Abstract
Background:
Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatments for major depressive disorders (MDD). It has been reported, however, that 30–40% of patients with MDD who received SSRIs failed to respond to treatment. Use of lithium (Li) to augment SSRIs seems to be the most common strategy in such cases. It was recently demonstrated that atypical antipsychotics are effective augmentation agents in MDD. Here, we present a randomized controlled study that compared augmentation with Li, olanzapine (OLA) or aripiprazole (ARI) in paroxetine-refractory patients with MDD.
Methods:
Participants were 30 patients who met Diagnostic and Statistical Manual of Mental Disorders IV criteria for MDD and refractory to paroxetine treatment. Treatment with Li, OLA or ARI was added to paroxetine in a randomized protocol for 4 weeks. We defined the patients whose scores on the Hamilton Rating Scale for Depression decreased 50% or more as responders.
Results:
Two patients dropped out because of adverse effects. Response rates to Li, OLA or ARI augmentation were 4/10 (40%), 3/10 (30%) and 4/10 (40%), respectively. In addition, Li, OLA and ARI did not influence plasma paroxetine concentrations.
Conclusions:
We concluded that OLA or ARI could be used as alternatives to Li as options for patients who do not respond to paroxetine treatment.
Keywords: aripiprazole, augmentation, lithium, major depressive disorder olanzapine, paroxetine
Introduction
Major depressive disorder (MDD) is one of the most prevalent mental disorders worldwide. Newer generation antidepressants are available for the treatment of MDD. According to the US National Institute of Mental Health (NIMH) Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, approximately 50% of patients fail to response to treatment with a first-line antidepressant. The response rate decreases with second- or third-line treatments [Rush et al. 2006]. Recently, adjunctive use of atypical antipsychotic drugs has increased. Lithium (Li) augmentation is the strategy with the most robust evidence for treatment of refractory depression [Bschor and Bauer, 2006]. A meta-analysis by Nelson and Papakostas (2009) demonstrated that the odds ratio for response with antipsychotic augmentation versus placebo was 1.65. Our own recent study showed that adding a low dose of atypical antipsychotic drug to ongoing treatment with a selective serotonin inhibitor (SSRI) or serotonin noradrenaline reuptake inhibitor (SNRI) brought a rapid improvement within 4 weeks [Yoshimura et al. 2010]. In addition, it significantly increased serum levels of brain-derived neurotrophic factor (BDNF). However, the ongoing first-line antidepressants studied in previous reports varied [Yoshimura et al. 2010]. Therefore, it remains uncertain whether atypical antipsychotic drugs improve symptoms in patients who do not respond to treatment specifically with paroxetine. Moreover, it is still not known which atypical drug produces the best response when added to ongoing paroxetine treatment.
The aim of the present study was to compare the impact of adding Li, olanzapine (OLA) and aripiprazole (ARI) to paroxetine in patients with MDD. We also measured serum levels of BDNF and plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG), a major metabolite of noradrenaline, as well as homovanillic acid (HVA), a major metabolite of dopamine, to elucidate their mechanisms.
Subjects and methods
The study initially enrolled 89 patients who met the Diagnostic and Statistical Manual of Mental Disorders IV Text Revision (DSM-IV-TR) criteria for MDD. There were 39 males and 50 females, ranging in age from 29 to 71 [mean ± standard deviation (SD), 46±14) years. All patients were physically healthy and free of current alcohol or drug abuse, comorbid anxiety and personality disorders. A total of 48 of 89 patients responded to treatment with paroxetine within 8 weeks. We defined ‘responded’ as a 50% or more decrease in score on the 17 items of the Hamilton Rating Scale for Depression (HAMD-17). We defined remission as HAMD-17 scores below 7. The remaining 30 patients were considered ‘nonresponders’ to paroxetine treatment. These patients were randomly administered Li, ARI or OLA in addition to their ongoing paroxetine treatment. The study protocol was approved by the Ethics Committee of the University of Occupational and Environmental Health [Kitakyushu, Japan). All patients signed informed consent forms after having been informed of the study’s purpose.
