Abstract
Up to 80% of colorectal, melanoma, and neuroendocrine liver metastases are unresectable due to excessive tumor burden. Isolated hepatic perfusion (IHP) administers intensive therapy to the liver while limiting systemic toxicity and thus may have an important role in the management of unresectable liver metastases. This review s describes the development of IHP, initial clinical results, open and percutaneous IHP techniques, and contemporary long-term treatment outcomes. IHP with melphalan or tumor necrosis factor α (TNFα) has been shown to achieve hepatic response rates of greater than 50% with progression-free survival of greater than 12 months among patients with refractory ocular melanoma liver metastases. The only series describing outcomes of IHP for neuroendocrine liver metastases notes an overall response rate of 50% and a median actuarial overall survival of 48 months after IHP treatment with melphalan or TNFα. The majority of studies that have evaluated IHP have been performed in patients with colorectal cancer liver metastases (CRCLM). In aggregate, survival results from retrospective studies and phase I/II clinical trials suggest that IHP demonstrated no significant survival benefit compared with systemic chemotherapy alone as first-line therapy. In contrast, IHP does improve outcomes relative to that provided by second-line chemotherapy for CRCLM, with overall response rates of 60% and median duration of liver response of 12 months. Continued evaluation of IHP for unresectable liver metastases is necessary to establish its role in multidisciplinary treatment approaches.
Keywords: colorectal cancer liver metastases, isolated hepatic perfusion, melanoma liver metastases, neuroendocrine liver metastases, ocular melanoma
Introduction
Liver metastases are commonly observed in various malignancies and are often the cause of substantial morbidity and mortality. In cases of isolated liver metastases, surgical resection (when possible) portends the best long-term survival. However, up to 80% of patients with liver metastases have unresectable disease due to combinations of excessive tumor burden, anticipated insufficient liver remnant with intact vascular inflow/outflow and biliary drainage, or medical comorbidity [Leporrier et al. 2006; Tzeng and Aloia 2013]. In these cases, systemic chemotherapy is often the only pursued treatment alternative. Yet long-term survival with chemotherapy treatment alone is rare, particularly for colorectal and melanoma liver metastases [Masi et al. 2011; Sanoff et al. 2008; Flaherty et al. 2012; Chapman et al. 2011; Eisen et al. 2010]. Moreover, durable disease response to second-line therapy is uncommon. For colorectal cancer liver metastases (CRCLM), second-line response rates are less than 25% and median survival after initiation of second-line chemotherapy is less than 15 months [Bidard et al. 2009; Giantonio et al. 2007; Peters et al. 2006; Rothenberg et al. 2008; van Cutsom et al. 2011]. While long-term survival is more common among patients with unresectable neuroendocrine liver metastases, symptoms due to disease burden or hormone secretion are usually refractory to systemic chemotherapy [Chamberlain et al. 2000]. Clearly alternative therapies are needed in a substantial portion of patients with colorectal, melanoma, and neuroendocrine liver metastases.
Liver-directed regional therapies administer intensive therapy to the cancer-burdened organ while limiting unnecessary systemic toxicities and thus may have an important role in the management of patients with unresectable liver metastases. Hepatic artery infusion (HAI), hepatic artery bland embolization and chemoembolizaton, radioembolization, and isolated hepatic perfusion (IHP) have the additional advantage of treating the entire liver to target macroscopic and microscopic disease. Recent meta-analyses on transarterial chemoembolization show partial response and stable disease rates of 16.7% and 48.2% and 1- and 2-year survival of 62% and 28% for CRCLM [Gruber-Rouh et al. 2013]. Corresponding data for radioembolization are 20–90% response rates and 1-year survival of 37–74% [Rosenbaum et al. 2013]. For neuroendocrine liver metastases, complete or partial response rates range from 12% to 100% with median survival of 18–70 months after first treatment after radioembolization [Yang et al. 2012]. While the experience is less for chemoembolization for neuroendocrine liver metastases, median progression-free survival (PFS) and overall survival (OS) of 18 and 69 months have been reported [Whitney et al. 2011]. While less extensive, small studies have shown disease response in the majority of patients after chemoembolization [Fiorentini et al. 2009a] and radioembolization [Kennedy et al. 2009] for neuroendocrine liver metastases. IHP can provide significant benefit in patients whose disease is refractory to other therapies and who have limited treatment options. The purpose of this review is to describe the development of and clinical results regarding IHP for liver metastases.
Development of IHP and initial clinical results
Liver metastases derive most of their blood supply from the hepatic artery whereas the majority of the blood source to benign hepatocytes is from the portal vein (PV) [Breedis and Young, 1954]. This discordance in blood supply is the basis for IHP and subsequent adjuvant HAI which allow for high concentrations of chemotherapy treatment to malignant tumors with limited toxicity to the background liver parenchyma. By isolating the liver and thereby limiting systemic chemotherapy administration, IHP further allows for delivery of substantially higher concentrations of chemotherapy at elevated temperatures that would otherwise be lethal if systemically administered [de Brauw et al. 1991].
Dr Robert Ausman published the first description of a technique for IHP [Ausman, 1961]. The technique was first refined in a canine model and was then tested in five patients with various hepatic malignancies. Although there was no long-term follow up and the morbidity was significant, therapeutic effect was likely observed in two patients. In 1969, Stehlin demonstrated the synergistic effects of hyperthermia and chemotherapy in regional perfusion [Stehlin, 1969]. Thus the combination of hyperthermia and chemotherapy became the standard approach which has been extrapolated to IHP. Because of the significant morbidity and potential mortality associated with IHP, this technique did not gain widespread acceptance over the following three decades. Several small, single institution series were published during this time, but patient selection criteria and perfusion parameters were variable, limiting the utility of these studies [Aigner et al. 1983; Schwemmle et al. 1987; Skibba and Quebbeman, 1986]. In the early 1990s, interest in the field of regional perfusion was renewed following a report by Lienard and Lejeune combining chemotherapy and tumor necrosis factor α (TNFα) for the treatment of extremity melanoma and sarcoma [Lienard et al. 1992]. Because of the potential for significant systemic toxicity associated with the use of TNFα, there was more focus on standardizing perfusion techniques. Emphasis was placed on ensuring complete vascular isolation and monitoring systems were developed to assess systemic leaks during perfusion.
