Abstract
Medical oncologists who treat men with castration-resistant prostate cancer (CRPC) have seen an abundance of new agents approved by the United States Food and Drug Administration in the last decade for a disease that was previously difficult to treat after becoming resistant to androgen-deprivation therapy. Advances in understanding of the mechanisms of castration-resistance and prostate cancer progression have highlighted several pathways and targets that appear promising to better treat CRPC. As the majority of CRPC appears to continue to rely on the androgen receptor for growth and progression, several of these agents directly or indirectly target the androgen receptor. A novel microtubule-targeted agent, cabazitaxel, has demonstrated an overall survival benefit following progression on docetaxel. Other agents target tumor immunogenicity and immune checkpoint pathways to attempt to harness the host immune system. The recently approved radiopharmaceutical, radium-223 dichloride, has demonstrated impressive results in patients with extensive bony metastases with minimal toxicity. Lastly, further understanding of the pathways underlying CRPC progression has led to late-phase clinical trials with the novel agents: custirsen, tasquinimod and cabozantinib. This article reviews the approved therapies for CRPC, the agents currently in late-phase clinical trials, and notable early-phase trials of novel therapies and their combinations, with particular attention to trials incorporating novel biomarkers and intermediate endpoints to better identify those men who may or may not benefit from specific therapies.
Keywords: cabozantinib; castration-resistant prostate cancer, custirsen, radium-223 dichloride, tasquinimod
Introduction
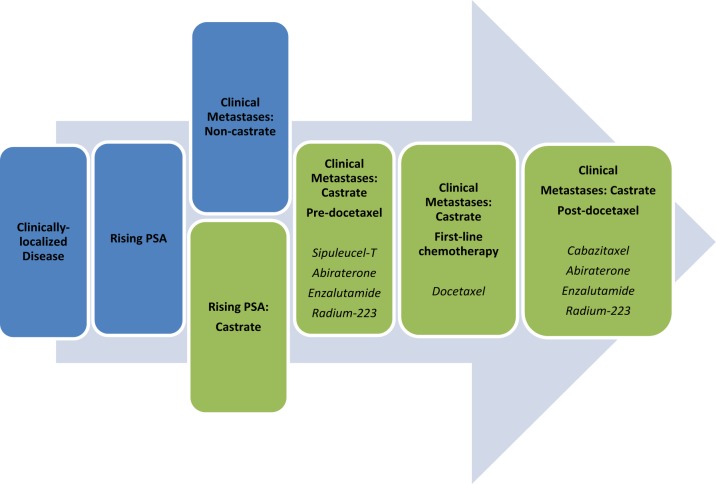
Prostate cancer is the second leading cause of cancer deaths in men. According to American Cancer Society estimates in 2013, as many as 238,590 American men will be diagnosed with prostate cancer and nearly 29,720 will die of the disease [Siegel et al. 2013]. The course of prostate cancer from diagnosis to death is best categorized as a series of clinical states (Figure 1) based on extent of disease, hormonal status (castrate versus noncastrate) and the presence/absence of detectable metastases on radiographic studies. Castration-resistant prostate cancer (CRPC) is defined by rising prostate-specific antigen (PSA) levels or progressive disease in the setting of serum testosterone levels within the castrate range (<50 ng/dl). Prior to the approval of docetaxel in 2004, therapeutic options for this disease state were limited and treatment was largely palliative. In the past several years, several new agents with a range of mechanisms have demonstrated overall survival benefits for men with CRPC and there are now six approved agents with overall survival benefits (Table 1). This paper reviews data regarding new approved agents and ongoing trials with these agents as well as with novel agents in earlier stages of clinical development.
Figure 1.
Clinical states of prostate cancer [adapted from Scher et al. 2008].
Table 1.
Agents with overall survival benefit in mCRPC.
| Agent | Trial | Patient population | Comparator | Overall survival | Hazard ratio |
|---|---|---|---|---|---|
| Docetaxel plus prednisone | TAX 327 [Tannock et al. 2004] | First-line mCRPC, chemotherapy-naïve | Mitoxantrone plus prednisone | 18.9 versus 16.5 months | 0.76 |
| Sipuleucel-T | IMPACT [Kantoff et al. 2010a] | First-line mCRPC, asymptomatic or minimally-symptomatic (15% were postdocetaxel) | Placebo | 25.8 versus 21.7 months | 0.78 |
| Abiraterone plus prednisone | COU-AA-302 [Fizazi et al. 2012] | Second-line mCRPC, postdocetaxel | Prednisone | 14.8 versus 10.9 months | 0.65 |
| Abiraterone plus prednisone | COU-AA-301 [Ryan et al. 2013] | First-line mCRPC, predocetaxel | Prednisone | 35.3 versus 30.1 months | 0.79 |
| Enzalutamide | AFFIRM [Scher et al. 2012] | Second-line mCRPC, postdocetaxel | Placebo | 18.4 versus 13.6 months | 0.63 |
| Enzalutamide | PREVAIL [Beer et al. 2014] | First-line mCRPC, predocetaxel | Placebo | 32.4 versus 30.2 months | 0.70 |
| Cabazitaxel plus prednisone | TROPIC [De Bono et al. 2010] | Second-line mCRPC, postdocetaxel | Mitoxantrone plus prednisone | 15.1 versus 12.7 months | 0.70 |
| Radium-223 dichloride | ALSYMPCA [Parker et al. 2013] | Second-line, postdocetaxel (or unable/unwilling to receive docetaxel), with symptomatic bone metastases and no visceral involvement | Placebo | 14.9 versus 11.2 months | 0.70 |
mCRPC, metastatic castration-resistant prostate cancer.