Dosages of antidepressants and atypical antipsychotics varied among patients and were not fixed for ethical reasons. However, doses of antidepressants were not altered during the comedication period. Benzodiazepines were the only hypnotics permitted and their dosages were kept constant throughout the study period. Clinical improvement of patients was evaluated using the HAMD-17 before the start of the study, and weekly after administration of Li or other atypical antipsychotic drugs had begun. Patients whose HAMD-17 scores decreased by ≥50% within 4 weeks after adding the atypical antipsychotic drug were defined as responders; those whose HAMD-17 scores decreased to 7 or less were defined as remissions; the remainder were defined as nonresponders.
Serum BDNF assay
All blood samples were taken at 7 a.m., before breakfast and at least 12 hours after the last dose of medication. Samples were drawn before the start of the study (T0), and then at four (T4) and eight weeks (T8) after treatment with paroxetine, sertraline or fluvoxamine. Venous blood (15 ml) was drawn with the patient lying in a supine position after resting overnight. Serum samples were quickly separated in a centrifuge (2000g, 10 min, 4 °C) and stored at −80 °C until assay.
Serum BDNF levels were measured using a BDNF Emax Immunoassay Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, 96-well microplates were coated with anti-BDNF monoclonal antibody and incubated at 4 °C for 18 hours. The plates were incubated in a blocking buffer for 1 h at room temperature. The samples were then diluted with assay buffer 100× and BDNF standards were kept at room temperature under conditions of horizontal shaking for 2 h, followed by washing with the appropriate buffer. The plates were incubated with antihuman BDNF polyclonal antibody at room temperature for 2 h, and then washed with the washing buffer. They were then incubated with anti-immunoglobulin Y antibody conjugated to horseradish peroxidase for 1 h at room temperature, and incubated in peroxidase substrate and tetramethylbenzidine solution to induce a color reaction. The reaction was stopped with 1 mol/l hydrochloric acid. The absorbance at 450 nm was measured with an Emax automated microplate reader. Measurements were performed in duplicate. The standard curve was linear from 5 pg/ml to 5000 pg/ml, and the detection limit was 10 pg/ml. Cross-reactivity to related neurotrophins (NT-3, NT-4, NGF) was less than 3%. Intra- and inter-assay coefficients of variation were about 5% and 7%, respectively. The recovery rate of the exogenous added BDNF in the measured plasma samples was more than 95%.
Plasma assay of MHPG and HVA
Plasma MHPG levels were analyzed by high performance liquid chromatography with electrochemical detection (HPLC-ECD), according to a previously described method [Minegishi and Ishizaki, 1984]. In brief, the plasma was separated by centrifugation at 600g at 4 °C. Extraction was performed under a vacuum using Bond-Elut columns (Varian, Palo Alto, CA, USA) prepacked with 100 mg of C18-bonded silica (40 μm) in a 1 ml capacity disposable syringe. The columns, which were inserted into a vacuum chamber connected to an aspirator, were prepared by washing with 1 ml methanol followed by 1 ml of water. After the addition of 50 μl of a solution of vanillyl alcohol [Minegishi and Ishizaki, 1984] (internal standard equivalent to 5 ng/ml) to 1 ml of plasma, the samples were passed through the columns; 0.75 ml water was then used to rinse off both residual samples and easily eluted hydrophilic compounds. The adsorbed materials were eluted with 200 μl of methanol to a 0.1M phosphate buffer (pH 4.8) mixture (40:60, v/v). A 20 μl portion of this solution was injected into the HPLC. The plasma HVA levels were also analyzed by HPLC-ECD according to the method detailed by Yeung and colleagues [Yeung et al. 1996]. In short, each cyano-bonded solid-phase extraction cartridge was preconditioned with methanol and then glass-distilled water. To each cartridge were added 0.3 ml of plasma sample or standard, and 0.1 ml of working internal standard solution (5 ng of 5-hydroxyindolecarboxylic acid in 0.01M KH2PO4, pH 7.2). The samples were allowed to pass slowly through the cartridge under a mild vacuum (15 mmHg), and the filtrate was collected. The cartridge was then washed with 0.2 ml of distilled water. The filtrate portions were combined and deproteinized with 1 ml of acetonitrile. After mixing by vortex and centrifugation (1760g, 48 °C for 10 min), an aliquot (5 ml) of the supernatant was injected into the HPLC.