Just over 20 years ago, several groups in the United States and Europe developed protocols to evaluate the safety and efficacy of IHP for unresectable liver malignancies [Lebhati et al. 1997; Hafström et al. 1994; Apple et al. 1999; Marinelli et al. 1998]. Investigators from the National Cancer Institute (NCI) conducted a prospective phase II clinical trial which evaluated the use of high-dose melphalan, TNFα, and moderate hyperthermia for the management of unresectable malignancies confined to the liver [Alexander et al. 1998; Lebhati et al. 1997]. The doses of melphalan and TNFα used were 1.5 mg/kg of ideal body weight and 1.0 mg, respectively, and established from a previously conducted phase I study. A total of 34 patients were treated and 33 patients were assessable for response. The majority of patients (76%) had CRCLM and 60% had received prior systemic or regional treatment. Other diagnoses included ocular melanoma (n = 4), leiomyosarcoma (n = 1), and liver metastases from an unknown primary adenocarcinoma (n = 2) and hepatocellular cancer (n = 1). There was one treatment-related mortality. Grade III or greater hepatic toxicity was observed in 75% of patients and was reversible in all but one patient. The overall response rate was 75% and was maintained in patients with advanced disease or those who had prior treatment (Table 1). This study established IHP using melphalan and TNF as a viable treatment option for patients with unresectable liver metastases.
Table 1.
Response to isolated hepatic perfusion based upon number of lesions, diameter of largest tumor, or percent hepatic replacement in 33 evaluable patients. (Modified from Alexander et al. [1998].)
| n | PR or CR | % | |
|---|---|---|---|
| Overall | 33 | 25 | 75% |
| Number (radiographically imageable lesions) | |||
| 1–4 | 9 | 7 | 78% |
| 5–19 | 13 | 9 | 69% |
| 20 | 11 | 9 | 81% |
| Diameter largest lesion (cm) | |||
| < 5 | 4 | 2 | 50% |
| 5–9.9 | 12 | 9 | 75% |
| 10 | 17 | 14 | 82% |
| % Hepatic replacement | |||
| <20 | 6 | 5 | 83% |
| 20–49 | 15 | 10 | 66% |
| ≥50 | 12 | 10 | 83% |
PR, partial response; CR, complete response.
Open technique for IHP
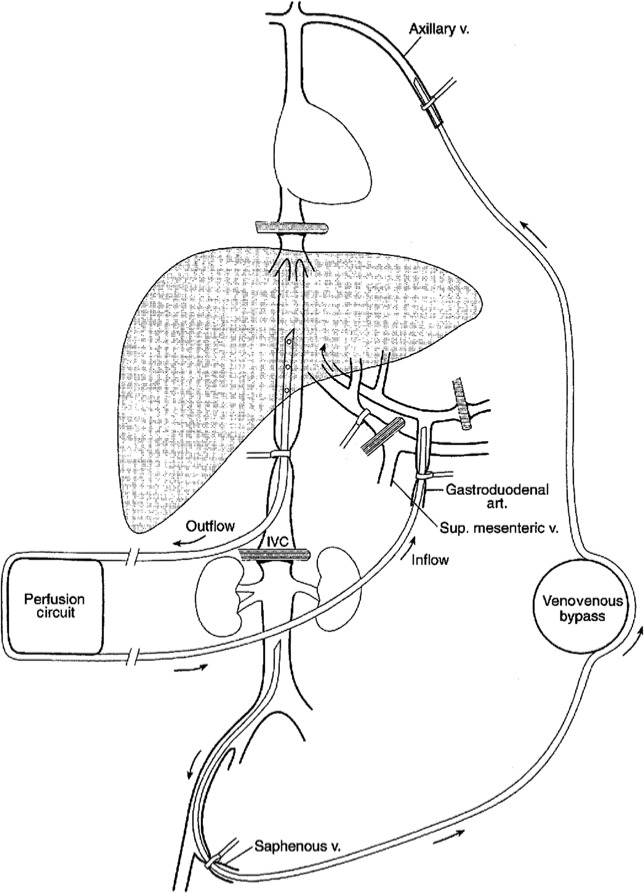
The abdomen is initially explored to evaluate for peritoneal dissemination or distant lymph node involvement which would be a contraindication to the procedure. Involvement of resectable lymph nodes limited to the porta hepatis is not a contraindication to IHP, since this has not been shown to adversely affect outcomes. The presence of background liver pathology, such as severe steatosis, steatohepatitis, and sinusoidal obstructive syndrome is a contraindication to IHP. Hepatic vascular isolation is obtained by extensively mobilizing the right and left lobes of the liver; at this time all collateral veins and accessory hepatic arteries to the liver are either clamped or ligated. The inferior vena cava (IVC) is exposed by performing a generous Kocher maneuver of the duodenum and then all venous tributaries from the retrohepatic IVC including the right adrenal vein and phrenic veins are ligated. The structures of the porta hepatis, including the proper hepatic artery, PV, and common bile duct are completely exposed. The gastroduodenal artery (GDA) is dissected and serves as the cannulation site for the perfusion. The IHP circuit is depicted in Figure 1.
Figure 1.
Illustration showing the isolated hepatic perfusion circuit. On the patient’s right is the extracorporeal perfusion circuit connected to an inflow cannula positioned in the gastroduodenal artery and an outflow cannula in an isolated segment of the retrohepatic inferior vena cava. Note the vascular occlusion clamps on the common hepatic artery and portal vein. A second venovenous bypass circuit is on the patient’s left to shunt inferior vena cava blood flow back to the heart during perfusion. art., artery; IVC, inferior vena cava; Sup, superior; v., vein.
Systemic anticoagulation using heparin is administered to maintain an activated clotting time of more than 350–400 s. A venovenous bypass circuit is then created from the saphenous vein to the axillary vein to maintain systemic venous return. This is necessary since flow in the retrohepatic vena cava will be interrupted with occluding clamps during the perfusion. The saphenous vein is cannulated with a 12–16 Fr catheter using an open or percutaneous insertion technique and the catheter tip is positioned just caudal to the renal veins. A 12–16 Fr catheter is then placed in the axillary vein or internal jugular vein with the tip positioned in the central circulation. These two cannulae are then attached to a centrifugal pump and form the venovenous bypass circuit.
To create the perfusion circuit, vascular clamps are placed across the infrahepatic IVC just above the renal veins a few centimeters apart. A venotomy is made in the IVC between the clamps and a 20–24 Fr catheter is advanced in the retrohepatic vena cava and secured with a Romel tourniquet; this cannula provides venous outflow for the hepatic perfusion circuit. Alternatively, the retrohepatic venous cannula can be inserted via the femoral vein percutaneously. The PV and common hepatic artery (CHA) are occluded with vascular clamps. As the dominant blood supply to the background liver is from the PV, clamping of the PV is necessary to prevent dilution of the chemotherapy and impairment of hyperthermia from the circuit. The inflow for the perfusion is then created by cannulating the GDA with a 3–4 mm arterial catheter with the tip positioned at the orifice of the CHA (Figure 2). Complete isolation of the liver is then achieved by placing a vascular clamp across the suprahepatic vena cava. Temperature probes are placed directly into the liver parenchyma on the right and left side to monitor hyperthermia during the procedure.
Figure 2.

Operative photograph which shows the porta hepatis during isolated hepatic perfusion. On the right a cannula is positioned in the gastroduodenal artery and there are atraumatic cross clamps on the portal vein and common bile duct. On the left is a cannula that is positioned in the retrohepatic vena cava.