Novel hormonal agents
Historically, prostate cancer progression after surgical or medical castration led to the belief that additional hormonal manipulations in the setting of CRPC would be futile. However, there has been a growing recognition that persistent androgen receptor (AR) signaling despite ‘castrate’ levels of serum androgens (testosterone <50 ng/dl) indicates that many tumors in this state remain sensitive to further targeting of this pathway. Recently, two drugs that ultimately function by decreasing AR signaling, abiraterone acetate and enzalutamide, have demonstrated an overall survival benefit and have been approved by the US Food and Drug Administration (FDA). Abiraterone has been approved both in the postdocetaxel space and in the first-line setting, and enzalutamide is currently approved in the postdocetaxel setting while it is also being tested in the prechemotherapy space. Several other promising compounds modulating the androgen–AR axis are currently in clinical trials (Table 2).
Table 2.
Notable ongoing phase II and III trials in CRPC and biochemical recurrence.
| Agent | Trial name (if available) | Phase | Patient population | Comparator | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Abiraterone acetate + prednisone + veliparib (ABT-888) | NCI-2012-01149 | II | Pre or postchemotherapy | Abiraterone acetate + predinsone | NCT01576172 |
| Orteronel + prednisone | ELM-PC 4 | III | Chemotherapy-naïve | Placebo + prednisone | NCT01193244 |
| Galeterone | ARMOR2 | II | Chemotherapy-naïve | None | NCT01709734 |
| ARN-509 ± LHRH agonist | II | Biochemical relapse | LHRH agonist | NCT01790126 | |
| Cabazitaxel 20mg/m2 + prednisone | PROSELICA | III | Post-docetaxel | Cabazitaxel 25mg/m2 + prednisone | NCT01308580 |
| Cabazitaxel at either 25mg/m2 or 20mg/m2 + prednisone | FIRSTANA | III | Chemotherapy-naïve | Docetaxel + prednisone | NCT01308567 |
| Docetaxel + prednisone followed by cabazitaxel + prednisone (if nonresponse) | TAXYNERGY | II | Chemotherapy-naive | Cabazitaxel + prednisone followed by docetaxel + predisone (if non-response) | NCT01718353 |
| ADT then sipuleucel-T | STAND | II | Biochemical relapse | Sipuleucel-T then ADT | NCT01431391 |
| Sipuleucel-T + concurrent abiraterone acetate + prednisone | P11-3 | II | Pre-abiraterone | Sipuleucel-T + sequential abiraterone acetate + predisone | NCT01338012 |
| ProstVac ± GM-CSF | PROSPECT | III | Chemotherapy-naive | Placebo | NCT01322490 |
| Ipilimumab | CA184-095 | III | Chemotherapy-naive | Placebo | NCT01057810 |
| Radium-223 dichloride + docetaxel + prednisone | I/IIa | Chemotherapy-naïve | Docetaxel + prednisone | NCT01106352 | |
| Custirsen + docetaxel + prednisone | SYNERGY | III | Chemotherapy-naive | Docetaxel + prednisone | NCT01188187 |
| Custirsen + cabazitaxel + prednisone | AFFINITY | III | Postdocetaxel | Cabazitaxel + prednisone | NCT01578655 |
| Tasquinimod | 10TASQ10 | III | Chemotherapy-naïve | Placebo | NCT01234311 |
| Tasquinimod | II | Postdocetaxel with response/stabilization | Placebo | NCT01732549 | |
| Cabozantanib | COMET-1 | III | Postdocetaxel and post-abiraterone or enzalutamide | Prednisone | NCT01605227 |
| Cabozantanib | COMET-2 | III | Postdocetaxel and post-abiraterone or enzalutamide; symptomatic bone metastases | Mitoxantrone + prednisone | NCT01522443 |
Note: bold indicates the active agent(s) being evaluated in the study.
Abiraterone
Building on evidence of the activity of ketoconazole through relatively nonspecific blockade of androgen synthesis pathways in the metastatic CRPC (mCRPC) setting, abiraterone acetate, a selective inhibitor of the CYP17 enzyme, was developed [Bryce et al. 2011]. Abiraterone has been shown to block androgen biosynthesis by the adrenal glands and testes (and within the prostate tumor) without antagonizing the CYP11B1 and CYP11B2 enzymes responsible for glucocorticoid and mineralocorticoid synthesis, unlike ketoconazole [Small et al. 2004]. Treatment with abiraterone reduces serum testosterone levels from 20–50 ng/dl with surgical castration or gonadotropin-release hormone analogues alone to 1–2 ng/dl, leading to a ‘super-castrate’ state. In addition to decreases seen in the serum, CYP17 inhibition also leads to decreased intratumoral testosterone synthesis within the prostate cancer cells themselves.