Plasma paroxetine assay
Plasma paroxetine was also analyzed by HPLC according to a previously described method [Gupta, 1994]. In short, a 0.5 ml aliquot of the sample was mixed with 100 ml of the working standard (dibucaine) and 0.5 ml of acetonitrile in a glass tube. After centrifugation at 1500g for 3 min, the supernatant was applied to a 1 ml Bond-Elut C18 extraction column which had been previously activated by washing serially, once with 1M HCl, twice with methanol and once with water. The sample was passed slowly through the column by mild suction. The column was then washed serially, twice with water and once with acetonitrile, making sure that each column was grained completely after every wash. An aliquot of 0.25 ml of methanol containing 2.5 ml/100 ml of 35% perchloric acid was applied to each column. The liquid was allowed to pass through the column. A 7 ml aliquot of the elute was injected into the HPLC.
Statistical analysis
The Bonferroni method was used for post hoc analysis; the three groups were compared using repeated measures analysis of variance (ANOVA) with Bonferroni correction. Pearson’s correlation coefficient was used for comparisons between two groups. The level of significance for all analyses was set at p < 0.05.
Results
The response rate with paroxetine within 8 weeks was 59/89 (66%). The remaining 30 patients were categorized as nonresponders. They were randomly assigned to receive Li, ARI or OLA in addition to paroxetine for 4 weeks. Demographic data for the three groups are shown in Table 1. Patients receiving Li, OLA and ARI all had decreased HAMD-17 scores at week 2 and week 4. There were no significant differences between the three groups regarding decrease in HAMD-17 scores (Figure 1). Two patients, one in the Li group and one in the ARI group, dropped out due to finger tremor and akathisia, respectively. Response rates at week 4 for the Li, OLA and ARI augmentation groups were 4/10 (40%), 3/10 (30%) and 4/10 (40%), respectively. In addition, rate of remission at week 4 for Li, OLA and ARI augmentation were 2/10 (20%), 1/10(10%), and 2/10 (20%), respectively (Figure 2). Augmenting paroxetine with Li, OLA and ARI for 4 weeks did not change serum BDNF levels (Figure 3). Serum BDNF levels for responders and nonresponders before and 4 weeks after beginning augmentation are presented in Table 2. Nor did these three drugs change plasma levels of MHPG and HVA when added to paroxetine (Figures 4 and 5). Li, OLA and ARI did not change plasma paroxetine concentrations (Figure 6).
Table 1.
Demographics of each augmentation group.
| Li | OLA | ARI | |
|---|---|---|---|
| # | 10 | 10 | 10 |
| Drop-out | 1 | 0 | 1 |
| Sex (male/female) | 4/6 | 5/5 | 3/7 |
| Age (year) | 39±8 | 42±7 | 40±10 |
| HAMD17 | 22±7 | 24±6 | 22±4 |
| Dose (mg/day) | 458±103 | 7±5 | 9±6 |
| Side effects | Tremor (2), | Drowsiness (1) | Akathisia (2) |
| Response | 4/10 (40%) | 3/10 (30%) | 4/10 (40%) |
| Remission | 2/10 (20%) | 1/10 (10%) | 2/10 (20%) |
ARI, aripiprazole; HAMD17, Hamilton Rating Scale for Depression 17-point scale; Li, lithium; OLA, olanzapine.
Figure 1.
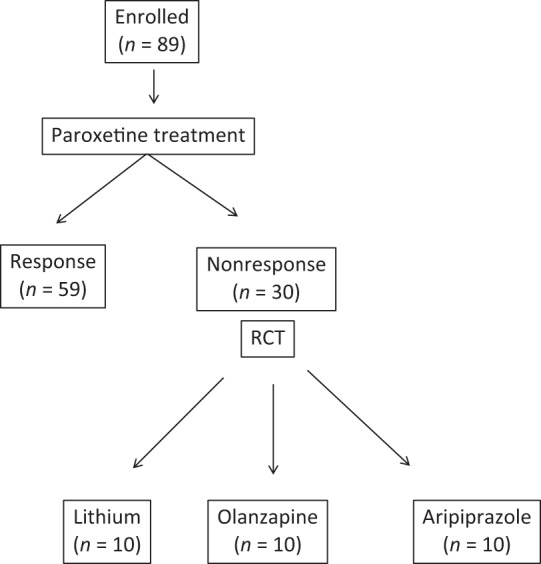
Protocol in the present study.
