Abstract
Otilonium bromide (OB) is a spasmolytic compound of the family of quaternary ammonium derivatives and has been successfully used in the treatment of patients with irritable bowel syndrome (IBS) due to its specific pharmacodynamic effects on motility patterns in the human colon and the contractility of colonic smooth muscle cells. This article examines how. OB inhibits the main patterns of human sigmoid motility in vitro, which are spontaneous rhythmic phasic contractions, smooth muscle tone, contractions induced by stimulation of excitatory motor neurons and contractions induced by direct effect of excitatory neurotransmitters. It does this mainly by blocking calcium influx through L-type calcium channels and interfering with mobilization of cellular calcium required for smooth muscle contraction, thereby limiting excessive intestinal contractility and abdominal cramping. OB also inhibits T-type calcium channels and muscarinic responses. Finally, OB inhibits tachykinin receptors on smooth muscle and primary afferent neurons which may have the joint effect of reducing motility and abdominal pain. All these mechanisms mediate the therapeutic effects of OB in patients with IBS and might be useful in patients with other spastic colonic motility disorders such as diverticular disease.
Keywords: gastrointestinal motility, irritable bowel syndrome, L-type calcium channel, otilonium bromide, smooth muscle relaxants, spasmolytics, tachykinin receptor, visceral sensitization
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional digestive disorders encountered in clinical practice. Prevalence of IBS in the western population has been estimated between 10% and 20% [Lovell and Ford, 2012]. Hallmark features of IBS include recurrent abdominal pain or discomfort, bloating and changes in bowel habit, which may be predominant or alternating diarrhea and constipation. Abdominal pain in IBS has been attributed to a combination of smooth muscle hypercontractility, visceral hypersensitivity, and changes in the central processing of visceral pain [Drossman et al. 2002]. The pathophysiology of IBS varies between patients and has been linked to several factors, including intestinal infection, inflammation, intestinal microflora, stress, genetic predisposition and diet [Drossman et al. 2002]. Despite considerable research, no biochemical or structural abnormalities have been related to the disease. Poor understanding of IBS pathophysiology limits the therapeutic strategies to treatment of patient’s symptoms. Spasmolytic compounds are in use as a treatment strategy based on the observations of disturbed gastrointestinal (GI) motility among patients with IBS [Drossman et al. 2002; Forte et al. 2012]. Among the various spasmolytic compounds, otilonium bromide (OB), a quaternary ammonium derivative, has demonstrated superior effectiveness in the treatment of IBS symptoms [Forte et al. 2012]. A recent review of clinical trials with OB concluded that OB was both safe and efficacious in improving abdominal pain and distension in patients with IBS [Clave et al. 2011]. Furthermore, OB has been proven to relieve pain in all IBS subtypes. Although the therapeutic properties of OB are well documented, the underlying mechanisms are less well understood. Studies on mechanisms of action of OB have provided a number of pharmacological properties which most likely operate together to produce the clinical effect. The mechanisms involved in the action of OB in the GI tract are reviewed here. These include modulation of smooth muscle cell (SMC) contractility, inhibition of main patterns of colonic contractions and possibly direct effects on sensory nerves. OB interacts with a variety of neurohormone receptors and calcium channels to produce its therapeutic effect.
Pharmacology and efficacy of OB in IBS
OB is not absorbed systemically on ingestion, and accumulates in the wall of the small bowel and colon [Evangelista et al. 2000]. This has been demonstrated mainly in animal studies which show that 97.8% of ingested OB is excreted in feces and only 0.71% in urine [Evangelista et al. 2000; Shin et al. 2008; Sutton et al. 1997]. At therapeutic dosages, the concentration of OB in intestinal and colonic smooth muscle is estimated to be around 10 µmol/liter while plasma concentration is at least 1000 times lower [Evangelista et al. 2000]. Thus, OB acts predominantly locally and, due to its poor systemic absorption and low bioavailability outside the colon, is devoid of serious side effects [Boeckxstaens et al. 2013; Evangelista, 2004]. Several studies have shown that OB modulates intestinal motility and visceral sensitivity and reduces the increased colonic motor responses associated with IBS [Evangelista, 2004; Narducci et al. 1986; Battaglia et al. 1998]. Recently, the efficacy of OB in the treatment of patients with IBS was demonstrated by an international placebo-controlled trial, ‘Otilonium Bromide in Irritable Bowel Syndrome (OBIS)’ [Clave et al. 2011]. In this study of 356 patients, 15 weeks of treatment with OB significantly reduced the frequency of episodes of abdominal pain and improved abdominal bloating. Adverse events did not differ between OB and placebo. Furthermore, the OBIS trial demonstrated long-lasting therapeutic effects and protection of symptom relapse even after discontinuation of treatment [Clave et al. 2011]. Taken together, these studies indicate that OB is suitable for use in patients with IBS and provides an important therapeutic option to treat bloating and abdominal pain.
Colonic motor patterns
GI motility is generated by a complex interaction of enteric motor neurons, SMCs and interstitial cells of Cajal (ICC) and is under the control of various hormones and inflammatory mediators [Wood et al. 1999; Sanders, 2008]. Two main types of contractions can be distinguished in the human small bowel and colon: myogenic rhythmic phasic contractions (RPCs), primarily responsible for mixing the luminal content; and nerve-mediated contractions such as giant migrating contractions, which propagate luminal content along the colon [Sarna, 2006; Bampton and Dinning, 2013; Dinning et al. 2013]. RPCs are facilitated by spontaneous periodic depolarization of SMCs, also called slow waves, which are generated and propagated by ICC. Slow waves cause brief periods of high and low excitability in the circular SMCs. Upon excitatory stimulation, these periods of high excitability raise the SMC depolarization above threshold levels and evoke rhythmic contractions. In contrast, giant migrating contractions are generated independent of slow waves by activity of enteric nerves and a sustained release of acetylcholine [Sanders, 2008; Sarna, 2006]. The binding of acetylcholine to muscarinic (M) receptors on circular SMCs initiates several signaling pathways that induce calcium influx through voltage-gated calcium channels located in the plasma membrane, and calcium channels located in intracellular membranes that delimitate internal calcium stores [Tobin et al. 2009]. The influx of calcium during action potentials activates the contractile proteins of SMCs resulting in large amplitude contractions [Sarna, 2006]. Relaxation is also dependent on calcium mobilization. Anal relaxations are evoked by inhibitory neurotransmitters which hyperpolarize SMCs thereby decreasing cytoplasmic mobilization of calcium. Cytoplasmic calcium influx thus determines the duration and amplitude of the contraction evoked by excitatory nerves and ICC.