The COU-AA-301 trial evaluated abiraterone plus prednisone in the postdocetaxel setting versus prednisone alone. In these men, who were previously thought to be hormone-refractory, abiraterone significantly improved overall survival [hazard ratio (HR) = 0.65] with side effects that were mild and limited largely to liver function test abnormalities and elevated levels of mineralocorticoids (i.e. hypokalemia, hypertension, fluid retention) [Fizazi et al. 2012]. The use of abiraterone following docetaxel was thus approved by the FDA in April 2011.
In the follow-up COU-AA-302 trial, men with mCRPC who were chemotherapy-naïve were again randomized either to abiraterone plus prednisone or placebo plus prednisone. The second planned interim analysis found that the study had exceeded its stopping rules after 43% of the expected deaths had occurred. Abiraterone demonstrated significantly prolonged progression-free survival (HR = 0.53) with a trend towards overall survival (HR = 0.75) that, given the number of events, did not reach statistical significance according to the O’Brien–Fleming boundary [Ryan et al. 2013]. With these data, and despite the absence of a statistically significant survival difference, the FDA expanded the use of abiraterone in chemotherapy-naïve patients with mCRPC in December 2012.
In the COU-AA-301 and COU-AA-302 trials, a significant minority of patients demonstrated primary resistance to abiraterone. Accordingly, there has been interest in identifying biomarkers that may identify abiraterone-sensitive tumors to allow more targeted therapy. One potential biomarker, the TMPRSS2-ERG fusion gene, is the most common gene fusion in prostate cancer, present in approximately 50% of newly diagnosed prostate cancer, and fuses an androgen-dependent growth factor to a transcription factor. A study of 77 patients treated with abiraterone found that 80% of patients who experienced a >90% decline in PSA levels were TMPRSS2-ERG fusion positive [Attard et al. 2009]. In a subsequent analysis (n = 497) of the COU-AA-302 study, the same investigators showed a trend towards superior progression-free survival in abiraterone-treated patients with ERG rearrangements in tumor specimens [22 versus 16 months; HR 0.59; 95% confidence interval (CI): 0.30–1.16; p = 0.12], while this was not the case in placebo-treated patients [Attard et al. 2013]. However, in a study that examined whether the fusion gene may play a role as a predictive biomarker of abiraterone, the investigators found that TMPRSS2-ERG status in circulating tumor cells did not predict abiraterone sensitivity, though this analysis was limited by a small number of patients [Danila et al. 2011]. Based in part on the finding that poly(ADP-ribose) polymerase-1 (PARP-1) may be required for the malignant phenotype in ERG positive cells [Schiewer et al. 2012], a phase II trial adding a PARP-inhibitor, veliparib, to abiraterone is currently underway. Men with mCRPC will be stratified for the presence of the TMPRSS2-ERG fusion and randomized either to abiraterone plus veliparib or abiraterone alone [ClinicalTrials.gov identifier: NCT01576172].
Enzalutamide
Enzalutamide is a next-generation anti-androgen with significantly increased affinity for AR compared with prior anti-androgens. Furthermore, enzalutamide prevents nuclear translocation and coactivator recruitment of the ligand–receptor complex. Compared with androgen synthesis inhibitors such as abiraterone, a potential advantage is that the use of corticosteroids is not required. The phase III AFFIRM trial evaluated enzalutamide versus placebo in the postdocetaxel setting. At a planned interim analysis, the trial was unblinded after demonstrating significantly improved overall survival (HR = 0.63). The drug was generally well-tolerated with fatigue, diarrhea and hot flushes more common in the enzalutamide group. Notably, about 1% of patients in the enzalutamide group developed seizures versus no patients in the placebo arm [Scher et al. 2012]. On the basis of these results, the FDA approved the use of enzalutamide in the postdocetaxel setting in August 2012. The PREVAIL trial, which evaluated the role of enzalutamide in the prechemotherapy setting, recently released the results of its first planned interim analysis, demonstrating an overall survival advantage with enzalutamide versus placebo (median 32.4 versus 30.2 months; HR 0.70; 95% CI: 0.59–0.83; p < 0.0001). In addition, there was a very significant improvement in progression-free survival with enzalutamide compared with placebo (HR 0.19; 95% CI: 0.15–0.23; p < 0.0001). As a result, the data and safety monitoring committee recommended stopping the trial. Complete results including safety data are pending, as is FDA approval for enzalutamide in the prechemotherapy setting [Beer et al. 2014].
