ≤ 0.001), stool consistency (p ≤ 0.001), and abdominal pain scores (p ≤ 0.05) |
| Phase IIb | Female patients = 92% | Proportion of patients who were CSBM responders was 25%, 19.5%, 32% and 24% respectively (all significant except for the 150 µg dose) | Proportion of CSBM responders Proportion of Global relief responders | Higher proportion of adequate relief responders | |
| Randomized, double-blind, parallel-group, multicenter, placebo-controlled trial | 12-week treatment period | Quality of life | Higher proportion of global relief responders | ||
| Placebo, 75, 150, 300 and 600 µg linaclotide | IBS-QOL scores increased ≥14 points in all linaclotide treatment groups |
CSBM, complete spontaneous bowel movement; IBS, irritable bowel syndrome; NA, not applicable; QOL, quality of life; SBM, spontaneous bowel movement.
Efficacy: phase II dose-ranging studies
In 2010, Johnston and colleagues studied the efficacy and safety of 12 weeks of linaclotide at a daily dose range of 75–600 µg in a phase IIb randomized, double-blind, parallel-group, multicenter, placebo-controlled trial in 420 patients with IBS-C (mean age = 44 years, female patients = 92%) [Johnston et al. 2010]. Patients had to meet Rome II criteria, with fewer than three spontaneous bowel movements (SBMs) per week, and straining, lumpy/hard stools, or sensation of incomplete evacuation more than 25% of the time, for at least 12 weeks in the 12 months preceding study entry. Additionally patients needed to have the presence of at least two of five severity of nonmenstrual pain/abdominal discomfort on a five-point ordinal scale, with less than three complete SBMs (CSBMs) (a combination of SBMs with sensation of complete evacuation, and no more than six SBMs per week). The primary endpoint was a change in the number of CSBMs. Secondary endpoints included effect on individual symptoms, QOL, the proportion of patients who were CSBM responders (at least three CSBMs/week, and increase of one CSBM from baseline for 75% of the study duration), and the proportion who were global relief responders (symptoms being somewhat, considerably, or completely relieved for 100% of the study duration, or completely relieved for 50% of the study duration).
For the 75, 150, 300 and 600 µg linaclotide doses, the mean change in CSBMs per week was 2.90, 2.49, 3.61 and 2.68, respectively (p < 0.01), and the proportion of patients who were CSBM responders was 25%, 19.5%, 32% and 24%, respectively. The CSBM responder proportions were significant for all doses except for the 150 µg dose. Compared with placebo, the linaclotide-treated group had a higher proportion of adequate relief responders (33–51% versus 22%) and global relief responders (44–55% versus 29%). Compared with placebo, all doses of linaclotide significantly improved bowel habits, including frequency of SBMs (p ≤ 0.001) and CSBMs (p ≤ 0.01), severity of straining (p ≤ 0.001), stool consistency (p ≤ 0.001), as well as abdominal pain scores (p ≤ 0.05). Patients with abdominal pain scores rated 4–5 (severe to very severe) for 50% of the days during the baseline period demonstrated the greatest reduction of abdominal pain during the treatment period, Abdominal discomfort, bloating, and global IBS-C measures were also improved, for all doses except for the 75 µg (abdominal discomfort) and 150 µg dose (bloating). Effects were present for the first week, and sustained throughout the 12 weeks of treatment (Table 1).
Efficacy: phase III trials
In 2012, there were two phase III randomized, double-blind, placebo-controlled trials published that studied linaclotide in patients with IBS-C, with entry criteria similar to Johnston and colleagues’ phase IIb trial [Chey et al. 2012; Rao et al. 2012]. The primary endpoints in both of these trials were based on US FDA guidance for IBS trials developed in 2012.
Rao and colleagues studied oral linaclotide 290 µg once daily versus placebo in a 12-week treatment period, followed by a 4-week randomized withdrawal period, in 800 patients with IBS-C (mean age = 43.5 years, female patients = 90.5%, white = 76.9%). There were four primary endpoints, which included the US FDA primary responder endpoint (which will be referred to as the ‘FDA endpoint’) of at least 30% improvement in the average daily worst abdominal pain score and increase by at least one CSBM from baseline, occurring in the same week for at least 6 of the 12 weeks of therapy. The other three primary endpoints, which will be referred to as ‘responder definitions’, needed to be met for more than 9 of the first 12 weeks of the treatment period. These responder definitions were as follows:
improvement of at least 30% in abdominal pain scores;
at least three CSBMs and an increase of at least one CSBM from baseline;
combined endpoint defined as a responder patient who met criteria for both (1) and (2) in the same week.