The perfusion circuit for the open technique consists of a roller pump, membrane oxygenator, and a heat exchanger. The perfusate consists of 700 ml of a balanced salt solution and 1 unit of packed red blood cells (roughly 300 ml). A unit of packed red blood cells is necessary to ensure adequate oxygen delivery to the hepatic parenchyma during the perfusion. Arterial and venous blood gases are monitored throughout the perfusion to maintain a perfusate pH between 7.2 and 7.3. This is achieved with the addition of sodium bicarbonate to the perfusate. The heat exchanger is utilized to warm the perfusate to maintain hepatic parenchymal temperatures between 38.5 and 40°C. Flow rates of over 400 ml/min should be achieved and optimal flow rates are 600–800 ml/min (Table 2). Uniform perfusion to both lobes of the liver can be observed by rapid and uniform increase in temperature in both lobes. Complete vascular isolation using continuous intraoperative leak monitoring with I-131 human serum albumin was used in initial clinical trials; however, because complete vascular isolation is almost always achieved with the technique as described, radioactive leak monitoring is no longer used. Nevertheless, during the perfusion the venous outflow reservoir is monitored for changes in volume and a significant change in the reservoir suggests incomplete vascular isolation. If this occurs, all vascular clamps should be evaluated and any additional collateral vessels to the liver should be identified and ligated. The perfusion continues for 60 min and then the liver is flushed with 1500 ml of crystalloid followed by 1500 ml of colloid. The cannulas are removed, vascular structures are repaired, and normal liver perfusion is restored.
Table 2.
Treatment and perfusion parameters used during isolated hepatic perfusion.
| Duration | 1 h |
|---|---|
| Hepatic tissue temp | 39.5–40ºC |
| Tumor necrosis factor# | 1.0 mg |
| Melphalan | 1.5 mg/kg |
| Flow rate | 600–1200 ml/min |
| Arterial line pressure | 110–200 mmHg* |
| Veno-venous bypass flow | 1.8–2.0 l/min |
| Perfusate volume | |
| Perfusate composition | 700 cm3 crystalloid |
| 300 cm3 packed red blood cells | |
| 2000 U heparin | |
| 20–40 meq NaHCO3 | |
| Post perfusion flush | |
| Hepatic artery | 1.5 l crystalloid |
| 1.5 l colloid | |
| Portal vein | 1.0 l crystalloid |
Not used currently
Measured pressure in circuit, actual delivered pressure into hepatic artery is lower.
Clinical results
Ocular melanoma metastases
Ocular melanoma accounts for approximately 3–6% of all cases of melanoma and 30–60% of patients with ocular melanoma will develop liver metastases [Cohen et al. 2003; Kujala et al. 2003; Lorigan et al. 1991; McLaughlin et al. 2005; Seregard and Kock, 1995; Singh and Topham, 2003]. Once liver metastases develop survival is very short, less than 1 year, and death is usually due to disease progression in the liver [Agarwala et al. 2004]. Therefore, aggressive regional therapy to control disease progression in the liver appears justified in this patient population. Several protocols were conducted at the NCI to evaluate IHP in patients with ocular melanoma liver metastases. A series of phase I–II studies of IHP in patients with ocular melanoma and unresectable hepatic metastases were conducted at the NCI, Bethesda between 1994 and 1999[Alexander et al. 2000]. Twenty-two patients were treated in two studies; an initial phase I trial testing escalating doses of melphalan with and without TNFα and a phase II study using fixed doses of melphalan and TNFα. Overall, half the patients received melphalan alone and the other half were treated with melphalan and TNFα. Patients generally had advanced disease with a median number of metastatic nodules of 25, a mean percentage of hepatic replacement of 25%, and the mean size of the largest lesion was greater than 7 cm. The overall radiographic response rate was 62% with two complete responses (CRs, 10%) and 11 partial responses (PRs, 52%). Of those patients treated with melphalan alone, 7 out of 10 (70%) had a response, while 6 out of 11 patients (54%) treated with melphalan and TNF had evidence of a radiographic response. There was one treatment mortality (5%). The median PFS was 9 months in all patients and was significantly longer in patients who received TNFα (14 months versus 6 months, p = 0.04). The OS for the 22 treated patients was 11 months. These results were very promising given the high response rate and the acceptable observed morbidity and mortality.
A follow-up study reported outcomes in 29 patients (seven from the previous report) with metastatic ocular melanoma to the liver treated at the NCI using IHP with melphalan alone. In this study conducted between 1997 and 2002 [Alexander et al. 2003), the overall response rate was 62% with three CRs (10%) and 15 PRs (52%). The actuarial median hepatic PFS in the 18 patients who demonstrated evidence of a response was 12 months and the OS in all patients was 12.1 months. There were no treatment-related deaths, and the most common side effect was transient grade III or greater hepatic toxicity, which occurred in 65% of patients. On multivariate analysis, only baseline lactate dehydrogenase (LDH) was identified as a significant independent prognostic factor for survival, suggesting that baseline LDH level may have a role in patient selection (Figure 3).
Figure 3.
Actuarial overall survival in patients with ocular melanoma liver metastases undergoing isolated hepatic perfusion with melphalan with or without tumor necrosis factor; survival for patients with baseline lactate dehydrogenase (LDH) below 160 is significantly greater than for patients with elevated baseline LDH levels.
Additional series in the literature have also reported similar results. Noter and colleagues treated eight patients with IHP using a fixed dose of 200 mg of melphalan for patients with ocular melanoma liver metastases [Noter et al. 2004]. The overall response rate was 50% (all partial), the median PFS was 6.7 months, and the median OS was 9.9 months. Transient hepatic toxicity was observed in three patients and veno-occlusive disease eventually developed in two patients. A follow-up report from the same center which included a total of 19 patients treated with IHP, 13 with ocular melanoma metastases, demonstrated a response rate of 33 % in patients with ocular melanoma metastases, a median time to hepatic progression of 8.2 months, and a median OS of 10 months [van Iersel et al. 2008b].
Based on these studies, response rates of greater than 50% can be obtained using IHP with melphalan with and without TNFα for unresectable ocular melanoma liver metastases. These results are better than those obtained with systemic therapy alone and appear to be comparable to those obtained with other regional therapies [Fiorentini et al. 2009b; Gupta et al. 2003; Huppert et al. 2010; Leyvraz et al. 1997; Mavligit et al. 1988; Peters et al. 2006; Sato et al. 2008]. Therefore, the use of IHP in these patients has the potential to provide substantial clinical benefit for a disease with a very low proportion of cure with any nonresectional liver therapy. Rather than being a substitute for established less invasive radiologically guided regional therapy, IHP should be viewed as an additional therapy in the armamentarium of treatments for ocular melanoma liver metastases For example, transarterial chemo- or radioembolization could be used as an additional line of therapy for disease progression after IHP.