In the hormone-naïve setting, Smith and colleagues are evaluating enzalutamide as monotherapy as an alternative to androgen-deprivation therapy. A phase II single-arm trial found that tumor response rates, as measured by PSA and radiographic responses, were roughly consistent with androgen-deprivation (92.5% rate of PSA decline of ≥80% at week 25). Adverse events were also essentially consistent with androgen-deprivation, although notably no decreases in bone mineral density were seen and adverse changes in lipid profiles were somewhat less than seen historically with androgen-deprivation [Smith et al. 2013b]. However, the short duration of the trial limited the evaluation of long-term adverse events such as changes in lipid profiles, cardiovascular risk and adiposity. A potential concern with the use of enzalutamide monotherapy is a feedback increase in circulating androgen levels, which was also seen in this study, although no information was available about the occurrence of a tumor flare when patients were taken off enzalutamide monotherapy (as has been seen with discontinuation of bicalutamide monotherapy).
One of the theoretical advantages of enzalutamide over other anti-androgens (such as bicalutamide) is that this agent was previously thought not to act as a partial agonist of the AR. However, recent data suggest that this is not uniformly true. To this end, an activating mutation (F876L) has now been identified in the ligand-binding domain of the AR that causes a conformational change in the binding site such that enzalutamide functions as an agonist in this setting [Balbas et al. 2013; Joseph et al. 2013]. The incidence of this activating mutation is not yet known, but it appears to result only after continued use of enzalutamide and may indicate selection pressure.
Other hormonal agents in development
There are several promising hormonal agents currently in development. Orteronel (formerly TAK-700) is an oral, nonsteroidal 17,20-lyase inhibitor with high specificity for its target. A phase I/II dose escalation trial demonstrated profound testosterone suppression with testosterone levels that decreased to <1 ng/dl in all but one patient. In the phase II portion of the trial, 52% of patients saw PSA decreases greater than 50% [Dreicer et al. 2014]. Orteronel was initially evaluated in this trial without corticosteroids at the 300mg twice daily (BID) dose, which has been taken forward as the therapeutic dose, without frequent signs of mineralocorticoid excess, raising the possibility of a steroid-free treatment regimen. Nevertheless, phase III trials did add concurrent prednisone. In a prespecified interim analysis of a phase III trial comparing orteronel plus prednisone versus prednisone alone in the postchemotherapy setting, the data and safety monitoring committee determined that the trial would be unlikely to meet the primary endpoint of overall survival (HR 0.89; p = 0.23) and the trial was subsequently halted and unblinded [Dreicer et al. 2014]. A phase III trial comparing orteronel plus prednisone versus prednisone alone is currently ongoing in the chemotherapy-naïve population.
Galeterone (formerly TOK-001) is a CYP17 inhibitor with three putative mechanisms of action including CYP17 inhibition, AR antagonism and decrease in intratumoral AR levels. Preclinical studies have demonstrated an IC50 for both wildtype and mutant AR superior to bicalutamide. In the ARMOR1 phase I trial, 22% of patients demonstrated a >50% decrease in PSA with an overall mild side effect profile [Montgomery et al. 2012]. A phase II trial (ARMOR2) that will randomize chemotherapy-naïve patients to galeterone (without corticosteroids) versus placebo is currently ongoing.
ARN-509 is a selective AR antagonist without agonist activity and demonstrated greater potency in preclinical trials than enzalutamide, and less central nervous system penetration [Clegg et al. 2012]. In the phase II portion of a phase I/II trial in men with high risk non-mCRPC, preliminary results of ARN-509 demonstrate PSA decreases of >50% in 91% of patients. In the mCRPC setting, 88% of chemotherapy and abiraterone-naïve patients demonstrated PSA decreases of >50% as did 29% of patients pretreated with abiraterone. In these small studies, ARN-509 was well-tolerated and notably no patients as yet had developed seizure [Rathkopf et al. 2012; Smith et al. 2012]. Mature data from the above-mentioned phase II trial are not yet available. ARN-509 is also being evaluated in combination with abiraterone in a phase Ib trial [ClinicalTrials.gov identifier: NCT01792687] and with and without a luteinizing hormone-releasing hormone (LHRH) agonist in men with biochemically-relapsed prostate cancer [ClinicalTrials.gov identifier: NCT01790126].
Additional hormonal agents in early stages of development include ODM-201, VT-464 and AZD-3514. ODM-201 is an AR antagonist with superior potency to enzalutamide and in preclinical models does not cross the blood–brain barrier, which may decrease the potential for seizure. Preliminary results from a phase I/II trial suggested a mild side-effect profile with PSA decreases of >50% in 87% of patients [Fizazi et al. 2013]. VT-464 is a CYP17 inhibitor currently in phase I/II trials and is being developed without concurrent corticosteroids due to preclinical evidence of minimal effect on upstream steroid levels [Eisner et al. 2012]. AZD-3514 is an AR antagonist that both inhibits AR nuclear translocation and has also been demonstrated to decrease intratumoral AR levels, unlike enzalutamide. In a phase I study, several PSA responses were observed using this agent, but clinical development of AZD-3514 has been halted due to toxicity concerns [Loddick et al. 2012; Omlin et al. 2013].
Chemotherapy
Docetaxel in combination with prednisone was approved in 2004 for the treatment of mCRCP and was the first agent to demonstrate an overall survival benefit in the castration-resistant setting, with a 2.4 month survival benefit compared with mitoxantrone plus prednisone (HR 0.76; 95% CI: 0.62–0.94) [Tannock et al. 2004]. However, the use of chemotherapy in the postdocetaxel setting has represented an area of unmet medical need until recently.