Secondary endpoints included a 12-week change from baseline in abdominal pain, abdominal discomfort, abdominal bloating, stool frequency (CSBM and SBM weekly rates), BSFS stool consistency, and severity of straining.
The linaclotide 290 µg treated group demonstrated statistically significant improvement for all primary and secondary efficacy endpoints compared with the placebo group. A total of 33.6% of patients receiving linaclotide treatment met the FDA endpoint versus 21% receiving placebo (p < 0.0001). For at least 6 out of the 12 weeks of the treatment period, 50.1% of patients receiving linaclotide treatment had a reduction in abdominal pain of at least 30% versus 37.5% in the placebo group (p = 0.0003), and 48.6% versus 29.6% had an increase of at least one CSBM from baseline (p < 0.0001). The NNT for the FDA endpoint was 7.9. A significantly greater proportion of the patients receiving linaclotide treatment also met the responder requirements for the three responder definition endpoints, with the NNT ranging from 7.6 to 14.3. Importantly, after the initial 12 weeks of therapy and subsequent randomization to placebo, the patients originally on linaclotide who remained on linaclotide showed continued improvement of symptoms, but a return of symptoms was seen in those patients who were switched to placebo therapy. Patients who were on placebo and switched to linaclotide showed improvement in abdominal pain symptoms during 1 week of therapy [Rao et al. 2012] (Table 2).
Table 2.
Phase III clinical trials of linaclotide in constipation-predominant irritable bowel syndrome.
| Study/phase/design | Number of patients/sex/duration/doses studied | Primary endpoints | Efficacy results | Selected secondary endpoints | Efficacy results |
|---|---|---|---|---|---|
| Rao et al. [2012] | N = 800 | FDA endpoint* | 33.6% versus 21% met the FDA endpoint (p < 0.0001) | 12-week change from baseline: | |
| Phase III, randomized, double-blind, placebo-controlled trial | Female patients = 90.5% | Three responder definitions** | NNT = 7.9 | Worst abdominal pain | 50.1% versus 37.5% had a ≥30% reduction in abdominal pain (p = 0.0003) |
| 12-week treatment period followed by a 4-week randomized withdrawal period | (>9 of the 12 weeks of therapy) | Greater proportion of linaclotide treated versus placebo treated met the three responder definitions | Abdominal discomfort | 48.1% versus 37% had a ≥30% reduction in abdominal discomfort (p = 0.0001) | |
| Placebo, 290 µg linaclotide | NNT range 7.6–14.3 | Abdominal bloating | 43.5% versus 29.9% had a ≥30% reduction in abdominal bloating (p < 0.0001) | ||
| EMA 12-week abdominal pain/discomfort endpoint | Stool frequency (CSBM rate) | 48.6% versus 29.6% had a ≥1 CSBM rate increase for ≥6/12 weeks (p < 0.0001) | |||
| NNT = 7.69 | Stool frequency (SBM rates) | 57.5% versus 29.4% had a ≥2 SBM rate increase for ≥6/12 weeks (p < 0.0001) | |||
| EMA 12-week IBS degree-of-relief endpoint | BSFS stool consistency | 79.4% versus 60.7% had a BSFS ≥3 (p < 0.0001) | |||
| NNT = 5.41 | Severity of straining | 85.3% versus 71.7% had strain score ≤3 (p < 0.0001) | |||
| Chey et al. [2012] | N = 804 | FDA endpoint* | 33.7% versus 13.9% met the FDA endpoint (p < 0.0001) | 12 week change from baseline: | |
| Phase III, randomized, double-blind, placebo-controlled trial | Female patients = 90% | Three responder definitions** | NNT = 5.1 | Worst abdominal pain | 48.9% versus 34.5% had a ≥30% reduction in abdominal pain (p < 0.0001) (NNT = 7) |
| 26-week treatment period | (>9 of the first 12 weeks of therapy) | Greater proportion of linaclotide treated versus placebo treated met the three responder definitions | Abdominal discomfort | 47.6% versus 30.8% had a ≥30% reduction in abdominal discomfort (NNT = 5.9) | |
| Placebo, 290 µg linaclotide | Efficacy parameters over 26-week time period | NNT range 5.2–10.3 | Abdominal bloating | 42.9% versus 23.8% had a ≥30% reduction in abdominal bloating (NNT = 5.2) | |
| EMA 12-week abdominal pain/discomfort endpoint | Stool frequency (CSBM rate) | 47.6% versus 22.6% had a ≥1 CSBM rate increase for ≥6/12 weeks (NNT = 4) | |||
| NNT = 6.41 | Stool frequency (SBM rates) | 55.4% versus 27.8% had a ≥2 SBM rate increase for ≥6/12 weeks (NNT = 3.6) | |||