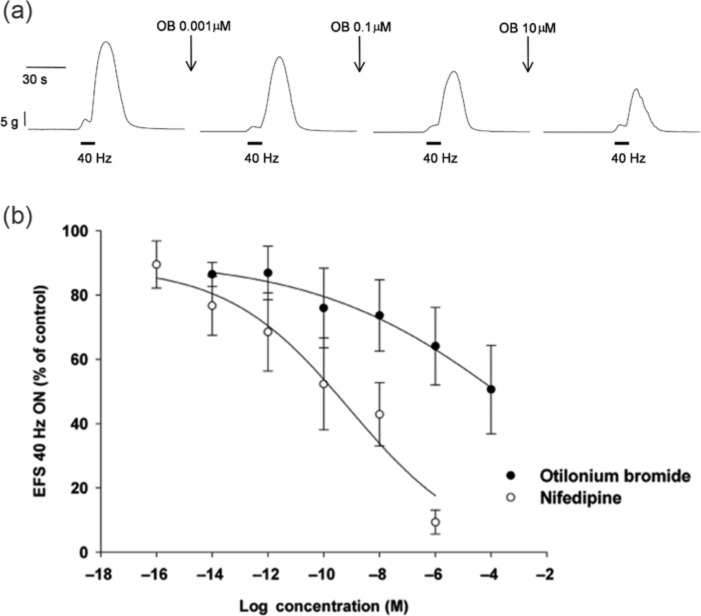
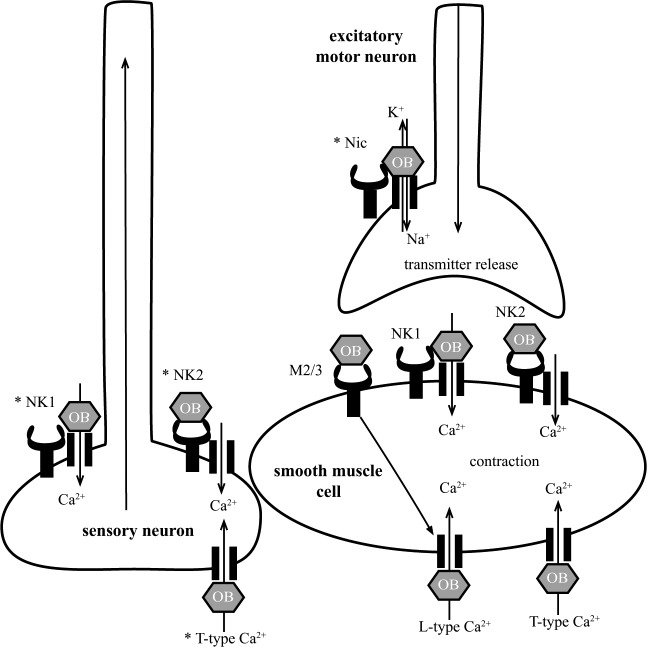
In vitro, patterns of contractility related to RPCs and giant migrating contractions can be observed in circular muscle strips from the human small bowel and colon and have been used to study the effect of OB [Gallego et al. 2010]. The results demonstrate that OB is a potent inhibitor of spontaneous RPCs and contractions induced by stimulation of excitatory motor neurons at submicromolar range (Figure 1). In addition, OB inhibits stretch-induced contractions. These data suggest that OB modulates the motility of the small bowel and colon predominantly by inhibiting SMC contractility and excitatory neurotransmission. OB appears to modify the basic patterns of motility, which explains the spasmolytic action and the therapeutic effect. The following sections focus on the mechanisms of OB in the GI tract with the aim of delineating major cell types, receptors and ion channels affected by OB and therefore responsible for the clinical effects of the drug (Figure 2).
Figure 1.
Effect of otilonium bromide (OB) on circular muscle contractions of the human sigmoid colon recorded in organ bath. (a) Electric stimulation (solid bars) of enteric motor neurons in a circular muscle strip evokes transient contraction with an amplitude of approx 25 g. Incubation with OB at increasing concentrations during 20 min decreased the contraction amplitude in a concentration-dependent manner. (b) Concentration–response curves of OB (solid circles) and the L-type Ca2+ channel antagonist nifedipine (open circles) on the amplitude of contractions evoked by electric field stimulation in human sigmoid colon circular muscle. Data are expressed as mean ± SEM, n = 5. Adapted from Gallego et al. [2010]. With permissions from Neurogastroenterology & Motility.
Figure 2.
Schematic summary of the interaction of otilonium bromide (OB) with neurohormone receptors and calcium (Ca2+) channels on smooth muscle cells, motor neurons, and sensory neurons in the gastrointestinal tract. Muscarinic receptor subtype 2 and 3 (M2/3), tachykinin receptor 1 (NK1) and tachykinin receptor 2 (NK2), L-type calcium channels (L-type Ca2+), T-type calcium channels (T-type Ca2+). OB inhibits mobilization of calcium from extracellular sources by direct blockade of neurohormone receptors and Ca2+ channels resulting in an attenuation of the contractile response of the smooth muscle cell and possibly (as indicated by asterisks) in diminished excitability of sensory neurons and excitatory motor neurons.