To address this need, a randomized phase III study compared a novel taxane agent, cabazitaxel, 25 mg/m2 intravenously (IV) every 3 weeks plus prednisone with mitoxantrone 12 mg/m2 IV every 3 weeks in 755 patients with mCRPC after treatment with docetaxel-containing chemotherapy. This trial demonstrated an increase in overall survival in favor of cabazitaxel compared with mitoxantrone (HR = 0.70; 95% CI: 0.59–0.83; p < 0.0001), the first time that a second-line chemotherapy had shown a survival benefit. Grade 3–4 neutropenia, however, was frequent with cabazitaxel compared with mitoxantrone (81.7% versus 58%, respectively) with a concomitant increase in severe infections including neutropenic infections [De Bono et al. 2010]. Pooled phase I/II safety data suggested that doses of cabazitaxel <25mg/m2 showed a significantly decreased incidence of neutropenia. A phase III randomized, open-label, noninferiority trial, PROSELICA, is currently underway comparing the efficacy and toxicity of cabazitaxel 20 mg/m2 plus prednisone versus cabazitaxel 25mg/m2 plus prednisone in the postdocetaxel space.
In the first-line setting, the FIRSTANA trial will examine cabazitaxel plus prednisone at 20mg/m2 and 25mg/m2 versus docetaxel (75 mg/m2) plus prednisone in patients with chemotherapy-naïve mCRPC. This study has recently completed enrollment. Another randomized phase II study, called TAXYNERGY, will also evaluate cabazitaxel versus docetaxel in the first-line setting. It is designed to determine whether an early switch from one taxane to another may provide clinical benefit and is powered to determine the superiority of an early switch with respect to the primary endpoint of PSA response rate (≥50% PSA reduction from baseline). In this trial, patients are randomized to either first-line docetaxel or cabazitaxel and, if they do not achieve a ≥30% PSA reduction after four cycles of treatment, they will switch to the alternative taxane agent [Antonarakis et al. 2013a]. This trial will also evaluate multiple markers of clinical taxane resistance, including interrogation of circulating tumor cells (CTCs) to determine if AR translocation, which is facilitated by microtubule-mediated nuclear transport, is taking place [Darshan et al. 2011].
Immunotherapies
Sipuleucel-T
Sipuleucel-T, an autologous prostatic acid phosphatase (PAP) directed cell-based immunotherapy product manufactured using patients’ own antigen-presenting cells, was approved by the FDA for men with minimally symptomatic or asymptomatic mCRPC. The IMPACT trial demonstrated a HR for overall survival of 0.78 (median survival 25.8 versus 21.7 months) for sipuleucel-T versus placebo in these patients despite no difference in proximal endpoints including PSA or radiographic tumor responses [Kantoff et al. 2010a].
Recent studies have focused on sequencing and concurrent administration of immunotherapies with other active agents in prostate cancer [Antonarakis and Drake, 2012]. Given that androgen deprivation therapy (ADT) has immunomodulatory effects [Drake et al. 2005], there has been interest in evaluating the optimal sequencing of sipuleucel-T and ADT. The STAND trial evaluated this sequence in men with biochemically recurrent prostate cancer with high risk for metastases secondary to rapid PSA doubling time (<12 months). A total of 68 men were randomized to receiving sipuleucel-T followed by ADT, or ADT followed by sipuleucel-T [Antonarakis et al. 2013b]. There was a greater increase in TH1 cytokines in the arm with ADT as initial therapy, suggesting that ADT may enhance T-cell activity and the combination may augment adaptive immunity. Follow up is ongoing to determine whether there will be differences in clinical responses.
To explore the question of whether concurrent prednisone use with abiraterone may prove sufficiently immunosuppressive to abrogate immunologic response to sipuleucel-T, the P11-3 trial was designed to evaluate the combination of sipuleucel-T and abiraterone acetate plus prednisone [Small et al. 2013]. Men with mCRPC were randomized to sipuleucel-T with either concurrent or sequential abiraterone/prednisone. A preliminary analysis demonstrated no difference in the magnitude of immunological response between the two arms, suggesting that the two therapies may be able to be administered concurrently without loss of efficacy. Lastly, because the IMPACT trial excluded patients who received docetaxel within the preceding 3 months, the PROCEED trial has enrolled men without restriction with regard to prior chemotherapy as part of a phase IV registry [Sartor et al. 2013]. Patients who received docetaxel prior to sipuleucel-T administration had similar numbers of antigen presenting cells in their product, although with a slightly lower number of activated cells. Further follow up will be needed to determine the clinical significance of these findings.
ProstVac-VF
A novel immunologic therapy, ProstVac-VF, is a PSA-targeted poxviral-based vaccine, administered with three costimulatory molecules (known as TRI-COM) to increase PSA-specific immune responses. A phase II trial demonstrated improved overall survival versus placebo in men with mCRPC with a HR of 0.56 without impact on proximal clinical endpoints, similar to sipuleucel-T [Kantoff et al. 2010b]. A randomized placebo-controlled multicenter phase III trial (PROSPECT) is currently ongoing and will evaluate three arms: ProstVac-VF plus adjuvant GM-CSF, ProstVac-VF plus placebo and placebo-only [ClinicalTrials.gov identifier: NCT01322490]. This study is powered to detect an overall survival difference in either of the two ProstVac-VF arms compared with the placebo arm.