| EMA 26-week abdominal pain/discomfort endpoint | BSFS stool consistency | 80.3% versus 61.1% had a BSFS ≥3 (NNT = 5.21) | |||
| NNT = 5.68 | Severity of straining | 82.4% versus 70.6% had strain score ≤3 (NNT=8.48) | |||
| EMA 12-week IBS degree-of-relief endpoint | 26-week change from baseline: | 49.1% versus 31.3% had a ≥30% reduction in abdominal pain (p < 0.0001) (NNT = 5.6) | |||
| NNT = 4.39 | Worst abdominal pain | 48.1% versus 28.8% had a ≥30% reduction in abdominal discomfort (NNT = 5.2) | |||
| EMA 26-week IBS degree-of-relief endpoint | Abdominal discomfort | 42.4% versus 25.1% had a ≥30% reduction in abdominal bloating (NNT = 5.8) | |||
| NNT=4.93 | Abdominal bloating | 43.6% versus 18.6% had a ≥1 CSBM rate increase for ≥6/12 weeks (NNT = 4) | |||
| Stool frequency (CSBM rate) | 49.6% versus 21.6% had a ≥2 SBM rate increase for ≥6/12 weeks (NNT = 3.6) | ||||
| Stool frequency (SBM rates) | 81% versus 62.1% had a BSFS ≥3 (NNT = 5.29) | ||||
| BSFS stool consistency | 83.5% versus 71.6% had strain score ≤3 (NNT = 8.40) | ||||
| Severity of straining |
BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; FDA, US Food and Drug Administration; EMA, European Medicines Agency; IBS, irritable bowel syndrome; NNT, number needed to treat; SBM, spontaneous bowel movement.
≥30% improvement in the average daily worst abdominal pain score and increase by ≥ 1 CSBMs from baseline, occurring in the same week for at least 6 of the 12 weeks of therapy **(i) ≥ 30% improvement in abdominal pain scores, (ii) ≥ 3 CSBMs and an increase ≥ 1 CSBM from baseline, and (iii) a combined end point defined as a responder patient who met criteria for both (i) and (ii) in the same week
Chey and colleagues studied oral linaclotide 290 µg once daily versus placebo in a 26-week treatment period in 804 patients with IBS-C (mean age = 44 years, female patients = 90%, white = 78%). The primary endpoints for this study included the same FDA endpoint for 6 of the first 12 weeks of therapy, and the three responder definitions for greater than 9 of the first 12 weeks of therapy. Secondary endpoints included 12-week change from baseline assessments of worst abdominal pain, abdominal discomfort, abdominal bloating, stool frequency (CSBM and SBM weekly rates), BSFS stool consistency, and severity of straining. Efficacy parameters measured as primary and secondary endpoints were also evaluated over 26 weeks of treatment as additional endpoints. Additionally, 12- and 26-week changes from baseline endpoints were measured for abdominal fullness and abdominal cramping, abdominal and bowel symptom responders, IBS symptom severity, constipation severity, adequate relief of IBS-C symptoms, degree of relief of IBS symptoms, and treatment satisfaction.
The linaclotide 290 µg treated group demonstrated statistically significant improvement for all primary and secondary efficacy endpoints compared with the placebo group. A total of 33.7% of linaclotide treated patients compared with 13.9% of patients receiving placebo met the FDA endpoint over the first 12 weeks of the treatment period (p < 0.0001). The NNT for the FDA endpoint was 5. Additionally 48.9% of patients receiving linaclotide versus 34.5% on placebo had at least 30% improvement in abdominal pain, and 47.6% of patients receiving linaclotide versus 22% on placebo had an increase in weekly CSBM rate of one or more for at least 6 of 12 treatment weeks (p < 0.0001). A greater proportion of the linaclotide-treated group versus the placebo group also met the three responder definition endpoints, which required improvement for at least 9 of the first 12 weeks of the treatment period. The NNT for the three responder definition endpoints ranged from 5.2 to 10.3. Over the 26-week treatment period, 32.4% of patients receiving linaclotide and 13.2% of patients receiving placebo met the weekly responder requirements for the FDA endpoint for at least 13 weeks of the 26-week treatment period (p < 0.0001). The secondary endpoints of abdominal pain, abdominal bloating, and bowel symptoms (SBM and CSBM rates, BSFS scores, and straining scores), were also improved with linaclotide versus placebo (p < 0.001). Statistically significant differences from placebo were observed for responder and continuous endpoints over 26 weeks of treatment (Table 2).