Muscarinic receptors
M receptors are classified into five subtypes (M1, M2, M3, M4 and M5) and are expressed by a variety of cell types, including intestinal SMCs and ICC. M receptors are the primary target for the excitatory neurotransmitter acetylcholine released by excitatory enteric motor neurons and binding between the two results in contraction of the smooth muscle [Tobin et al. 2009]. OB also binds with M receptors, as demonstrated inradioligand binding assays, which measured interference of OB with binding of a radiolabeled ligand to its specific receptor. In the rat colon, competitive M2 receptor binding of OB was demonstrated at a half maximal inhibitory concentration (IC50) of 1220 nmol/liter [Evangelista et al. 1998]. Human muscarinic receptors M1, M2, M4 and M5 also bound with OB at submicromolar affinity [Evangelista et al. 1998]. This indicates that OB interacts with M receptors, possibly inhibiting signal transduction.
The antimuscarinic component in the action of OB was further evaluated in an electrophysiological study in the guinea pig colon [Santicioli et al. 1999]. In this study, stimulation of M receptors by the agonist methacholine, and by cholinergic excitatory junction potential, resulted in smooth muscle membrane depolarization and contraction. OB inhibited both these responses in a concentration-dependent manner. Furthermore, OB reduced the methacholine-induced membrane depolarization and the subsequent SMC contraction at similar IC50 values (4.1 and 3.7 μmol/liter respectively), indicating a blockade of the receptor rather than interference with downstream signaling events [Santicioli et al. 1999].
Similar direct antimuscarinic properties of OB were demonstrated by Martinez-Cutillas and colleagues in human colonic SMCs and rat colon [Martinez-Cutillas et al. 2013]. Using organ bath and electrophysiological studies, they showed that electrically evoked excitatory junction potentials and contractions of the rat colonic circular muscle were inhibited by OB. Both the contractile and the electrical response were sensitive to atropine, a muscarinic receptor antagonist, suggesting that the muscarinic receptor was the target of inhibition by OB. In human SMCs, nifedipine-resistant calcium transients evoked by the M-receptor agonist carbachol were also inhibited by OB, suggesting interaction between OB and M receptors [Martinez-Cutillas et al. 2013].
A direct antagonistic effect of OB on human M3 was also observed in colonic crypts obtained from the sigmoid colon and in a Chinese hamster ovary transfection system expressing the human recombinant M3 muscarinic receptor [Lindqvist et al. 2002]. In this study, OB selectively inhibited M3-coupled calcium signaling at IC50 of 880 nmol/liter, a similar range as the IC50 for M-receptor binding. Interestingly, in this study, OB did not inhibit the mobilization of calcium induced by other receptors that share the same calcium signaling route. This is thought to demonstrate that OB may target the M3 receptor directly or via a downstream signaling component specific to the M receptor and not shared with other receptors [Lindqvist et al. 2002]. Because the M3 receptor plays a role in intestinal fluid secretion [Hirota and McKay, 2006], its inhibition by OB on colonic crypts also suggests that this drug may possess antisecretory properties which could be of therapeutic value especially for patients with diarrhea-predominant IBS. In short, the binding and inhibition of muscarinic receptors on SMCs by OB can be expected to counteract activation by acetylcholine released from enteric motor neurons and thereby block the contractile response.
Tachykinin receptors
Tachykinins (TKs) are widely distributed excitatory neurotransmitters in the GI tract and have also been implicated in IBS [Holzer and Holzer-Petsche, 1997, 2001]. Members of the TK family include substance P (SP) and neurokinin A (NKA). Within the enteric nervous system, SP and NKA are expressed by extrinsic primary afferents, ascending interneurons and by myenteric and submucosal intrinsic primary afferent neurons. Myenteric excitatory motor neurons innervating the circular and longitudinal muscle layers express SP and NKA and release both as excitatory cotransmitters of acetylcholine [Auli et al. 2008]. In addition, TK expression has been described on several non-neuronal cells in the GI tract [Santicioli et al. 1999; Holzer and Holzer-Petsche, 2001]. TKs act through interaction with neurokinin receptor 1 (NK1), 2 (NK2) and 3(NK3), each characterized by the specific agonists and antagonists acting on them [Santicioli et al. 1999]. The NK1 receptor has been localized on SMCs, neurons and ICC, but also on glands and enterocytes in the GI tract. The NK2 receptor is predominantly localized on smooth muscle, while the NK3 receptor is expressed by neurones [Holzer and Holzer-Petsche, 1997; Costa et al. 1996; Furness and Sanger, 2002]. The interaction between OB and NK receptors was initially investigated by Santicioli and colleagues who demonstrated an inhibitory action of OB on circular muscle contraction of the guinea pig colon evoked by the NK1 receptor agonist [Sar9]SP sulphone, and the NK2 receptor agonists [bAla8] NKA(4–10) [Santicioli et al. 1999]. The results obtained from this study indicate that the inhibitory mechanisms of OB differ between NK1 receptor and NK2 receptor.
The activation of NK receptors on SMCs results in the opening of nonselective cation channels, producing membrane depolarization followed by activation of voltage-dependent calcium channels and influx of intra- and extracellular calcium. This generates the action potential required for contraction [Sanders, 2000]. OB was shown to eliminate the SMC action potential and contractile response evoked by NK1 receptor activation (IC50 43 μmol/liter) without significant effect on the membrane depolarization [Santicioli et al. 1999]. Therefore it appears that OB does not interact directly with the NK1 receptor but rather inhibits the entry of extracellular calcium possibly by blocking voltage-gated calcium channels downstream of the NK1 receptor activation. This is further supported by the lack of effect of OB on the membrane depolarization induced by NK1 receptor activation in the presence of the voltage-dependant, L-type Ca2+ channel antagonist nifedipine [Santicioli et al. 1999].