Neuroendocrine liver metastases
Approximately 75% of patient with neuroendocrine tumors will have metastatic disease at presentation and the most common site for metastasis is the liver. While surgical resection is effective and can improve 5-year survival rates to greater than 50%, complete surgical resection is usually difficult since patients often present with multifocal or bilateral disease. Even with diffuse liver metastases, 5-year survival rates of approximately 30% have been observed without treatment [Chamberlain et al. 2000; Moertel, 1987; Norheim et al. 1987; Benevento et al. 2000; Chen et al. 1998; Pingpank et al. 2005]. However, patients may develop debilitating local and systemic symptoms related to tumor burden and hormone production. In addition, liver-directed therapies have the potential to improve long-term outcomes by controlling progression of disease. Therefore, treatment of hepatic metastases has become an important component in the overall management of these patients.
The largest report in the literature which documents the use of IHP to treat neuroendocrine hepatic metastases is a study from the NCI [Grover et al. 2004]. This report details treatment and outcomes in 13 patients with neuroendocrine liver metastases treated with IHP on various protocols between 1993 and 2003. Ten patients were treated with melphalan alone, two patients received a combination of melphalan and TNFα, and one patient was treated with TNFα alone. Reversible grade III/IV hepatic toxicity was observed in 62% of patients, which is consistent with toxicity observed in other IHP studies. There was one treatment-related mortality. Overall response was 50% and the median actuarial OS was 48 months. Given the effectiveness of surgical resection and other liver-directed therapies in the management of patients with neuroendocrine liver metastases, it is likely that IHP will only play a significant role in the management of patients with quite advanced disease.
Colorectal cancer liver metastases
Because of the relatively high frequency of CRCLM, the majority of studies that have evaluated IHP have been performed in patients with CRCLM. These studies have utilized multiple types of chemotherapy, including mitomycin C, oxaliplatin, and melphalan with and without TNFα [Zeh et al. 2009; Alexander et al. 2002, 2005, 2009; Rothbarth et al. 2003; van Iersel et al. 2008a, 2010].
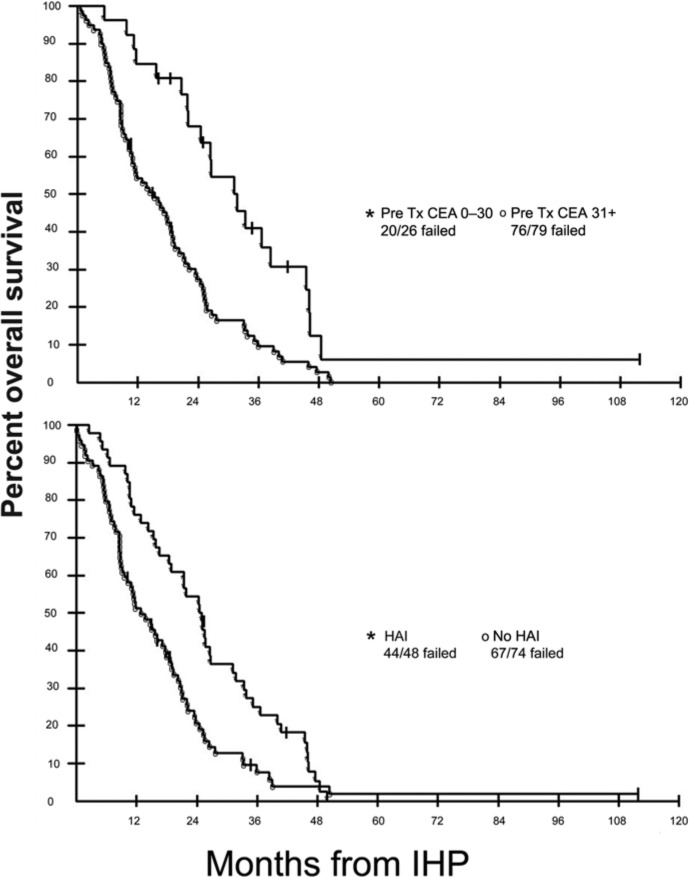
A total of 120 patients with CRCLM were treated on sequential prospective clinical trials at the NCI using IHP with melphalan alone (n = 69), melphalan and TNFα (n = 41), or TNFα alone (n = 10); 46 patients also received additional HAI therapy using floxuridine [Alexander et al. 2009]. The majority of patients (80%) had been treated with previous chemotherapy prior to IHP. There were five (4%) treatment-related mortalities; three of the mortalities occurred in patients treated on phase I dose-seeking studies. Response was evaluable in 114 patients. The overall radiographic response rate was 59% with a median time to hepatic progression of 7.0 months. Median OS was 17.4 months. Patients who received HAI therapy had a longer time to hepatic progression than those who did not receive HAI therapy: 13.0 versus 5.8 months respectively (Table 3). The most common toxicities were transient elevations in serum transaminases and total bilirubin. Factors associated with response were higher doses of melphalan and the use of TNF. With respect to OS, only the use of HAI therapy and a preoperative carcinoembryonic antigen level of up to 30 ng/ml were significant on multivariate analysis (Figure 4).
Table 3.
Results with IHP for patients with colorectal cancer liver metastases treated with IHP.
| Treatment regimen | Numberof evaluable patients | CR | PR | Median PFS months |
|---|---|---|---|---|
| Overall | 114 | 2 | 67 | 7.0 |
| 59% | ||||
| IHP–no HAI | 58 | 0 | 33 | 5.8 |
| 57% | ||||
| IHP–HAI | 46 | 2 | 30 | 13.0 |
| 65% | ||||
| IHP (TNF alone) | 10 | 0 | 4 | 3.0 |
IHP, isolated hepatic perfusion; PR, partial response; CR, complete response; TNF, tumor necrosis factor; HAI, hepatic artery infusion.
Figure 4.
Actuarial overall survival in 120 patients with diffuse colorectal cancer liver metastases who underwent isolated hepatic perfusion (IHP) based on baseline carcinoembryonic antigen (CEA) level (top panel) or with or without hepatic artery infusion (HAI) therapy (bottom panel) following IHP. Tx, treatment.
Similar outcomes were reported by van Iersel and colleagues who treated 105 patients with unresectable CRCLM over a 10-year period [van Iersel et al. 2008a]. All patients were treated with a fixed high dose of 200 mg of melphalan and almost all patients were perfused simultaneously through the hepatic artery and PV (of note, therapy via the PV is not the norm for IHP). Treatment-related morbidity and mortality were similar to those observed in the NCI study. The median PFS was 7.4 months, while the median duration of hepatic response was 11.4 months. The overall response rate was 50% (52/105 patients). The median OS was 24.8 months. On multivariate analysis, the use of adjuvant chemotherapy was associated with response and PFS while a greater number of hepatic metastases, PV perfusion alone, and postoperative complications were associated with decreased OS.