Adverse effects
In Johnston and colleagues’ phase IIb dose-ranging trial, diarrhea of mild to moderate severity was the primary dose-dependent adverse effect noted. This was reported by 11.4%, 12.2%, 16.5%, and 18.0% of patients in the 75, 150, 300, and 600 µg linaclotide dose groups, respectively, compared with 1.2% in the placebo group. No cases of dehydration or electrolyte disturbances were noted, although one instance of fecal impaction occurred [Johnston et al. 2010]. In the phase III trials by Rao and colleagues and Chey and colleagues, diarrhea resulted in discontinuation of the study medication in 4.5–5.7% of the linaclotide-treated group and 0.2–0.3% of the placebo-treated group [Chey et al. 2012; Rao et al. 2012].
Linaclotide: IBS-C endpoints and meta-analysis
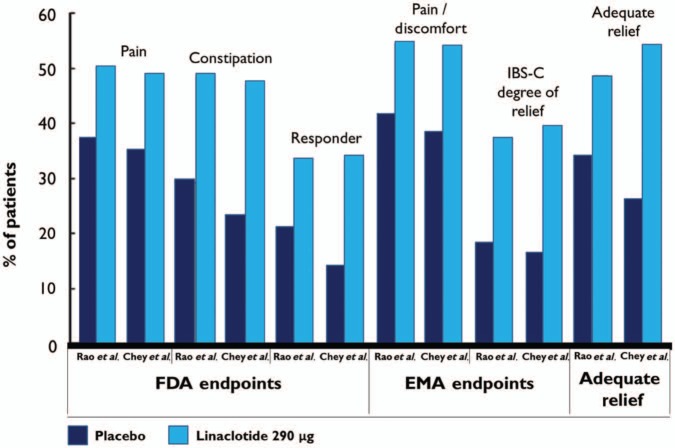
The complexity of IBS as an entity, primarily because it is a symptom-based condition without biomarkers that can be used for diagnostic or monitoring purposes, makes its evaluation and outcome assessments equally complex. MacDougall and colleagues studied the FDA endpoint in the two phase III clinical trials of linaclotide in IBS-C by Rao and colleagues and Chey and colleagues, and found that it demonstrated a sensitivity of 60.7%, a specificity of 93.5%, and an accuracy of 82%. Changing the number of weeks required to be a responder or the percentage improvement in abdominal pain criteria did not significantly change the accuracy of the FDA endpoint, affirming the excellent specificity and reasonable sensitivity of this metric [MacDougall et al. 2013]. The EMA studied the data from these two phase III trials based on EMA-recommended coprimary endpoints:12-week abdominal pain/discomfort responders (30% reduction in mean abdominal pain or discomfort score on an 11-point scale, with neither worsening from baseline for 6 weeks); and 12-week IBS degree of relief responders (symptoms ‘considerably’ or ‘completely’ relieved for 6 weeks). Linaclotide treatment significantly improved abdominal pain/discomfort and degree of relief of IBS-C symptoms compared with placebo over 12 and 26 weeks based on the EMA endpoints. For the EMA abdominal pain/discomfort responder coprimary endpoint, the NNT from Rao and colleagues was 7.69 at 12 weeks, and from Chey and colleagues it was 6.41 at 12 weeks and 5.68 at 26 weeks. For the EMA IBS degree-of-relief responder coprimary endpoint, the NNT from Rao and colleagues was 5.41 at 12 weeks, and from Chey and colleagues it was 4.39 at 12 weeks and 4.93 at 26 weeks [Quigley et al. 2013].