In contrast to the indirect inhibition of NK1 signaling, inhibition of NK2 signaling by OB is the result of direct interaction with the receptor. This was initially suggested by competitive binding of OB to human NK2 receptor in radioligand binding experiments [Santicioli et al. 1999]. In this study, OB was found to displace [125I]NKA in a concentration-dependent manner with a Ki of 7.2 ± 0.83 μmol/liter. Binding of another NK2 receptor agonist, [3H]SR 48968, was inhibited by OB with a Ki of 2.2 μmol/liter [Santicioli et al. 1999]. Moreover, in the guinea pig colon, SMC membrane depolarization and the mechanical response evoked by NK2 receptor ligand were equally inhibited by OB, suggesting blockade at the level of the receptor and not via effects on cytoplasmic calcium mobilization.
Finally, the OB IC50 values for inhibition of membrane depolarization and mechanical response evoked by NK2 receptor activation were not affected by nifedipine, which supports the hypothesis that OB blocks the NK2 receptor signaling upstream of the voltage-dependant L-type Ca2+ channel [Santicioli et al. 1999]. Recent studies on human tissue support the findings from animal experiments. As NK2 receptors internalize as a consequence of their activation, internalization is used as an indirect measure of ligand binding. A study by Cipriani and colleagues quantified NK2 receptor internalization induced by the selective agonist [bAla8]NKA(4–10) in SMC of the human colon. The results demonstrated that OB inhibits, in a concentration-dependent manner, NK2 receptor internalization in the presence of the agonist [Cipriani et al. 2011]. It was concluded that OB exerts its effect (although not exclusively) via direct interaction with NK2 receptor [Cipriani et al. 2011]. Further work on human SMCs showed that stimulation of NK2 receptor with NKA (0.1 μmol/liter) evokes calcium transients which are inhibited by OB in a dose-dependent manner [Martinez-Cutillas et al. 2013]. This inhibition persisted in the presence of nifedipine, supporting the notion of direct inhibition of the NK2 receptor by OB [Martinez-Cutillas et al. 2013]. In summary, the above studies indicate that OB can inhibit NK1 receptor signaling at the level of voltage-gated calcium channels while it blocks NK2 receptor activation via a direct interaction with the receptor. However, it remains to be established to what extent the spasmolytic effects of OB in vivo are related to modulation of tachykinergic signaling.
L-type Ca2+ channels
L-type Ca2+ channels are expressed in GI SMCs and mediate the influx of intracellular calcium when smooth muscles depolarize after excitatory (muscarinic and tachykinergic) stimulation [Holzer and Holzer-Petsche, 2001]. The multiple involvements of L-type Ca2+ channels in smooth muscle contractility makes them a strategic target for spasmolytic compounds but makes interactions of OB with the receptor and interactions at the calcium channel difficult to distinguish. OB was first shown to have direct Ca2+ channel blocking properties through the observed inhibition of K+-induced Ca2+ mobilization in rat colon SMCs [Maggi et al. 1983] and neuronal cells [Gandia et al. 1996]. Subsequent studies using radioligand binding assays demonstrated that OB indeed interacts with L-type Ca2+ channels at the molecular level [Evangelista et al. 1998]. Specifically, OB was found to competitively bind the verapamil binding site on the L-type Ca2+ channels in the rat colon with an IC50 of 1020 nmol/liter. OB also bound the diltiazem binding site in rat cerebral cortex at higher concentrations (1490 nmol/liter) [Evangelista et al. 1998]. The functional consequences of this interaction were studied by Martin and colleagues in the rat colon using patch-clamp techniques and organ bath [Martin et al. 2004]. Their work demonstrated L-type Ca2+ channel-specific inhibition of SMC inward calcium currents and contractile response in the presence of OB. Studies on human tissue supported and expanded these findings. Using patch clamp techniques, Strege and colleagues recorded whole cell currents in human jejunal circular SMCs and on L-type Ca2+ channels expressed heterologously in HEK293 cells [Strege et al. 2004]. OB-inhibited SMC whole cell currents by 88% at 9 μmol/liter and HEK293 cell currents by 29% at 0.9 μmol/liter. The functional consequence of L-type Ca2+ channel blockade by OB has also been studied in human colon. In organ bath studies, Gallego and colleagues observed that OB inhibited RPCs and stretch-induced tone and contractions induced by electrical stimulation of excitatory motor neurons [Gallego et al. 2010]. The effects of OB were largely reversed by the L-type Ca2+ channel agonist, BayK8644, but persisted in the presence of nicotinic and muscarinic receptor antagonists, NK2 receptor antagonist or depletion of intracellular Ca2+ stores. This suggests that inhibition of the main patterns of motility in the human colon by OB is a result of L-type Ca2+ channel blockade. Interestingly, this study found that OB did not affect inhibitory neuromuscular transmission, suggesting that OB mainly acts as an inhibitor of excitatory signaling in the human gut [Gallego et al. 2010].
Blockade of L-type Ca2+ channels was also demonstrated in human colonic SMCs using calcium imaging and electrophysiology [Martinez-Cutillas et al. 2013]. Here, OB inhibited K+-evoked calcium transients (half maximal effective concentration of 3.6 μmol/liter) sensitive to the L-type Ca2+ channel inhibitor nifedipine. Similarly, calcium transients evoked by the L-type Ca2+ channel agonist BayK8644 were blocked by OB in a concentration-dependent manner. In organ bath experiments, OB also inhibited nifedipine-sensitive, calcium-dependent, rhythmic contractions of the rat colonic circular muscle [Martinez-Cutillas et al. 2013].