RCT, Randomised Control Trial.
Figure 2.
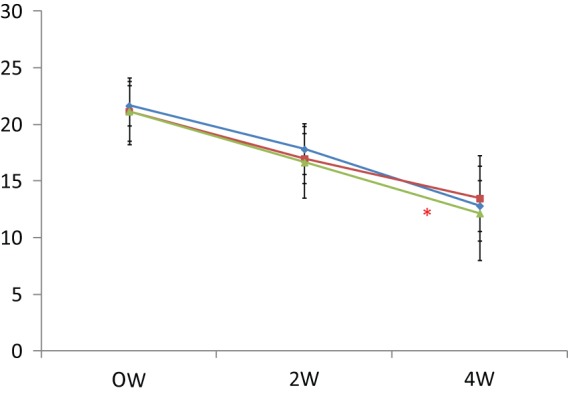
Changes in HAMD-17 scores after augmentation.
ARI, aripiprazole; HAMD-17, Hamilton Rating Scale for Depression 17 point scale; Li, lithium, OLA, olanzapine.
*p < 0.001
Blue line: ARI; Red line: Li; Green line: OLA.
Vertical bar shows standard deviation.
Figure 3.
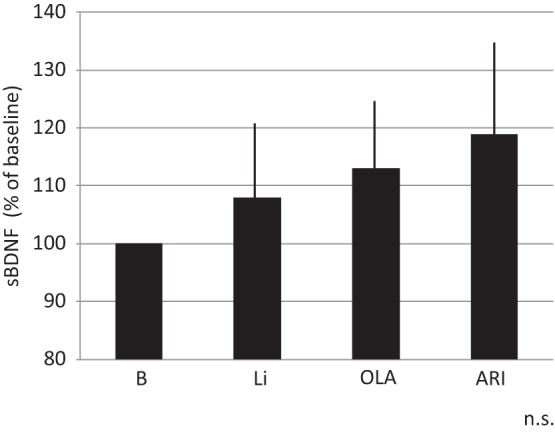
Changes in serum BDNF levels 4 weeks after augmentation.
ARI, aripiprazole; BDNF, brain-derived neurotrophic factor; Li, lithium; OLA, olanzapine; n.s., not significant.
Vertical bar shows standard deviation.
Table 2.
Serum BDNF levels and plasma levels of MHPG and HVA before and after augmentations between responders and nonresponders.
| Serum BDNF | ||
|---|---|---|
| Responders | Nonresponders | |
| Before | 7.8±5.3 ng/ml | 8.4±6.7 ng/ml |
| 4 weeks | 11.9±6.4 ng/ml* | 9.2±7.1 ng/ml |
p = 0.041
Figure 4.
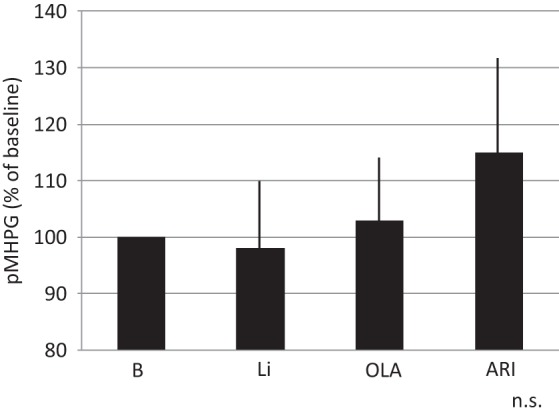
Changes in plasma MHPG levels 4 weeks after augmentation.
ARI, aripiprazole; Li, lithium; MHPG, 3-methoxy-4-hydroxyphenylglycol; OLA, olanzapine; n.s., not significant.