Corsetti and Tack thoughtfully editorialized the discussion of FDA and EMA endpoints, citing the major changes over the past two decades that have culminated in the 2012 FDA guidance that has influenced trial endpoints. There is particular discordance because 50% of patients with IBS-C treated with linaclotide are considered abdominal pain responders, and a similar proportion are CSBM responders. However, when the two endpoints are combined, only one-third of patients with IBS-C qualify as dual responders, suggesting that there are a number of patients treated with linaclotide who experience improvement in abdominal pain intensity without improvement in bowel frequency and vice versa (see Figure 2). They note that the FDA endpoint yields similar responder rates as the EMA overall degree-of-relief endpoint, while the binary endpoint of adequate relief generates higher response rates. It is not clear if the FDA endpoint, limited to abdominal pain and disordered defecation, is able to integrate the multiple aspects of IBS-C, making other clinical assessments essential, and emphasizing the need for further studies on overlapping symptom components in IBS treatment trials. Additionally, there is concern that the FDA-proposed focus on CSBM evaluation contributes to a loss of distinction between treatments for IBS-C and for chronic constipation [Corsetti and Tack, 2013].
Figure 2.
Responder rates to placebo and linaclotide 290 μg in studies by Rao and colleagues and Chey and colleagues in irritable bowel syndrome with constipation (IBS-C) according to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) guidelines, and using the adequate relief endpoint.
Camilleri and colleagues studied the adequate relief endpoint in relation to the FDA endpoint in the phase III trials by Rao and colleagues and Chey and colleagues [Camilleri et al. 2013]. Thresholds of clinical meaningfulness for the abdominal and bowel symptoms were estimated using receiver operating characteristic methods with adequate relief as an anchor on the pooled 12-week data from both trials, and a comparison between linaclotide and placebo was performed using these thresholds to define 12-week responder endpoints. The distribution of the agreement between weekly adequate relief and the FDA’s weekly responder criteria was also assessed. The NNT ranged from 5.1 to 6.4 for abdominal symptoms and from 2.4 to 3.7 for bowel symptoms. Responder rates for both adequate relief and the FDA criteria were greater in the linaclotide versus placebo groups. Responder endpoint analyses based on these thresholds showed that linaclotide treatment versus placebo resulted in a higher percentage of patients experiencing a clinically meaningful improvement in abdominal and bowel symptoms. An analysis of weekly responder rates revealed considerable agreement between adequate relief and the FDA responder endpoints.
Effects of linaclotide on IBS-C symptoms rated as severe
Rao and colleagues further evaluated the effect of linaclotide on abdominal symptoms in patients with IBS-C, and explored its efficacy in patients with IBS-C with severe symptoms. A post hoc analysis of pooled data from the Rao and Chey phase III trials was used to determine the prevalence of severe abdominal symptoms in patients with IBS-C and the effects of linaclotide on abdominal symptoms, global measures, and QOL in this subpopulation of patients [Rao et al. 2014]. The daily reports by patients for both studies were collected using an interactive voice response system (IVRS). All abdominal symptoms were measured using an 11-point numerical rating scale. (Example question: ‘How would you rate your abdominal discomfort over the last 24 hours? Enter a number from 0 to 10, where 0 represents no abdominal discomfort and 10 represents very severe abdominal discomfort’.) Patients in the intention-to-treat (ITT) population with a baseline score of at least 7 at baseline were included in the ‘severe symptom’ subpopulation for their respective symptom. Weekly IVRS assessments of global measures of improvement included adequate relief of IBS-C symptoms (yes/no) and degree of relief of IBS symptoms (seven-point scale; 1 = completely relieved, 4 = unchanged, 7 = as bad as I can imagine). At baseline and at week 1, the IBS-QOL, a self-administered QOL instrument yielding an overall score ranging from 0 (poor quality of life) to 100 (maximum quality of life), was assessed and was expectedly worse in the severe subpopulations compared with the ITT population (50–56 versus 61 on a 100-point scale).
The pooled ITT population consisted of 1602 patients, and the abdominal symptoms with the highest prevalence of severe symptoms were fullness (44%) and bloating (44%), followed by discomfort (32%), pain (23%), and cramping (22%). Significant overlap was seen for the severe bloating/fullness (90%) and severe pain/cramping (80%) populations. Efficacy data were reported for the pain, discomfort, and bloating subpopulations, as well as a fourth subpopulation of ITT patients with baseline severe symptoms for all three symptoms (see Figure 3). Across the severe subpopulations, 59–61% of patients treated with linaclotide reported adequate relief of IBS symptoms at week 12 compared with 28–32% of the placebo group (p < 0.0001), with NNTs from 3.0 to 3.7. Additionally, 73–75% of patients treated with linaclotide reported that their symptoms were ‘somewhat’, ‘considerably’, or ‘completely relieved’ at week 12 compared with 43–47% of the placebo-treated group (p < 0.0001), with NNTs from 3.2 to 3.8. Similarly, 70–77% of patients treated with linaclotide reported being ‘moderately’, ‘quite’, or ‘very satisfied’ with treatment at week 12 compared with 41–43% of placebo-treated patients (p < 0.0001), with NNTs from 2.9 to 3.6. The IBS-QOL analysis showed 62–68% of patients treated with linaclotide were IBS-QOL responders versus 45–47% of the placebo group (p < 0.01), with NNTs from 4.7 to 6.0. These post hoc analyses further demonstrate that linaclotide is effective in the treatment of patients with IBS-C with severe abdominal symptoms, resulting in greater symptom improvement from baseline than in the ITT population.