Using this same group of patients treated with IHP, van Iersel and colleagues recently reported a case–control study that compared the use of IHP with melphalan to systemic chemotherapy in patients with unresectable CRCLM [van Iersel et al. 2010]. The IHP group consisted of 99 patients treated between August 1994 and December 2004. The systemic chemotherapy group consisted of 111 patients who were enrolled in the Capecitabine, Irinotecan, Oxaliplatin (CAIRO) study of the Dutch Colorectal Cancer Group and received either sequential chemotherapy with first-line capecitabine, followed by second-line irinotecan, and then third-line capecitabine and irinotecan or combination chemotherapy with first-line capecitabine and irinotecan followed by second-line capecitabine and oxaliplatin. Patient characteristics in both groups were similar except that the patients receiving IHP were significantly younger. In the IHP group, major postoperative complications were observed in 35% and perioperative mortality was 6%. In the systemic chemotherapy group, grade III/IV toxicity was observed in 52% of patients and treatment-related mortality was 2%. The overall response rate for IHP was 47% and the median time to disease progression was 7.3 months. The overall response rate to first-line therapy was 37% and the median time to disease progression was 7.9 months. There was no significant difference in OS between the two groups: 25.0 months for those treated with IHP and 21.7 months for patients treated with systemic chemotherapy alone. Those patients who were treated with IHP as first-line therapy (50 patients) had an OS of 28.9 months which was not significantly different compared with the systemic therapy group (p = 0.24).
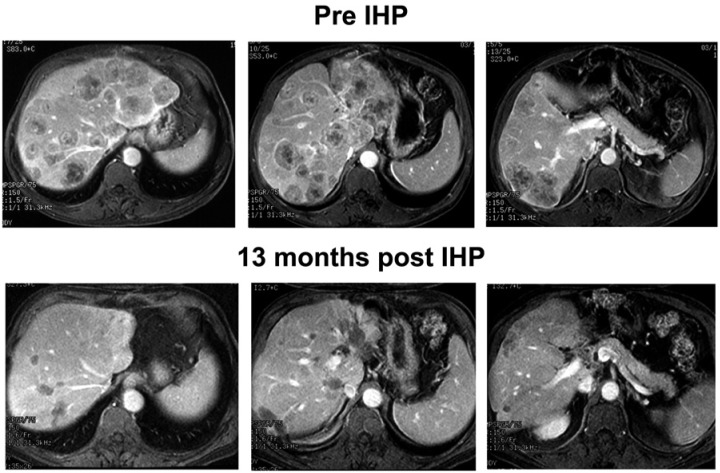
Together these data raise important questions about the management of patients with unresectable CRCLM. The role for IHP in patients who experience disease progression on systemic chemotherapy was tested by Alexander and colleagues who reported the outcomes of 25 patients with unresectable CRCLM refractory to chemotherapy [Alexander et al. 2005]. All patients received 5-fluorouracil (5-FU) based therapy with (n = 3) or without (n =22) irinotecan as first-line treatment; however, all 22 patients did receive irinotecan-based therapy as second-line treatment for CRCLM. Similar to other IHP studies, the patients had significant tumor burden with a median number of 10 hepatic metastases and a median percent hepatic replacement by tumor of 25%. The overall response rate was 60% (1 complete, 14 partial), and the median duration of response in the liver was 12 months. Systemic progression occurred in 13 patients (54%) at a median of 5 months. The median OS was 12 months, with a 2-year survival of 28%. These results are quite favorable compared with second-line chemotherapy, which is associated with response rates of generally less than 25% and a median OS of usually less than 15 months. Taken together these data suggest that IHP demonstrated no significant survival benefit compared with systemic chemotherapy alone as first-line therapy. There does appear to be a role for IHP as second-line or third-line therapy in selected patients with unresectable CRCLM whose condition is refractory to systemic chemotherapy (Figure 5). IHP should be considered in the context of prospective clinical trials evaluating an integrated multimodal management approach.
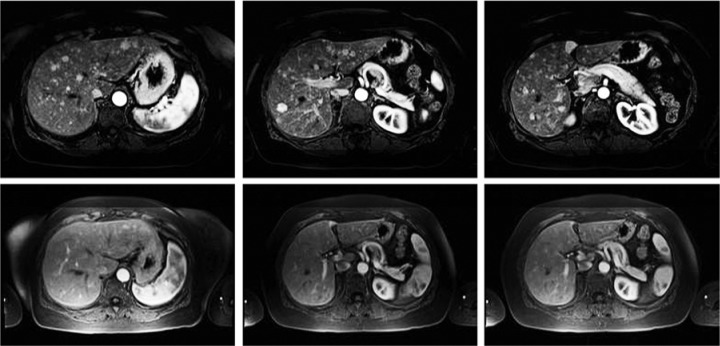
Figure 5.
Gadolinium-enhanced magnetic resonance imaging study from a patient with extensive hepatic metastases from colorectal cancer who had been previously treated with systemic and regional chemotherapy. The patient had a marked response to a 60 min isolated hepatic perfusion (IHP) as reflected in the lower images obtained more than 1 year after treatment.
Percutaneous hepatic perfusion
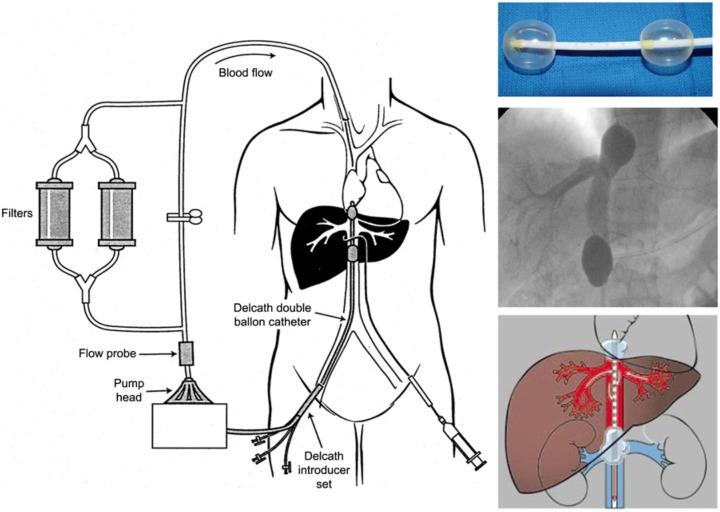
Percutaneous hepatic perfusion (PHP) utilizes a double-balloon catheter system (produced by Delcath Systems, New York, NY, USA) positioned percutaneously in the retrohepatic vena cava under fluoroscopic guidance. The double-balloon catheter has a unique construction with a large central lumen, three accessory lumina, and fenestrations throughout its length that allows for collection of hepatic venous outflow (Figure 6). The two balloons on either end of the catheter are positioned inferior and superior to the hepatic veins and are independently inflated under fluoroscopy. The venous outflow from the liver is filtered through an extracorporeal filtration system and is then returned to the systemic circulation through a catheter in the internal jugular vein. The arterial catheter is placed percutaneously from the femoral artery and is positioned in the proper hepatic artery under fluoroscopic guidance. Accessory hepatic arteries are embolized to minimize dilution of chemotherapy infusion to the liver. Once vascular isolation is confirmed, chemotherapy is administered as a continuous infusion over 30 min. The filtration circuit is then continued for an additional 30 min after the perfusion to ensure that all the chemotherapy is removed. Anticoagulation is required during the perfusion and is reversed using protamine and fresh frozen plasma at the end of the perfusion. Temporary use of vasopressors is necessary after balloon inflation to maintain hemodynamic stability.