Vertical bar shows standard deviation.
Figure 5.
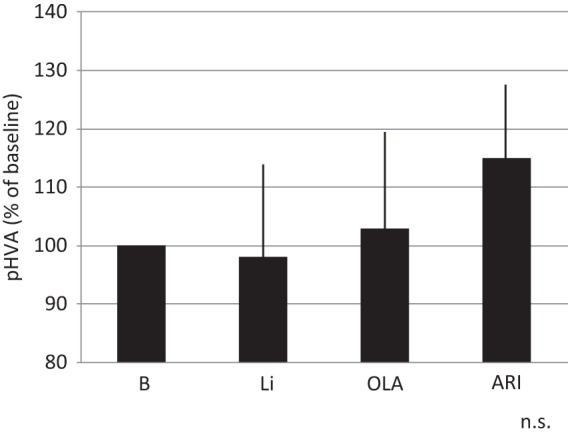
Changes in plasma HVA levels 4 weeks after augmentation.
ARI, aripiprazole; HVA, homovanillic acid; Li, lithium; OLA, olanzapine ; n.s., not significant.
Blue line: ARI; Red line: Li, Green line: OLA’
Vertical bar shows standard deviation.
Figure 6.
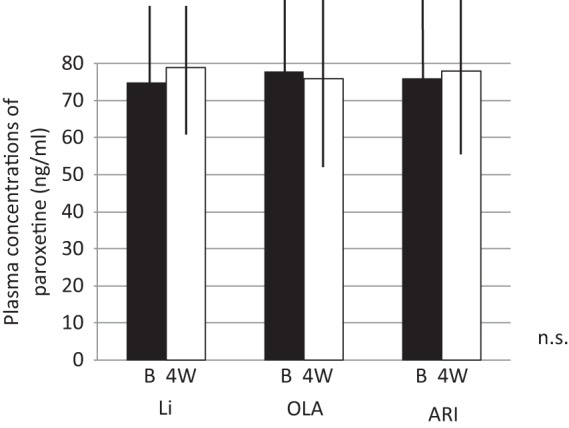
Changes in plasma paroxetine levels before and after augmentation.
ARI, aripiprazole; Li, lithium; OLA, olanzapine; n.s., not significant.
Vertical bar shows standard deviation.
| Plasma MHPG | ||
|---|---|---|
| Responders | Nonresponders | |
| Before | 4.1±3.6 ng/ml | 4.9±2.7 ng/ml |
| 4 weeks | 4.2±3.0 ng/ml | 4.8±3.4 ng/ml |
n.s.
| Plasma HVA | ||
|---|---|---|
| Responders | Nonresponders | |
| Before | 5.0±3.8 ng/ml | 4.3±3.9 ng/ml |
| 4 weeks | 4.3±3.7 ng/ml | 4.8±3.6 ng/ml |
n.s.
BDNF, brain-derived neurotrophic factor; HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; n.s., not significant.
Discussion
The most important finding in the present study is that augmentation of paroxetine with Li, OLA or ARI were equally effective in patients with MDD. Generally, Li is the most popular augmentation strategy with antidepressants [Bschor and Bauer, 2006; Fleurence et al. 2009]; it is effective and well tolerated by refractory patients. Therapeutic drug monitoring should be performed and other drugs, such as nonsteroidal anti-inflammatory agents, should be used cautiously because of the possibility of raising plasma Li levels. While there is growing evidence of the efficacy of adding atypical antipsychotic drugs to antipsychotic treatments, few studies have compared the efficacy of Li with that of OLA or ARI in addition to paroxetine.
This study first reviewed current literature on Li augmentation in patients who did not respond to SSRIs. A significant proportion of depressive patients do not respond to a first antidepressant treatment, independent of the class of drugs used. According to a review [Zullino and Bauman, 2001], several case reports during the past 10 years have looked at open and controlled studies on the use of Li augmentation in patients who were nonresponders to SSRIs. The main underlying hypothesis is a synergistic effect between SSRIs and Li, as both act on serotonergic neurotransmission. Most studies show substantial effects after 1–2 weeks and some after 6 weeks. There is as yet no other clear evidence for a pharmacokinetic interaction between Li and SSRIs with pharmacodynamic consequences.