In summary, these data imply OB blocks L-type Ca2+ channels on SMCs. The blockade of SMC L-type Ca2+ channels results in the inhibition of the contractile response to various excitatory stimuli and is thus likely to explain the observed spasmolytic properties of the drug.
T-type Ca2+ channels
Although L-type Ca2+ channels are mainly responsible for the calcium entry into SMCs, recent studies have also revealed the importance of T-type Ca2+ channels in regulation of GI motility [Beyder and Farrugia 2012; Lee et al. 2007]. T-type Ca2+ channels have been identified in various cell types, including SMCs and ICC in both the small intestine and the colon [Gibbons et al. 2009; Huizinga et al. 1991; Smirnov et al. 1992; Xiong et al. 1995]. Activation of T-type Ca2+ channels has been shown to be involved in the propagation of the slow wave from ICC to SMCs, modulating their excitability [Lee et al. 2007]. Thus, T-type Ca2+ channels are involved in the regulation of motility, which makes them a potential target for spasmolytic compounds such as OB. Indeed, studies on the effect of OB on T-type Ca2+ channels have revealed specific inhibitory properties. This was demonstrated in human embryonic kidney cells transfected with the three T-type Ca2+ channel types: CaV3.1, CaV3.2 and CaV3.3 [Strege et al. 2010]. In these cells, OB reversibly blocked whole cell, T-type Ca2+ currents, recorded by the standard patch-clamp technique. OB inhibited all three channel types at IC50 values between 1 and 10 μmol/liter, which is the therapeutic concentration of OB observed in GI smooth muscle [Evangelista et al. 2000]. Importantly, the measured IC50 values were lower than those observed previously for the L-type Ca2+ channel inhibition by OB (2.3 ± 0.5 μmol/liter) [Strege et al. 2004]. This indicates that at therapeutic concentrations of the drug, both L- and T-type Ca2+ channels are blocked, which is relevant to understanding the in vivo effect of OB.
An interaction of OB with the T-type Ca2+ channel was also demonstrated in human cultured SMCs [Martinez-Cutillas et al. 2013]. In these cells, calcium transients were induced by CaCl2 and measured by calcium imaging. A significant fraction of the evoked calcium transients were resistant to the L-type Ca2+ channel blocker nifedipine and sensitive to the T- and L-type Ca2+ channel antagonist mibefradil, suggesting involvement of T-type Ca2+ channels. OB inhibited these responses with an IC50 of 17.5 μmol/liter, indicating direct modulation of cellular calcium entry by T-type Ca2+ channels. This study suggests that inhibition of T-type Ca2+ channels by OB canproduce inhibitory effects on the contractility of human colonic muscle, possibly promoting spasmolysis.
In summary, studies indicate the blockade of T-type Ca2+ channels by OB at concentrations well within the range of those observed in the colon. Such inhibition is expected to decrease the smooth muscle contractility, possibly via alternations in slow wave propagation. It remains to be established whether this is a relevant mechanism underlying the spasmolytic actions of OB observed in the clinical setting. Recently, SMC sodium channels (Nav1.5) have also been implicated in the mechanisms of action of OB [Strege et al. 2010]. However, the exact role of these channels in GI motility and their interactions with OB are largely unknown.
Nicotinic receptors
The mode of action of OB has been studied predominantly in SMCs. However, evidence suggests that enteric nerves are also affected by OB, which could contribute to its spasmolytic properties. OB has been shown to inhibit signaling by nicotinic acetylcholine (ACh) receptors, which are expressed by many enteric nerves and which facilitate enteric neurotransmission. This was demonstrated in bovine adrenal chromaffin cells stimulated by the synthetic ACh receptor agonist dimethylphenylpiperazinium (DMPP) [Gandia et al. 1996]. OB inhibited the DMPP-evoked calcium uptake and catecholamine release with IC50 values of 96 and 7.4 nmol/liter, respectively. This inhibition was not caused by blockade of L-type Ca2+ channels downstream of the ACh receptor since K+-depolarization-evoked responses were inhibited by OB at a much lower potency (IC50 = 7.6 and 10 µmol/liter for calcium uptake and catecholamine release respectively) [Gandia et al. 1996]. Therefore, OB appears to interact directly with the nicotinic ACh receptor on a molecular level. The exact mechanism of this interaction is not known but the electrophysiological characteristics observed in chromaffin cells indicate an insertion and attachment of the OB molecule to the channel pore of the ACh receptor [Gandia et al. 1996]. Such obstruction may impair the diffusion of potassium and sodium ions through the conducting pore during receptor activation thereby suppressing neuronal excitability.
In short, in addition to its inhibitory properties on L- and T-type Ca2+ channels, OB appears to antagonize neuronal nicotinic receptors by blocking the ion-conducting pore. Such nicotinic blockade operating at the level of parasympathetic ganglia of the myenteric plexus could reduce colonic hypermotility [Gandia et al. 1996]. However, more studies need to be done to confirm this. Interestingly, the blockade of nicotinic receptors by hexamethonium in in vitro studies on human sigmoid colon muscle strips did not prevent the strong inhibition of RPCs induced by OB. This still occurred following the blockade of muscarinic acetylcholine receptors with atropine, further suggesting a mechanism of action on RPCs independent of cholinergic blockade [Gallego et al. 2010]. So, although OB potentially inhibits nicotinic receptors, its main effect on RPCs is likely to result from inhibition of L-type Ca2+ channels.