Figure 6.
Diagram of the Delcath Catheter System. Melphalan is administered directly into the hepatic artery through an infusion catheter placed percutaneously via the femoral artery. Hepatic venous outflow is isolated via a double balloon catheter in the retrohepatic inferior vena cava (IVC) (shown top right). Blood is drawn out of the retrohepatic IVC through multiple fenestrations located along the length of the catheter between the cranial and caudal balloons. The blood is then pumped through a pair of activated charcoal filters prior to return to the systemic circulation via an internal jugular vein catheter. Fluoroscopic image of the isolated, retrohepatic IVC segment obtained by retrograde injection of contrast through the intraballoon fenestrations to confirm the absence of systemic leak is shown in the middle right.
PHP has several attractive features compared with open IHP. First, the open technique has the potential for significant morbidity and the technique cannot be repeated. While hemodynamic perturbations can occur during PHP (including decreases in mean arterial and central venous pressure) which may require inotropic or vasopressor support, these changes usually are transient [Miao et al. 2008]. Decreased venous return due to inflation of the IVC balloon catheter and depletion of catecholamine observed with extracorporeal hemofiltration are the reasons for decreasing mean arterial blood pressure. A total of 70–80% of patients treated required some type of pressor or intropic support [Miao et al. 2008; Ravikumar et al. 1994]. Given the excellent response rates that are observed after a single perfusion, it is possible that multiple perfusion treatments as done with PHP may provide more durable responses and improve OS. Initial experience and results using PHP were first reported in the early 1990s [Beheshti et al. 1992; Curley et al. 1994; Ravikumar et al. 1994]. Ravikumar and colleagues reported a series of 58 PHPs that were performed in 21 patients using escalating doses of 5-FU or doxorubicin. The majority of patients experienced significant transient hypotension at the time of IVC occlusion. In this study, the extraction efficiency of the filter ranged from 64% to 91% resulting in sufficient systemic exposure of the therapeutic agents to result in transient grade III/IV toxicities. There were no mortalities. As an example of the benefits of repeat PHP, one patient with scalp melanoma liver metastases in this study had a 96% reduction in liver disease burden after four treatments with complete symptom resolution [Ravikumar et al. 1994]. Curley and colleagues reported similar outcomes using PHP with escalating doses of doxorubicin in 10 patients with hepatocellular carcinoma. Importantly, one patient died after IVC balloons were inflated [Curley et al. 1994]. Both of these studies demonstrated that the technique was feasible; however, the clinical efficacy of the procedure was not rigorously evaluated.
In 2005, Pingpank and colleagues reported a phase I study of escalating melphalan dose administered via PHP in patients with unresectable hepatic malignancies [Pingpank et al. 2005]. A total of 74 procedures were performed in 28 patients. Twelve patients were treated at an initial melphalan dose of 2.0 mg/kg which was then escalated to a maximum tolerated dose (MTD) of 3.5 mg/kg. Seventy-five percent of patients had received previous treatment for liver metastases. As experienced by previous investigators, the most common grade III/IV toxicities were myelosuppression, mainly neutropenia and thrombocytopenia, which were observed at all dose levels. Grade III/IV hepatic toxicity occurred infrequently and was transient. The filtration efficiency ranged from 58.2% to 94.7%, with a mean of 77%. The overall response rate in 27 assessable patients was 30%. In the 10 patients with ocular melanoma primaries, the objective tumor response was 50%. This study established the MTD of melphalan at 3.0 mg/kg, demonstrated that PHP could be performed with manageable toxicities, and also showed antitumor effects across various tumor histologies. Preliminary results from a phase III trial comparing the efficacy of PHP with melphalan with standard of care for melanoma liver metastases have been published [Pingpank et al. 2010]. Median hepatic PFS was 254 days among 44 patients treated with PHP compared with 49 days among 49 patients treated with standard of care. The overall response rate after PHP was 34.1%. Marked responses may be obtained after PHP, as shown in Figure 7.
Figure 7.
Gadolinium-enhanced magnetic resonance images of a patient with diffuse liver metastases from ocular melanoma (top panels) and corresponding images taken 20 months after three percutaneous hepatic perfusion treatments.
As of now, the catheter delivery systems for PHP have not been approved by the US Food and Drug Administration for use in the United States.
Conclusion
IHP is a liver-directed therapy that may be utilized to treat patients with unresectable liver metastases. Numerous studies in patients with various tumor histologies have generally demonstrated response rates of greater than 50% with transient morbidity and acceptable mortality rates. While systemic therapy remains the standard of care for treatment of patients with unresectable CRCLM, there may be a role for IHP in patients who are refractory to systemic therapy. For patients with unresectable ocular melanoma liver metastases, the role for IHP may be more significant as another component in the armamentarium of therapies for a disease with relatively rapid disease progression after nonresectional therapy. The ability to perform PHP may increase the role of hepatic perfusion for treatment of multiple tumor histologies since this procedure allows for sequential treatments to be delivered with lower morbidity. The management of unresectable liver metastases is a significant clinical problem that requires the combined efforts of multiple providers to develop an integrated approach for each patient. Continued evaluation of hepatic perfusion in these patients is necessary so that its role in this integrated approach can be more clearly defined.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Srinevas K. Reddy, Division of General and Oncologic Surgery, Department of Surgery and the Marlene and Stewart Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD, USA
Susan B. Kesmodel, Division of General and Oncologic Surgery, Department of Surgery and the Marlene and Stewart Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD, USA
H. Richard Alexander, Jr, Division of Surgical Oncology, Department of Surgery, University of Maryland Medical Center, 22 South Greene Street, Baltimore, MD 21201, USA.