Ipilimumab
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) blockade via the monoclonal antibody ipilimumab has attracted attention due to positive results in patients with metastatic melanoma [Hodi et al. 2010] as well as promising activity in renal cell carcinoma, non-Hodgkin lymphoma, as well as several other malignancies [Ansell et al. 2009; Yang et al. 2007]. Toxicities are typically immune-related and most frequently involve the gut, skin and liver as well as auto-immune endocrine adverse events. Tumor cells have been demonstrated to stimulate regulatory T cells in the microenvironment leading to immune tolerance despite antigen-specific immune responses [Kiniwa et al. 2007]. Ipilimumab is thought to break this tolerance by reducing intratumoral regulatory T-cells [Selby et al. 2013] and has demonstrated anti-tumor activity in preclinical evaluation in prostate cancer. [Kwon et al. 1997] Unlike the other active immunotherapies discussed above, the use of ipilimumab in men with mCRPC has been associated with a 20% PSA response rate and a 5% objective response rate among men with measurable disease [Slovin et al. 2013]. Ipilimumab is thus currently being evaluated in the prechemotherapy and postchemotherapy settings in men with mCRPC. In the postchemotherapy space, the agent has been combined with localized radiotherapy to promote a putative abscopal effect (i.e. immune-mediated anti-tumor activity at a site distant to a locally-radiated tumor) [Postow et al. 2012]. Promising evidence of activity was seen in a phase I/II trial of ipilimumab following a single fraction of radiation per bone lesion, suggesting that increased delivery of tumor antigens to antigen-presenting cells in this fashion may augment the immune response [Slovin et al. 2013]. However, the phase III trial of this regimen in men with previously treated CRPC failed to demonstrate a significant overall survival benefit (HR 0.85; 95% CI: 0.72–1.00; p = 0.053), although there was a notable progression-free survival benefit (HR 0.70; 95% CI: 0.61–0.82; p < 0.0001), as well as improved survival in a prespecified subset with lower disease burden (normal hemoglobin, normal alkaline phosphatase, no visceral metastases) [Gerritsen et al. 2013]. In the first-line setting, a phase III trial investigating ipilimumab versus placebo prior to chemotherapy is also underway and completed enrollment in January 2013 [ClinicalTrials.gov identifier: NCT01057810].
Nivolumab
Programmed death 1 (PD-1) is another immune-checkpoint receptor expressed by activated T cells which mediates immunosuppression. An anti-PD-1 antibody (nivolumab) that blocks the binding of PD-1 to PD-L1 (as well as PD-L2) was tested in several solid tumors in a phase I dose escalation trial [Topalian et al. 2012]. A small number of men with CRPC were enrolled in this trial. Although this therapy was well-tolerated, no objective responses were observed in mCRPC patients, although one man had a 28% reduction in measurable lesions. Of note, in the two CRPC pretreatment tumor specimens tested for PD-L1 expression, both were negative. Nevertheless, given the dramatic and durable responses demonstrated in other tumor types, this may be an area deserving further study. Finally, the combination of anti-CTLA4 and anti-PD1 therapies appears to produce unprecedented clinical activity in patients with advanced melanoma [Wolchok et al. 2013] and this approach may be warranted in men with mCRPC.
Radiopharmaceuticals
Radium-223 dichloride is a radiopharmaceutical that acts as a calcium mimetic, targeting new bone growth near bone metastases. Unlike other radioisotopes, i.e. strontium-89 and samarium-153, which emit β particles, radium-223 emits α particles which penetrate a relatively shorter distance to deliver higher energy to tumor tissues, in theory leading to double-strand DNA breaks in tumor cells with relatively minimal damage to surrounding normal tissue [Goyal and Antonarakis, 2012]. In the phase III ALSYMPCA trial, men with symptomatic mCRPC and extensive bony metastatic disease (but without visceral disease) were randomized to radium-223 versus placebo. Patients either had to have previously received docetaxel chemotherapy, or they had to be unfit for docetaxel, or they had to turn down docetaxel. Ongoing osteoclast-inhibitory therapy was permitted, together with best standard of care.
The trial demonstrated a significant overall survival advantage for radium-223 (HR = 0.70) as well as improved time to symptomatic skeletal events (HR = 0.66), with relatively minimal toxicity manifested primarily by thrombocytopenia [Parker et al. 2013]. The survival benefit was maintained regardless of prior docetaxel use and regardless of concurrent bisphosphonate use. On the basis of this trial, the FDA approved the use of radium-223 on 15 May 2013 for men with symptomatic bone mCRPC without known visceral metastases. Ongoing trials with this agent include a phase I/IIa study of the efficacy and safety of radium-223 when used concurrently with docetaxel [ClinicalTrials.gov identifier: NCT01106352] [Morris et al. 2013].