Figure 3.
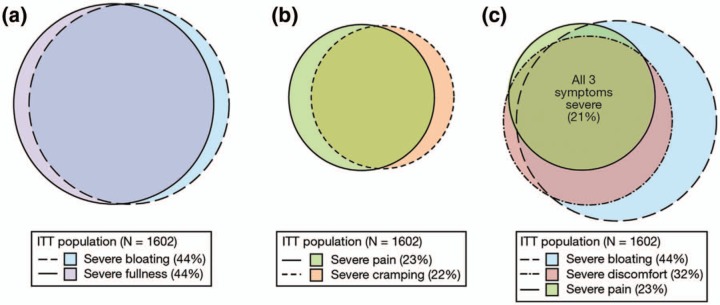
The effect of linaclotide on severe abdominal symptoms in constipation-predominant irritable bowel syndrome (IBS-C) intention-to-treat (ITT) subpopulations (mean baseline severity score ≥ 7.0). (a) The severe bloating (44%) and severe fullness (44%) subpopulations of the ITT population overlap by 90%. (b) The severe pain (23%) and severe cramping (22%) subpopulations represent over 80% of the ITT population overlap. (c) The severe bloating (44%), severe discomfort (32%), and severe pain (23%) subpopulations of the ITT population were chosen for analyses. The intersection of these three subpopulations represents 21% of the ITT population. From Rao et al. [2014].
Linaclotide has been studied in meta-analyses of IBS-C and chronic constipation (CC) [Videlock et al. 2013], as well as in IBS-C alone [Atluri et al. 2013]. The meta-analysis by Videlock and colleagues showed benefit of linaclotide over placebo in large, high-quality, and homogeneous trials, with improvement in bowel function, abdominal pain, and global outcomes in both IBS-C and CC. Although the Rome criteria used for recruitment offer a definite separation between these two disorders, the authors note that in daily clinical practice the approach to these disease entities is similar. The pooled effect estimate was larger in comparison with currently available pharmacologic therapies for constipation. The meta-analysis by Atluri and colleagues showed that there is moderate confidence that linaclotide is reasonably effective compared with placebo for improving typical symptoms of IBS-C. The linaclotide-treated group achieved subjective endpoints, such as an improvement in abdominal pain or discomfort, adequate relief response, global relief response, a clinically meaningful improvement in IBS QOL, and more conservative endpoints such as the FDA and EMA endpoints. The strengths of this analysis include use of the GRADE framework and incorporation of the QOL data.
Conclusion
IBS-C is a complex and challenging disorder with limited treatment options. The development of linaclotide provides a targeted approach that addresses the complexity of symptoms inherent to the syndrome. Linaclotide has demonstrated the ability to safely improve IBS-C abdominal pain severity, bowel movement quality, and bowel movement frequency, as well as key symptoms of abdominal fullness, bloating, and discomfort, with associated improvements in QOL. Based on guidance from the US FDA and the EMA, linaclotide meets the recommended endpoints with a NNT ranging from 4.39 to 7.9. It is safe and effective, with diarrhea reported as the most common adverse effect that leads to discontinuation of the medication in approximately 5% of patients. Based on recent clinical evidence, linaclotide should be considered for patients with IBS-C because it improves abdominal pain and bowel symptoms.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Rao has participated in a phase III clinical trial of linaclotide and has served as an advisory board member for Ironwood Pharmaceuticals and Forest Research Labs.