References
- Agarwala S., Panikkar R., Kirkwood J. (2004) Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with ocular melanoma metastatic to the liver. Melanoma Res 14: 217–222 [DOI] [PubMed] [Google Scholar]
- Aigner K., Walther H., Tonn J., Wenzl A., Hechtel R., Merker G., et al. (1983) First experimental and clinical results of isolated liver perfusion with cytotoxics in metastases from colorectal primary. Recent Results Cancer Res 86: 99–102 [DOI] [PubMed] [Google Scholar]
- Alexander H., Bartlett D.L., Libutti S.K., Fraker D.L., Moser T., Rosenberg S.A. (1998) Isolated hepatic perfusion with tumor necrosis factor and melphalan for unresectable cancers confined to the liver. J Clin Oncol 16: 1479–1489 [DOI] [PubMed] [Google Scholar]
- Alexander H., Jr, Bartlett D., Libutti S., Pingpank J., Fraker D., Royal R., et al. (2009) Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol 16: 1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander H., Jr, Libutti S., Bartlett D., Pingpank J., Kranda K., Helsabeck C., et al. (2002) Hepatic vascular isolation and perfusion for patients with progressive unresectable liver metastases from colorectal carcinoma refractory to previous systemic and regional chemotherapy. Cancer 95: 730–736 [DOI] [PubMed] [Google Scholar]
- Alexander H.R., Libutti S.K., Bartlett D.L., Puhlmann M., Fraker D.L., Bachenheimer L.C. (2000) A Phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res 6: 3062–3070 [PubMed] [Google Scholar]
- Alexander H., Jr, Libutti S., Pingpank J., Bartlett D., Helsabeck C., Beresneva T. (2005) Isolated hepatic perfusion for the treatment of patients with colorectal cancer liver metastases after irinotecan-based therapy. Ann Surg Oncol 12: 138–144 [DOI] [PubMed] [Google Scholar]
- Alexander H., Jr, Libutti S., Pingpank J., Steinberg S., Bartlett D., Helsabeck C., et al. (2003) Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res 9: 6343–6349 [PubMed] [Google Scholar]
- Apple S., Hecht J., Lewin D., Jahromi S., Grody W., Nieberg R. (1999) Immunohistochemical evaluation of K-ras, p53, and HER-2/neu expression in hyperplastic, dysplastic, and carcinomatous lesions of the pancreas: evidence for multistep carcinogenesis. Hum Pathol 30: 123–129 [DOI] [PubMed] [Google Scholar]
- Ausman R. (1961) Development of a technic for isolated perfusion of the liver. N Y State J Med 61: 3393–3397 [PubMed] [Google Scholar]
- Beheshti M., Denny D., Jr, Glickman M., Bodden W., Marsh J., Strair R., et al. (1992) Percutaneous isolated liver perfusion for treatment of hepatic malignancy: preliminary report. JVIR 3: 453–458 [DOI] [PubMed] [Google Scholar]
- Benevento A., Boni L., Frediani L., Ferrari A., Dionigi R. (2000) Result of liver resection as treatment for metastases from noncolorectal cancer. J Surg Oncol 74: 24–29 [DOI] [PubMed] [Google Scholar]
- Bidard F., Tournigand C., Andre T., Mabro M., Figer A., Cervantes A., et al. (2009) Efficacy of FOLFIRI-3 (irinotecan D1,D3 combined with LV5-FU) or other irinotecan-based regimens in oxaliplatin-pretreated metastatic colorectal cancer in the GERCOR OPTIMOX1 study. Ann Oncol 20: 1042–1047 [DOI] [PubMed] [Google Scholar]
- Breedis C., Young G. (1954) Blood supply of neoplasms of the liver. Am J Pathol 30: 969–985 [PMC free article] [PubMed] [Google Scholar]
- Chamberlain R., Canes D., Brown K., Saltz L., Jarnagin W., Fong Y., et al. (2000) Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 190: 432–445 [DOI] [PubMed] [Google Scholar]
- Chapman P., Hauschild A., Robert C., Haanen J., Ascierto P., Larkin J., et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Hardacre J., Uzra A., Cameron J., Choti M. (1998) Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 187: 88–92 [DOI] [PubMed] [Google Scholar]
- Cohen V., Carter M., Kemeny A., Radatz M., Rennie I. (2003) Metastasis-free survival following treatment for uveal melanoma with either stereotactic radiosurgery or enucleation. Acta Ophthalmol Scand 81: 383–388 [DOI] [PubMed] [Google Scholar]
- Curley S., Newman R., Dougherty T., Fuhrman G., Stone D., Mikolajek J., et al. (1994) Complete hepatic venous isolation and extracorporeal chemofiltration as treatment for human hepatocellular carcinoma: a phase I study. Ann Surg Oncol 1: 389–399 [DOI] [PubMed] [Google Scholar]
- de Brauw L., Marinelli A., Van de Velde C., Hermans J., Tjaden U., Erkelens C., et al. (1991) Pharmacological evaluation of experimental isolated liver perfusion and hepatic artery infusion with 5-fluorouracil. Cancer Res 51: 1694–1700 [PubMed] [Google Scholar]
- Eisen T., Trefzer U., Hamilton A., Hersey P., Millward M., Knight R., et al. (2010) Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer 116: 146–154 [DOI] [PubMed] [Google Scholar]
- Fiorentini G., Aliberti C., Del C., Tilli M., Rossi S., Ballardini P., et al. (2009a) Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo 23: 131–137 [PubMed] [Google Scholar]
- Fiorentini G., Aliberti C., Del C., Tilli M., Rossi S., Ballardini P., et al. (2009b) Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo 23: 131–137 [PubMed] [Google Scholar]
- Flaherty K., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., et al. (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367: 107–114 [DOI] [PubMed] [Google Scholar]
- Giantonio B., Catalano P., Meropol N., O’Dwyer P., Mitchell E., Alberts S., et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25: 1539–1544 [DOI] [PubMed] [Google Scholar]
- Grover A., Libutti S., Pingpank J., Helsabeck C., Beresnev T., Alexander H., Jr (2004) Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery 136: 1176–1182 [DOI] [PubMed] [Google Scholar]
- Gruber-Rouh T., Naguib N., Eichler K., Ackermann H., Zangos S., Trojan J., et al. (2013) Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer 134: 1225–1231 [DOI] [PubMed] [Google Scholar]
- Gupta S., Yao J., Ahrar K., Wallace M., Morello F., Madoff D., et al. (2003) Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J 9: 261–267 [DOI] [PubMed] [Google Scholar]
- Hafström L., Holmberg S., Naredi P., Lindnér P., Bengtsson A., Tidebrant G., et al. (1994) Isolated hyperthermic liver perfusion with chemotherapy for liver malignancy. Surg Oncol 3: 103–108 [DOI] [PubMed] [Google Scholar]
- Huppert P., Fierlbeck G., Pereira P., Schanz S., Duda S., Wietholtz H., et al. (2010) Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol 74: e38–e44 [DOI] [PubMed] [Google Scholar]
- Kennedy A., Nutting C., Jakobs T., Cianni R., Notarianni E., Ofer A., et al. (2009) A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest 27: 682–690 [DOI] [PubMed] [Google Scholar]
- Kujala E., Makitie T., Kivela T. (2003) Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 44: 4651–4659 [DOI] [PubMed] [Google Scholar]