Visceral pain
Abdominal pain and discomfort are common symptoms of IBS and have been related to a sensitization of intestinal afferent nerves and alternations in central processing [Drossman et al. 2002; Boeckxstaens et al. 2013]. As a consequence of this visceral hypersensitivity, signals from the GI tract which do not evoke sensation in healthy subjects are experienced as painful in patients with IBS [Drossman et al. 2002]. Treatment with OB has been shown to decrease the incidence of abdominal pain and the severity of abdominal bloating [Clave et al. 2011; Battaglia et al. 1998; Chang et al. 2011]. Several mechanisms could underlie this therapeutic effect. It is possible that the smooth muscle relaxing action of OB may reduce abdominal pain and discomfort as these could be triggered by exacerbated colonic and small bowel contractions [Chey et al. 2001]. In addition, OB has been shown to affect visceral sensitivity independent of motility. A study by Czimmer and colleagues demonstrated decreased visceral sensitivity to balloon distention in the rectosigmoid in patients with IBS following treatment with OB [Czimmer et al. 2001]. While this study is not a randomized, controlled trial, the increase in the threshold for pain seems to suggest a direct interaction of OB with sensory afferent nerves. Part of this effect could be due to inhibition of NK receptors and L- and T-type Ca2+ channels within the GI tract on tissue other than smooth muscles. Activation of neuronal NK1 and NK2 receptors has been shown to stimulate and sensitize visceral afferent nerves and is involved in abdominal hypersensitivity [Holzer and Holzer-Petsche 2001; Toulouse et al. 2000; Maggi 1997]. An inhibition of tachikinergic signaling by OB could thus reduce abdominal pain and discomfort in patients with IBS. Likewise, L-type Ca2+ channels are expressed by dorsal root ganglia which provide sensory innervation to the gut [Mendelowitz et al. 1995]. In animal models, IBS evoked upregulation of L-type Ca2+ channels while their pharmacological blockade inhibited visceral pain, indicating involvement of the channel in hypersensitivity [Qian et al. 2013]. Since OB behaves as an L-type Ca2+ channels antagonist, it is possible that part of its effect on pain symptoms originates through this mechanism. Recently, T-type Ca2+ channels have been highlighted as another possible target in the modulation of visceral pain [Marger et al. 2011]. Specifically, the expression of the CaV3.2 T-type Ca2+ channel isoform was demonstrated on colonic nociceptive primary afferent neurons of the rat. In the same study, using a rat model of IBS, genetic or pharmacological inhibition of the channel resulted in fewer pain symptoms [Marger et al. 2011]. Thus, T-type Ca2+ channel blocking in colonic sensory afferents may alleviate abdominal pain and discomfort in patients with IBS treated with OB.
In short, the improved pain symptoms in patients with IBS following treatment with OB could be the consequence of normalization of motility patterns and the inhibition of neuronal NK receptors, and L- and T-type Ca2+ channels on nociceptive primary afferent neurons.
Discussion
The mechanisms of action of OB have been examined at the molecular, cellular and organ level, demonstrating various interactions with SMCs and the enteric nervous system, which could explain the therapeutic properties of this compound. Organ bath experiments have revealed OB inhibition of the main patterns of motility, such as RPCs, stretch-induced smooth muscle tone and contractile response to stimulation of enteric motor neurons. At the molecular and cellular level, OB binds and inhibits M and TK receptors, and L- and T-type Ca2+ channels in SMCs at therapeutically relevant concentrations. In addition, OB may inhibit TK receptors and L- and T-type Ca2+ channels expressed by enteric sensory nerves. Taken together, OB displays properties of a receptor antagonist and an inhibitor of calcium mobilization. As a result, OB is also expected to antagonize the acetylcholinergic and tachikinergic excitation of SMCs which generates the contractile response of the human GI tract. These two properties of OB, independently or together, are most likely involved in the inhibition of SMC contractility following stimulation of excitatory motor neurons.
Fewer data are available on the effects of OB on enteric nerves. However, both cell types share some of the signaling machinery used for excitation. This includes L- and T- type calcium channels and NK receptors. The observed effects of OB on visceral sensitivity may be the result of an interaction with these receptors and channels. In addition, inhibition of Ach receptors on sensory nerves could provide neuron-specific mechanisms by which OB modulates visceral sensation. Furthermore, OB behaves as a neuronal nicotinic receptor antagonist in bovine adrenal chromaffin cells, demonstrating an 18-fold potency over its inhibition of Ca2+ channels [Gandia et al. 1996]. Whether such nicotinic blockade also occurs in the human gut and to what extent it mediates spasmolysis remain to be demonstrated. The alternations of motility patterns of the human ileum and colon in vitro by OB support its role as receptor antagonist and SMC calcium channel blocker. Here, OB reduces the nerve-mediated contractions by interfering with excitatory neurotransmission and, in addition, spontaneous non-neural contractions by limiting smooth muscle calcium entry. The contractile patterns inhibited by OB can be considered in vitro homologues of mixing and propagating contractions in the human colon [Rao et al. 2001]. Inhibition of these motility patterns is therefore most likely responsible for the reduction of colonic hypermotility following OB treatment. The combined action of OB on motility patterns and sensory afferents is likely to underlie the therapeutic properties of this drug in patients with IBS. The spasmolytic properties of OB are most effective for treatment of patients with IBS with abdominal bloating and painful cramping associated with colonic hypermotility. However, other GI disorders with similar symptoms could also benefit from treatment with this drug. Recently, diverticular disease has been associated with IBS [Jung et al. 2010; Spiller, 2012]. In addition, in vitro observations demonstrated an increased contractile response, possibly related to symptom generation, of colonic circular muscle obtained from patients with diverticular disease [Gallego et al. 2013]. This could provide the basis for the therapeutic use of OB in this condition.
In summary, OB displays several pharmacological properties which act locally and in concert to produce spasmolytic effects and pain relief in IBS (Figure 2). Further research and pharmacological modulation of the underlying mechanisms are likely to produce improved treatments for IBS and other GI disorders associated with colonic hypermotility
Footnotes
Funding: Jakub Rychter and Diana Gallego are funded by CIBERehd (Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Instituto de Salud Carlos III). Agencia de Gestió d’Ajuts Universitaris i de Recerca (2009 SGR 708).