Contributor Information
Siegfried W.B. Yu, Division of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, Augusta, GA, USA
Satish S.C. Rao, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BBR2540, 1120 15th Street, Augusta, GA 30912, USA
References
- Abdulmajeed A., Rabab M., Sliem H., Hebatallah N. (2011) Pattern of irritable bowel syndrome and its impact on quality of life in primary health care center attendees, Suez governorate, Egypt. Pan Afr Med J 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen V., Camilleri M., Busciglio I., Grudell A., Burton D., McKinzie S., et al. (2007) Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133: 761–768 [DOI] [PubMed] [Google Scholar]
- Atluri D., Chandar A., Bharucha A., Falck-Ytter Y. (2013) Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysis. Neurogastroenterol Motil 26: 499–509 [DOI] [PubMed] [Google Scholar]
- Bharucha A., Linden D. (2010) Linaclotide – a secretagogue and antihyperalgesic agent – what next? Neurogastroenterol Motil 22: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S., Martinez V., Lao L., Larsson H., Mayer E. (2009) Involvement of vasopressin 3 receptors in chronic psychological stress-induced visceral hyperalgesia in rats. Am J Physiol Gastrointest Liver Physiol 296: G302–G309 [DOI] [PubMed] [Google Scholar]
- Brandt L., Chey W., Foxx-Orenstein A., Schiller L., Schoenfeld P., Spiegel B., et al. (2009) An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 104(Suppl. 1): S1–S35 [DOI] [PubMed] [Google Scholar]
- Brierley S. (2012) Guanylate cyclase-C receptor activation: unexpected biology. Curr Opin Pharmacol 12: 632–640 [DOI] [PubMed] [Google Scholar]
- Bryant A., Busby R., Bartolini W., Cordero E., Hannig G., Kessler M., et al. (2010) Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci 86: 760–765 [DOI] [PubMed] [Google Scholar]
- Busby R., Bryant A., Bartolini W., Cordero E., Hannig G., Kessler M., et al. (2010) Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 649: 328–335 [DOI] [PubMed] [Google Scholar]
- Busby R., Kessler M., Bartolini W., Bryant A., Hannig G., Higgins C., et al. (2013) Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther 344: 196–206 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Anthony L., Lavins B., MacDougall J., Shiff S., Jia X., et al. (2013) Assessing abdominal and bowel symptoms using adequate relief based thresholds: results from 2 phase 3 trials of linaclotide in patients with irritable bowel syndrome with constipation. Gastroenterology 144(5 Suppl. 1): S-728-S-729 [Google Scholar]
- Carrithers S., Ott C., Hill M., Johnson B., Cai W., Chang J., et al. (2004) Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int 65: 40–53 [DOI] [PubMed] [Google Scholar]
- Cash B., Sullivan S., Barghout V. (2005) Total costs of IBS: employer and managed care perspective. Am J Manag Care 11(1 Suppl.): S7-S16 [PubMed] [Google Scholar]
- Castro J., Harrington A., Hughes P., Martin C., Ge P., Shea C., et al. (2013) Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-c and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145: 1334–1346 [DOI] [PubMed] [Google Scholar]
- Chang F., Lu C., Chen T. (2010) The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil 16: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W., Lembo A., Lavins B., Shiff S., Kurtz C., Currie M., et al. (2012) Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 107: 1702–1712 [DOI] [PubMed] [Google Scholar]
- Corsetti M., Tack J. (2013) FDA and EMA end points: which outcome end points should we use in clinical trials in patients with irritable bowel syndrome? Neurogastroenterol Motil 25: 453–457 [DOI] [PubMed] [Google Scholar]
- Currie M., Kurtz C., Mahajan-Miklos S., Busby R., Fretzen A., Geis S., et al. (2005) Effects of a single dose administration of MD-1100 on safety, tolerability, exposure, and stool consistency in healthy subjects. Am J Gastoenterol 100: S328 [Google Scholar]
- Drossman D., Camilleri M., Mayer E., Whitehead W. (2002) AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131 [DOI] [PubMed] [Google Scholar]
- Eutamene H., Bradesi S., Larauche M., Theodorou V., Beaufrand C., Ohning G., et al. (2010) Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 22: 312-e384 [DOI] [PubMed] [Google Scholar]
- Feng B., Kiyatkin M., La J., Ge P., Solinga R., Silos-Santiago I., et al. (2013) Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci 33: 9831–9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., La J., Schwartz E., Gebhart G. (2012) Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: G1085-G1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Fan J., Kemmerer E., Evans S., Li Y., Wiley J. (2009) Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 58: 202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J., Kurtz C., Macdougall J., Lavins B., Currie M., Fitch D., et al. (2010) Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 139: 1877–1886 e1872. [DOI] [PubMed] [Google Scholar]