Non-hormonal small molecule inhibitors
Custirsen
Clusterin is a cytoprotective chaperone protein induced by various stressors including chemotherapy, androgen ablation and radiation, as well as by AR signaling. It may promote prostate cancer treatment-resistance and progression through anti-apoptotic properties including Bcl-2 and Bax signaling [Zhang et al. 2005]. Increased clusterin expression has been correlated with increased Gleason score and androgen deprivation [July et al. 2002; Steinberg et al. 1997]. Custirsen (formerly OGX-011) is a second-generation antisense oligonucleotide complementary to the clusterin mRNA translation initiation site that forms RNA duplexes and inhibits clusterin expression. In a phase II trial in which men with mCRPC were randomized to docetaxel plus prednisone with or without custirsen, the addition of custirsen did not appear to add significant toxicity to docetaxel plus prednisone and resulted in self-limited fevers and rigors as well as lymphopenia. PSA responses and objective responses were similar between the two arms. However, the custirsen-containing arm demonstrated a trend towards superior progression-free survival (HR = 0.86) and superior overall survival (HR = 0.61), although survival was an exploratory analysis in this study. [Chi et al. 2010] Based on these encouraging results, two phase III trials are currently ongoing. The SYNERGY trial randomizes men with mCRPC to first-line docetaxel plus prednisone with or without custirsen and completed enrollment in November 2012 [ClinicalTrials.gov identifier: NCT01188187]. The AFFINITY trial randomizes men in the second-line setting to cabazitaxel plus prednisone with or without custirsen, and is currently recruiting patients [ClinicalTrials.gov identifier: NCT01578655].
Tasquinimod
Tasquinimod is an oral quinolone-3-carboxamide derivative with anti-angiogenic and preclinical tumor growth inhibition properties. Although its target has previously been unclear, recent research suggests that tasquinimod binds the histone deacetylase HDAC4 to block the action of client factors including hypoxia-inducible factor 1-alpha (HIF-1α), which mediates angiogenesis in a hypoxic tumor microenvironment [Isaacs et al. 2013]. An additional mechanism may be induction of thrombospondin-1, an endogenous angiogenesis inhibitor, possibly via inhibition of S100A, an immunomodulatory protein expressed on myeloid-derived suppressor cells [Olsson et al. 2010].
In a phase II study, tasquinimod demonstrated significantly improved progression-free survival in men with mCRPC in the chemotherapy-naïve setting (HR = 0.49) [Pili et al. 2011]. Side effects were moderate although 22% of patients (versus 1% for placebo) discontinued therapy due to toxicity, predominantly muscle/joint pain with grade 3–4 toxicities most commonly manifested as asymptomatic laboratory abnormalities including elevated lipase and anemia. A phase III trial in asymptomatic to mildly symptomatic men with mCRPC in the chemotherapy-naïve setting is currently ongoing [ClinicalTrials.gov identifier: NCT01234311] and enrollment is now complete. Additionally, a phase II trial is recruiting patients with mCRPC following response or stabilization on first-line docetaxel to determine if tasquinimod maintenance therapy may further improve outcomes [ClinicalTrials.gov identifier: NCT01732549].
Cabozantinib
Cabozantinib (formerly XL-184) is an oral tyrosine kinase inhibitor of mesenchymal-epithelial transition factor (MET) and vascular endothelial growth factor receptor 2 (VEGFR2), both of which have been implicated in the progression of CRPC [George et al. 2001; Knudsen et al. 2002]. In a phase II randomized discontinuation trial, patients with mCRPC received cabozantinib for 12 weeks and those without evidence of progression of disease were then randomized to cabozantinib or placebo. The randomization was halted due to early evidence of activity of cabozantinib with respect to improved bone scans and pain. Progression-free survival was significantly increased compared with the placebo arm (HR = 0.12) with improvement in bone pain, suggesting that the clinical efficacy of cabozantinib may be secondary to effects on the bone microenvironment. Notably, PSA changes did not correlate with other markers of clinical activity with this agent. The most common grade-3 or greater adverse events included fatigue, hypertension and hand-foot syndrome [Smith et al. 2013a].
Encouraged by these results, two phase III trials are currently ongoing. The COMET-1 trial is designed to evaluate a potential survival benefit in men with previously treated mCRPC randomly assigned to cabozantinib or prednisone [ClinicalTrials.gov identifier: NCT01605227]. Patients in this trial are required to have previously received docetaxel, as well as either abiraterone or enzalutamide. The COMET-2 trial will compare the palliative effects of cabozantinib versus mitoxantrone-prednisone in men with previously-treated symptomatic mCRPC [ClinicalTrials.gov identifier: NCT01522443]. Patients in this trial are also required to have received docetaxel as well as abiraterone/enzalutamide, and are also required to have cancer-related pain graded ≥4/10 despite the use of both a long-acting and short-acting narcotic analgesic. Finally, a phase I study combining abiraterone plus cabozantinib is currently underway [ClinicalTrials.gov identifier: NCT01574937].