- Lebhati R., Cadiot G., Marmuse J., Vissuzaine C., Petegnief Y., Courillon-Mallet A., et al. (1997) False-positive somatostatin receptor scintigraphy due to an accessory spleen. J Nucl Med 38: 1979–1981 [PubMed] [Google Scholar]
- Leporrier J., Maurel J., Chiche L., Bara S., Segol P., Launoy G. (2006) A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 93: 465–474 [DOI] [PubMed] [Google Scholar]
- Leyvraz S., Spataro V., Bauer J., Pampallona S., Salmon R., Dorval T., et al. (1997) Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol 15: 2589–2595 [DOI] [PubMed] [Google Scholar]
- Lienard D., Ewalenko P., Delmotti J., Renard N., Lejeune F. (1992) High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 10: 52–60 [DOI] [PubMed] [Google Scholar]
- Lorigan J., Wallace S., Mavligit G. (1991) The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. AJR 157: 1279–1281 [DOI] [PubMed] [Google Scholar]
- Marinelli A., Vahrmeijer A., van de Velde C. (1998) Phase I/II studies of isolated hepatic perfusion with mitomycin C or melphalan in patients with colorectal cancer hepatic metastases. Recent Results Cancer Res 147: 83–94 [DOI] [PubMed] [Google Scholar]
- Masi G., Vasile E., Loupakis F., Cupini S., Fornaro L., Baldi G., et al. (2011) Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst 103: 21–30 [DOI] [PubMed] [Google Scholar]
- Mavligit G., Charnsangavej C., Carrasco C., Patt Y., Benjamin R., Wallace S. (1988) Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA 260: 974–976 [PubMed] [Google Scholar]
- McLaughlin C., Wu X., Jemal A., Martin H., Roche L., Chen V. (2005) Incidence of noncutaneous melanomas in the U.S. Cancer 103: 1000–1007 [DOI] [PubMed] [Google Scholar]
- Miao N., Pingpank J., Alexander H., Steinberg S., Beresneva T., Quezado Z. (2008) Percutaneous hepatic perfusion in patients with metastatic liver cancer: anesthetic, hemodynamic, and metabolic considerations. Ann Surg Oncol 15: 815–823 [DOI] [PubMed] [Google Scholar]
- Moertel C. (1987) An odyessy in the land of small tumors. J Clin Oncol 5: 1502–1522 [DOI] [PubMed] [Google Scholar]
- Norheim I., Oberg K., Theodorsson-Norheim E., Lindgren P., Lundqvist G., Magnusson A., et al. (1987) Malignant carcinoid tumors. Ann Surg 206: 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noter S., Rothbarth J., Pijl M., Keunen J., Hartgrink H., Tijl F., et al. (2004) Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res 14: 67–72 [DOI] [PubMed] [Google Scholar]
- Peters S., Voelter V., Zografos L., Pampallona S., Popescu R., Gillet M., et al. (2006) Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol 17: 578–583 [DOI] [PubMed] [Google Scholar]
- Pingpank JR. (2010) Mephalan for colorectal metastases to the liver. Clin Adv Hematol Oncol 8: 175–7 [PubMed] [Google Scholar]
- Pingpank J., Libutti S., Chang R., Wood B., Neeman Z., Kam A., et al. (2005) Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol 23: 3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar T., Pizzorno G., Bodden W., Marsh J., Strair R., Pollack J., et al. (1994) Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J Clin Oncol 12: 2723–2736 [DOI] [PubMed] [Google Scholar]
- Rosenbaum C., Verkooijen H., Lam M., Smits M., Koopman M., van Seeters T., et al. (2013) Radioembolization for treatment of salvage patients with colorectal cancer liver metastases: a systematic review. J Nucl Med 54: 1890–1895 [DOI] [PubMed] [Google Scholar]
- Rothbarth J., Pijl M., Vahrmeijer A., Hartgrink H., Tijl F., Kuppen P., et al. (2003) Isolated hepatic perfusion with high-dose melphalan for the treatment of colorectal metastasis confined to the liver. Br J Surg 90: 1391–1397 [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Cox J., Butts C., Navarro M., Bang Y., Goel R., et al. (2008) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Sanoff H., Sargent D., Campbell M., Morton R., Fuchs C., Ramanathan R., et al. (2008) Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 26: 5721–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Eschelman D., Gonsalves C., Terai M., Chervoneva I., McCue P., et al. (2008) Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 26: 5436–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle K., Link K., Rieck B. (1987) Rationale and indications for perfusion in liver tumors: current data. World J Surg 11: 534–540 [DOI] [PubMed] [Google Scholar]
- Seregard S., Kock E. (1995) Prognostic indicators following enucleation for posterior uveal melanoma. Acta Opthalmol Scand 73: 340–344 [DOI] [PubMed] [Google Scholar]
- Singh A., Topham A. (2003) Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 110: 956–961 [DOI] [PubMed] [Google Scholar]
- Skibba J., Quebbeman E. (1986) Tumoricidal effects and patient survival after hyperthermic liver perfusion. Arch Surg 121: 1266–1271 [DOI] [PubMed] [Google Scholar]
- Stehlin J. (1969) Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg Gynecol Obstet 129: 305–308 [PubMed] [Google Scholar]
- Tzeng C., Aloia T. (2013) Colorectal liver metastases. J Gastrointest Surg 17: 195–201 [DOI] [PubMed] [Google Scholar]
- van Iersel L., Gelderblom H., Vahrmeijer A., van Persijn van Meerten E., Tijl F., Putter H., et al. (2008a) Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol 19: 1127–1134 [DOI] [PubMed] [Google Scholar]
- van Iersel L., Hoekman E., Gelderblom H., Vahrmeijer A., van Persijn van Meerten E., Tijl F., et al. (2008b) Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Ann Surg Oncol 15: 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel L., Koopman M., van de Velde C., Mol L., van Persijn van Meerten E., Hartgrink H., et al. (2010) Management of isolated nonresectable liver metastases in colorectal cancer patients: a case-control study of isolated hepatic perfusion with melphalan versus systemic chemotherapy. Ann Oncol 21: 1662–1667 [DOI] [PubMed] [Google Scholar]
- van Cutsom E., Bajetta E., Valle J., Kohne C., Hecht J., Moore M., et al. (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 29: 2004–2010 [DOI] [PubMed] [Google Scholar]
- Whitney R., Valek V., Fages J., Garcia A., Narayanan G., Tatum C., et al. (2011) Transarterial chemoembolization and selective internal radiation for the treatment of patients with metastatic neuroendocrine tumors: a comparison of efficacy and cost. Oncologist 16: 594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Chua T., Morris D. (2012) Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases - a systematic review. Surg Oncol 21: 299–308 [DOI] [PubMed] [Google Scholar]
- Zeh H., III, Brown C., Holtzman M., Egorin M., Holleran J., Potter D., et al. (2009) A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol 16: 385–394 [DOI] [PubMed] [Google Scholar]