- Krogsgaard L., Engsbro A., Bytzer P. (2013) The epidemiology of irritable bowel syndrome in Denmark. A population-based survey in adults </=50 years of age. Scandinavian J Gastroenterol 48: 523–529 [DOI] [PubMed] [Google Scholar]
- Kurtz C., Fitch D., Busby R., et al. (2006) Effects of multidose administration of MD-1100 on safety, tolerability, exposure, and pharmacodynamics in healthy subjects. Gastroenterology 130: A26 [Google Scholar]
- Lacy B., Levenick J., Crowell M. (2012) Chronic constipation: new diagnostic and treatment approaches. Therap Adv Gastroenterol 5: 233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Valentino M., Marszalowicz G., Magee M., Li P., Snook A., et al. (2010) Bacterial heat-stable enterotoxins: translation of pathogenic peptides into novel targeted diagnostics and therapeutics. Toxins (Basel) 2: 2028–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth G., Thompson W., Chey W., Houghton L., Mearin F., Spiller R. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491 [DOI] [PubMed] [Google Scholar]
- MacDougall J., Johnston J., Lavins B., Nelson L., Williams V., Carson R., et al. (2013) An evaluation of the FDA responder endpoint for IBS-C clinical trials: analysis of data from linaclotide phase 3 clinical trials. Neurogastroenterol Motil 25: 481–486 [DOI] [PubMed] [Google Scholar]
- Mayer E., Tillisch K. (2011) The brain–gut axis in abdominal pain syndromes. Annu Rev Med 62: 381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke E., Ladep N., Adah S., Bupwatda P., Agaba E., Malu A. (2009) Prevalence of irritable bowel syndrome: a community survey in an African population. Ann Afr Med 8: 177–180 [DOI] [PubMed] [Google Scholar]
- Pare P., Gray J., Lam S., Balshaw R., Khorasheh S., Barbeau M., et al. (2006) Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 28: 1726–1735; discussion 1710–1721. [DOI] [PubMed] [Google Scholar]
- Potter L. (2011) Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther 130: 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley E., Tack J., Chey W., Rao S., Fortea J., Falques M., et al. (2013) Randomised clinical trials: linaclotide phase 3 studies in IBS-C – a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 37: 49–61 [DOI] [PubMed] [Google Scholar]
- Rao S., Lembo A., Shiff S., Lavins B., Currie M., Jia X., et al. (2012) A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 107: 1714–1724; quiz p 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Quigley E., Shiff S., Lavins B., Kurtz C., MacDougall J., et al. (2014) Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol 12: 616–623 [DOI] [PubMed] [Google Scholar]
- Ringel Y., Maharshak N. (2013) Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 305: G529-G541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fandino O., Hernandez-Ruiz J., Lopez-Vidal Y., Charua L., Bandeh-Moghaddam H., Minzoni A., et al. (2013) Intestinal recruiting and activation profiles in peripheral blood mononuclear cells in response to pathogen-associated molecular patterns stimulation in patients with IBS. Neurogastroenterol Motil 25: 872-e699 [DOI] [PubMed] [Google Scholar]
- Saito Y., Schoenfeld P., Locke G., 3rd (2002) The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 97: 1910–1915 [DOI] [PubMed] [Google Scholar]
- Schwetz I., Bradesi S., McRoberts J., Sablad M., Miller J., Zhou H., et al. (2004) Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol 286: G683-G691 [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I., Hannig G., Eutamene H., Ustinova E., Bernier S., Ge P., et al. (2013) Gastrointestinal pain: unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 154: 1820–1830 [DOI] [PubMed] [Google Scholar]
- Stasi C., Bellini M., Costa F., Mumolo M., Ricchiuti A., Grosso M., et al. (2013) Neuroendocrine markers and psychological features in patients with irritable bowel syndrome. Int J Colorectal Dis 28: 1203–1208 [DOI] [PubMed] [Google Scholar]
- Vaandrager A., Smolenski A., Tilly B., Houtsmuller A., Ehlert E., Bot A., et al. (1998) Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl- channel activation. Proc Natl Acad Sci U S A 95: 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videlock E., Cheng V., Cremonini F. (2013) Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol 11:1084–1092 e1083; quiz e1068. [DOI] [PubMed] [Google Scholar]
- Whitaker T., Witte D., Scott M., Cohen M. (1997) Uroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestine. Gastroenterology 113: 1000–1006 [DOI] [PubMed] [Google Scholar]