Conflict of interest statement: Pere Clavé has served as a speaker for Menarini International. Pere Clavé and Marcel Jiménez have received research funding from Laboratorios Menarini SA-Menarini Group, Badalona, Spain and Menarini International, Florence, Italy.
Contributor Information
Jakub Rychter, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Barcelona, Spain.
Francisco Espín, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Barcelona, Spain; Department of Surgery, Hospital de Mataró, Mataró, Spain.
Diana Gallego, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Barcelona, Spain.
Patri Vergara, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Barcelona, Spain; Department of Cell Biology, Physiology and Immunology, Universitat Autònoma de Barcelona, Barcelona, Spain.
Marcel Jiménez, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Barcelona, Spain; Department of Cell Biology, Physiology and Immunology, Universitat Autònoma de Barcelona, Barcelona, Spain.
Pere Clavé, Department of Surgery, Hospital de Mataró, Universitat Autónoma de Barcelona, C/ Cirera s/n, Mataró, Barcelona 08304, Spain.
References
- Auli M., Martinez E., Gallego D., Opazo A., Espin F., Marti-Gallostra M., et al. (2008) Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol 155: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bampton P., Dinning P. (2013) High resolution colonic manometry – what have we learnt? A review of the literature 2012. Curr Gastroenterol Rep 15: 328. [DOI] [PubMed] [Google Scholar]
- Battaglia G., Morselli-Labate A., Camarri E., Francavilla A., De Marco F., Mastropaolo G., et al. (1998) Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther 12: 1003–1010 [DOI] [PubMed] [Google Scholar]
- Beyder A., Farrugia G. (2012) Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap Adv Gastroenterol 5: 5–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G., Corazziari E., Mearin F., Tack J. (2013) IBS and the role of otilonium bromide. Int J Colorectal Dis 28: 295–304 [DOI] [PubMed] [Google Scholar]
- Chang F., Lu C., Luo J., Chen T., Chen M., Chang H. (2011) The evaluation of otilonium bromide treatment in Asian patients with irritable bowel syndrome. J Neurogastroenterol Motil 17: 402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W., Jin H., Lee M., Sun S., Lee K. (2001) Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol 96: 1499–1506 [DOI] [PubMed] [Google Scholar]
- Cipriani G., Santicioli P., Evangelista S., Maggi C., Riccadonna S., Ringressi M., et al. (2011) Effect of otilonium bromide and ibodutant on the internalization of the NK2 receptor in human colon. Neurogastroenterol Motil 23: 96–102 [DOI] [PubMed] [Google Scholar]
- Clave P., Acalovschi M., Triantafillidis J., Uspensky Y., Kalayci C., Shee V., et al. (2011) Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther 34: 432–442 [DOI] [PubMed] [Google Scholar]
- Costa M., Brookes S., Steele P., Gibbins I., Burcher E., Kandiah C. (1996) Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75: 949–967 [DOI] [PubMed] [Google Scholar]
- Czimmer J., Suto G., Kiraly A., Mozsik G. (2001) Otilonium bromide enhances sensory thresholds of volume and pressure in patients with irritable bowel syndrome. J Physiol Paris 95: 153–156 [DOI] [PubMed] [Google Scholar]
- Dinning P., Wiklendt L., Gibbins I., Patton V., Bampton P., Lubowski D., et al. (2013) Low-resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber-optic high-resolution manometry studies. Neurogastroenterol Motil 25: 640–649 [DOI] [PubMed] [Google Scholar]
- Drossman D., Camilleri M., Mayer E., Whitehead W. (2002) AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131 [DOI] [PubMed] [Google Scholar]
- Evangelista S. (2004) Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 10: 3561–3568 [DOI] [PubMed] [Google Scholar]
- Evangelista S., Cochet P., Bromet N., Criscuoli M., Maggi C.A. (2000) A distribution study with (14)C-otilonium bromide in the rat: evidence for selective tropism for large intestine after oral administration. Drug Metab Dispos 28: 643–647 [PubMed] [Google Scholar]
- Evangelista S., Giachetti A., Chapelain B., Neliat G., Maggi C. (1998) Receptor binding profile of otilonium bromide. Pharmacol Res 38: 111–117 [DOI] [PubMed] [Google Scholar]
- Forte E., Pizzoferrato M., Lopetuso L., Scaldaferri F. (2012) The use of anti-spasmodics in the treatment of irritable bowel syndrome: focus on otilonium bromide. Eur Rev Med Pharmacol Sci 16: 25–37 [PubMed] [Google Scholar]
- Furness J., Sanger G. (2002) Intrinsic nerve circuits of the gastrointestinal tract: identification of drug targets. Curr Opin Pharmacol 2: 612–622 [DOI] [PubMed] [Google Scholar]
- Gallego D., Auli M., Aleu J., Martinez E., Rofes L., Marti-Rague J., et al. (2010) Effect of otilonium bromide on contractile patterns in the human sigmoid colon. Neurogastroenterol Motil 22: e180–e191 [DOI] [PubMed] [Google Scholar]
- Gallego D., Espin F., Mikulka J., Smirg O., Gil V., Faundez-Zanuy M., et al. (2013) In vitro motor patterns and electrophysiological changes in patients with colonic diverticular disease. Int J Colorectal Dis 28: 1413–1422 [DOI] [PubMed] [Google Scholar]
- Gandia L., Villarroya M., Lara B., Olmos V., Gilabert J., Lopez M., et al. (1996) Otilonium: a potent blocker of neuronal nicotinic ACh receptors in bovine chromaffin cells. Br J Pharmacol 117: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons S., Strege P., Lei S., Roeder J., Mazzone A., Ou Y., et al. (2009) The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J Cell Mol Med 13: 4422–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota C., McKay D. (2006) Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149: 463–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Holzer-Petsche U. (1997) Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther 73: 173–217 [DOI] [PubMed] [Google Scholar]