Our findings suggest that Li augmentation in depressive patients who do not respond to SSRIs may be an efficacious and generally well tolerated treatment, with a response rate of at least 50% after a period of 1–2 weeks. Berman and colleagues reported that significant improvement in depressive symptoms as assessed by decreases in the Montgomery–Asberg Depression Rating Scale total score were greater with adjunctive ARI than with placebo; remission rates were also greater for adjunctive ARI than for placebo [Berman et al. 2007]. Completion rates with adjunctive ARI and placebo were high, and discontinuations due to adverse events were low. Boku and colleagues reported that in 11 patients who received OLA with milnacipran, HAMD and Clinical Global Impression scores improved significantly from baseline to endpoint [Boku et al. 2011]. This improvement occurred in week 1. At endpoint, seven of the 11 (64%) were responders on HAMD. There were no severe adverse effects. OLA augmentation of milnacipran for stage 2 treatment of refractory depression might be effective and well tolerated. The results in the present study were basically in accordance with previous reports [Berman et al. 2007; Boku et al. 2011].
Serum BDNF levels and plasma levels of MHPG and HVA were not altered in the period between the start of the study and 4 weeks after augmentation. These results indicate that Li, OLA and ARI had little influence on blood levels of BDNF, MHPG and HVA. In other words, the mechanisms of combined treatment with paroxetine and Li, OLA or ARI for improvement of refractory depression were intact with synthesis and/or secretion of BDNF, noradrenaline and dopamine. We recently reported that the plasma BDNF levels in responders (those showing a decline in HAMD scores of 50% or more) were significantly increased 4 weeks after administration of each atypical antipsychotic drug, while the levels in nonresponders were not changed. Furthermore, there was a significant correlation between the changes in HAMD scores and the changes in plasma BDNF levels. These results suggest that adding an atypical antipsychotic drug to ongoing treatment with an antidepressant or mood stabilizer is useful and well tolerated for refractory depressed patients, although the efficacy of atypical antipsychotics as an adjuvant might involve an increase in plasma BDNF levels. Thus, there is a discrepancy between the results of the present study and those of our previous study [Yoshimura et al. 2010].
In the previous study, the increase of plasma BDNF levels was observed only in responders to atypical antipsychotic drugs augmentation, patients were diagnosed with either MDD or bipolar I disorder, and ongoing drugs were various antidepressants and mood stabilizers. The increase in serum BDNF levels was found only in responders to Li, OLA and ARI used in addition to paroxetine. We recently reported two patients with psychotic depression who were successfully treated with Li in addition to ongoing paroxetine treatment. In both cases, plasma BDNF levels increased about two-fold after Li augmentation compared with paroxetine treatment alone. Plasma paroxetine levels did not change after the addition of Li. These results suggest that the increases in plasma BDNF levels reflect recovery from depressive symptoms in psychotic depression [Yoshimura et al. 2007]. The increase in serum BDNF levels was found in responders after 4 weeks of augmentation with Li, OLA or ARI. We could not, however, compare the difference in BDNF levels before and 4 weeks after each drug augmentation due to small samples of each group. These findings should be taken into account; the results in the present study indicate that paroxetine plus Li, OLA and ARI increases serum BDNF levels in responders to the treatment.
Li, OLA and ARI did not change plasma levels of MHPG and HVA. We previously reported that OLA and ARI monotherapy increased plasma MHPG levels and decreased plasma HVA levels in schizophrenia patients. The results suggest that OLA and ARI might enhance noradrenergic activity in schizophrenic patients. We could not use ANCOVA for the data analysis throughout the study because each level of covariates was parallel.
To the best of our knowledge, this is the first report to examine the effects of augmentation of paroxetine with Li, OLA and ARI on plasma catecholamine metabolites. It indicates that the mechanism of alleviating depressive symptoms seems to be independent of catecholamine dynamics.
Li, OLA and ARI did not influence plasma paroxetine levels. Paroxetine is mainly metabolized by cytochrome (cyp) 2D6 and 3A4 [Cozza et al. 2003]. Li, OLA and ARI do not inhibit cyp 2D6 or cyp 3A4 [Cozza et al. 2003]. Therefore, it is reasonable that these three drugs did not affect plasma paroxetine levels.