- Holzer P., Holzer-Petsche U. (2001) Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol 1: 583–590 [DOI] [PubMed] [Google Scholar]
- Huizinga J., Farraway L., Den Hertog A. (1991) Effect of voltage and cyclic AMP on frequency of slow-wave-type action potentials in canine colon smooth muscle. J Physiol 442: 31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Choung R., Locke G., 3rd, Schleck C., Zinsmeister A., Talley N. (2010) Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol 105: 652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Hennig G., Fleming N., Keef K., Spencer N., Ward S., et al. (2007) The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology 132: 1852–1865 [DOI] [PubMed] [Google Scholar]
- Lindqvist S., Hernon J., Sharp P., Johns N., Addison S., Watson M., et al. (2002) The colon-selective spasmolytic otilonium bromide inhibits muscarinic M(3) receptor-coupled calcium signals in isolated human colonic crypts. Br J Pharmacol 137: 1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell R., Ford A. (2012) Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10: 712–721 [DOI] [PubMed] [Google Scholar]
- Maggi C. (1997) Tachykinins as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacol Res 36: 153–169 [DOI] [PubMed] [Google Scholar]
- Maggi C., Manzini S., Meli A. (1983) Octylonium bromide: a smooth muscle relaxant which interferes with calcium ions mobilization. Arch Int Pharmacodyn Ther 264: 305–323 [PubMed] [Google Scholar]
- Marger F., Gelot A., Alloui A., Matricon J., Ferrer J., Barrere C., et al. (2011) T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A 108: 11268–11273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Hove-Madsen L., Jimenez M. (2004) Otilonium bromide inhibits muscle contractions via L-type calcium channels in the rat colon. Neurogastroenterol Motil 16: 175–183 [DOI] [PubMed] [Google Scholar]
- Martinez-Cutillas M., Gil V., Gallego D., Mane N., Martin M., Jimenez M. (2013) Mechanisms of action of otilonium bromide (OB) in human cultured smooth muscle cells and rat colonic strips. Neurogastroenterol Motil 12: 803–812 [DOI] [PubMed] [Google Scholar]
- Mendelowitz D., Reynolds P., Andresen M. (1995) Heterogeneous functional expression of calcium channels at sensory and synaptic regions in nodose neurons. J Neurophysiol 73: 872–875 [DOI] [PubMed] [Google Scholar]
- Narducci F., Bassotti G., Granata M., Pelli M., Gaburri M., Palumbo R., et al. (1986) Colonic motility and gastric emptying in patients with irritable bowel syndrome. Effect of pretreatment with octylonium bromide. Dig Dis Sci 31: 241–246 [DOI] [PubMed] [Google Scholar]
- Qian A., Song D., Li Y., Liu X., Tang D., Yao W., et al. (2013) Role of voltage gated Ca2+ channels in rat visceral hypersensitivity change induced by 2,4,6-trinitrobenzene sulfonic acid. Mol Pain 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Sadeghi P., Beaty J., Kavlock R., Ackerson K. (2001) Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280: 629–639 [DOI] [PubMed] [Google Scholar]
- Sanders K. (2000) Postjunctional electrical mechanisms of enteric neurotransmission. Gut 47(Suppl. 4): 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. (2008) Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil 20(Suppl. 1): 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P., Zagorodnyuk V., Renzetti A., Maggi C. (1999) Antimuscarinic, calcium channel blocker and tachykinin NK2 receptor antagonist actions of otilonium bromide in the circular muscle of guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol 359: 420–427 [DOI] [PubMed] [Google Scholar]
- Sarna S. (2006) Molecular, functional, and pharmacological targets for the development of gut promotility drugs. Am J Physiol Gastrointest Liver Physiol 291: 545–555 [DOI] [PubMed] [Google Scholar]
- Shin B., Kim J., Kim J., Hu S., Kim H., Hong S., et al. (2008) Oral bioavailability and enterohepatic recirculation of otilonium bromide in rats. Arch Pharm Res 31: 117–124 [DOI] [PubMed] [Google Scholar]
- Smirnov S., Zholos A., Shuba M. (1992) Potential-dependent inward currents in single isolated smooth muscle cells of the rat ileum. J Physiol 454: 549–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R. (2012) Is it diverticular disease or is it irritable bowel syndrome? Dig Dis 30: 64–69 [DOI] [PubMed] [Google Scholar]
- Strege P., Evangelista S., Lyford G., Sarr M., Farrugia G. (2004) Otilonium bromide inhibits calcium entry through L-type calcium channels in human intestinal smooth muscle. Neurogastroenterol Motil 16: 167–173 [DOI] [PubMed] [Google Scholar]
- Strege P., Sha L., Beyder A., Bernard C., Perez-Reyes E., Evangelista S., et al. (2010) T-type Ca(2+) channel modulation by otilonium bromide. Am J Physiol Gastrointest Liver Physiol 298: 706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J., Kilminster S., Mould G. (1997) The clinical pharmacology of single doses of otilonium bromide in healthy volunteers. Eur J Clin Pharmacol 52: 365–369 [DOI] [PubMed] [Google Scholar]
- Tobin G., Giglio D., Lundgren O. (2009) Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol 60: 3–21 [PubMed] [Google Scholar]
- Toulouse M., Coelho A., Fioramonti J., Lecci A., Maggi C., Buéno L. (2000) Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br J Pharmacol 129: 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J., Alpers D., Andrews P. (1999) Fundamentals of neurogastroenterology. Gut 45(Suppl. 2): 6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Sperelakis N., Noffsinger A., Fenoglio-Preiser C. (1995) Ca2+ currents in human colonic smooth muscle cells. Am J Physiol 269: 378–385 [DOI] [PubMed] [Google Scholar]