In conclusion, augmentation of paroxetine with Li, OLA and ARI were equally effective for treatment-refractory depression. The mechanisms by which this occurs might be involved in BDNF.
Footnotes
Funding and conflict of interests: Professor Jun Nakamura has received grant support from Astellas Pharma, Janssen Pharmaceutical, Eli Lily, Glaxo Smith Kline, Pfizer, Dainippon Sumitomo Pharma, Otsuka Pharmaceutical, and Chugai Pharmaceutical.
Contributor Information
Reiji Yoshimura, Department of Psychiatry, University of Occupational and Environmental Health, 1-1Iseigaoka, Yahatanishi-ku, Kitakyushu, Fukuoka 80785555, Japan.
Hikaru Hori, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
Wakako Umene-Nakano, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
Atsuko Ikenouchi-Sugita, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
Asuka Katsuki, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
Kiyokazu Atake, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
Jun Nakamura, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
References
- Berman R., Marcus R., Swanink R., McQuade R., Carson W., Corey-Lisle P., et al. (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind placebo-controlled study. J Clin Psychiatry 68: 843–853 [DOI] [PubMed] [Google Scholar]
- Boku S., Inoue T., Honma H., Honma H., Nakagawa S., Koyama T. (2011) Olanzapine augmentation of milnacipran for stage 2 treatment-resistant major depression: an open study. Hum Psychopharmacol 26: 237–241 [DOI] [PubMed] [Google Scholar]
- Bschor T., Bauer M. (2006) Efficacy and mechanisms of action of lithium augmentation in refractory major depression. Curr Pham Des 12: 2985–2992 [DOI] [PubMed] [Google Scholar]
- Cozza K., Armstrong S., Oesterheld J. (2003) Drug Interaction Principle for Medical Practice. Arlington, VA: American Psychiatric Publishing [Google Scholar]
- Fleurence R., Williamson R., Jing Y., Kim E., Tran Q., Pikalov A., et al. (2009) A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull 42: 57–90 [PubMed] [Google Scholar]
- Gupta R. (1994) Column liquid chromatographic determination of paroxetine in human serum using solid-phase extraction. J Chromatgr B Biomed Appl 661: 362–365 [DOI] [PubMed] [Google Scholar]
- Minegishi A., Ishizaki T. (1984) Determination of free 3-methoxy-4-hydroxyphenylglycol with several other monoamine metabolites in plasma by high-performance liquid chromatography with amperometric detection. J Chromatgr 311: 51–57 [DOI] [PubMed] [Google Scholar]
- Nelson J., Papakostas G. (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166: 980–991 [DOI] [PubMed] [Google Scholar]
- Rush A., Trivedi M., Winsniewski S., Nierenberg A., Stewart J., Warden D., et al.(2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917 [DOI] [PubMed] [Google Scholar]
- Yeung P., Buckley S., Pedder S., Dingemanse J. (1996) Determination of 3,4-dihydroxyphenylacetic acid and 5-hydroxyindoleacetic acid in human plasma by a simple and rapid high-performance liquid chromatography assay. J Pharm Sci 85: 451–453 [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Ikenouchi-Sugita A., Hori H., Umene-Nakano W., Katsuki A., Hayashi K., et al. (2010) Adding a low dose atypical antipsychotic drug to an antidepressant induced a rapid increase of plasma brain-derived neurotrophic factor levels in patients with treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 34: 308–312 [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Tsuji K., Ueda N., Nakamura J. (2007) Increase of plasma brain-derived neurotrophic factor levels in two psychotic depressed patients responding to lithium addition to paroxetine treatment. Neuropsychiatr Dis Treat 3: 683–685 [PMC free article] [PubMed] [Google Scholar]
- Zullino D., Baumann P. (2001) Lithium augmentation in depressive patients not responding to selective serotonin reuptake inhibitors. Pharmacopsychiatry 34: 119–127 [DOI] [PubMed] [Google Scholar]