Conclusion
There have been rapid advancements in the treatment of CRPC with five new life-prolonging agents approved in the last 3 years alone. Further research is needed with respect to sequencing of therapy to determine the optimal series of treatments for an individual patient. There is also a need to evaluate combinations of these therapies that may prove more effective than any agent given alone. Lastly, a role for biomarkers to select patients that may (or may not) benefit from a particular therapy will need to be elucidated. Ongoing trials using agents with novel mechanisms of action including immunotherapies and nonhormonal small molecules inhibitors may further revolutionize the therapeutic landscape, but will also raise further questions. Results of several trials over the next few years promise an exciting era in the field which will hopefully further improve the outcomes of men with advanced prostate cancer.
Footnotes
Funding: E.S.A. is partially funded by NIH grant P30 CA006973.
Conflict of interest statement: D.S. declares no conflicts of interest in preparing this article. E.S.A. is a paid consultant/advisor to Janssen Biotech, and has received research funding from Janssen. E.S.A. is also a paid consultant/advisor to Dendreon and Sanofi.
Contributor Information
Daniel L. Suzman, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, USA
Emmanuel S. Antonarakis, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans Street, CRB1-1M45, Baltimore, MD 21287, USA
References
- Ansell S., Hurvitz S., Koenig P., Laplant B., Kabat B., Fernando D., et al. (2009) Phase I study of ipilimumab, an anti–CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 15: 6446–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis E., Drake C. (2012) Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol 24: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis E., Giannakakou P., Kirby B., Nicacio L., Eisenberger M., Nanus D., et al. (2013a) Taxynergy (NCT01718353): a randomized phase II trial examining an early switch from first-line docetaxel to cabazitaxel, or cabazitaxel to docetaxel, in men with metastatic castration-resistant prostate cancer (MCRPC). ASCO Meeting Abstracts 31: TPS5100. [Google Scholar]
- Antonarakis E., Kibel A., Adams G., Karsh L., Elfiky A., Shore N., et al. (2013b) A randomized phase II study evaluating the optimal sequencing of sipuleucel-T and androgen deprivation therapy (ADT) in biochemically recurrent prostate cancer (BRPC): immune results. ASCO Meeting Abstracts 31: 5016 [Google Scholar]
- Attard G., De Bono J., Li W., Molina A., Griffin T., Kheoh T., et al. (2013) ERG rearrangements and association with clinical outcome in patients (PTS) receiving abiraterone acetate (AA): results from the COU-AA-302 study in chemotherapy (chemo)-naive metastatic castration-resistant prostate cancer (MCRPC). ASCO Meeting Abstracts 31: 5004 [Google Scholar]
- Attard G., Swennenhuis J., Olmos D., Reid A., Vickers E., A’hern R., et al. (2009) Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 69: 2912–2918 [DOI] [PubMed] [Google Scholar]
- Balbas M., Evans M., Hosfield D., Wongvipat J., Arora V., Watson P., et al. (2013) Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2: e00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer T., Armstrong A., Sternberg C., Higano C., Iverson P., Loriot Y., et al. (2014). Enzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC): Results of phase III PREVAIL study. ASCO Meeting Abstracts 32: LBA1 [Google Scholar]
- Bryce A., Ryan C. (2011) Development and Clinical Utility of Abiraterone Acetate as an Androgen Synthesis Inhibitor. Clinical Pharmacology & Therapeutics. 91: 101–108 [DOI] [PubMed] [Google Scholar]
- Scher H., Fizazi K., Saad F., Taplin M., Sternberg C., Miller K., et al. (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367: 1187–1197 [DOI] [PubMed] [Google Scholar]
- Chi K., Hotte S., Evan Y., Tu D., Eigl B., Tannock I., et al. (2010) Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. ASCO Meeting Abstracts 28:4247–4254 [DOI] [PubMed] [Google Scholar]
- Clegg N., Wongvipat J., Joseph J., Tran C., Ouk S., Dilhas A., et al. (2012) ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res 72: 1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila D., Anand A., Sung C., Heller G., Leversha M., Cao L., et al. (2011) TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol 60: 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darshan M., Loftus M., Thadani-Mulero M., Levy B., Escuin D., Zhou X., et al. (2011) Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res 71: 6019–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono J., Oudard S., Ozguroglu M., Hansen S., Machiels J., Kocak I., et al. (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376: 1147–1154 [DOI] [PubMed] [Google Scholar]
- Drake C., Doody A., Mihalyo M., Huang C., Kelleher E., Ravi S., et al. (2005) Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 7: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreicer R., Jones R., Oudard S., Efstathiou E., Saad F., De Wit R., et al. (2014) Results from a phase 3, randomized, double-blind, multicenter, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel-based therapy (ELM-PC 5 trial). ASCO Meeting Abstracts 32: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner J., Abbott D., Bird I., Rafferty S., Moore W., Schotzinger R. (2012) VT-464: A novel, selective inhibitor of P450c17(CYP17)-17,20 lyase for castration-refractory prostate cancer (CRPC). ASCO Meeting Abstracts 30: 198 [Google Scholar]
- Fizazi K., Massard C., James N., Culine S., Jones R., Oksala R., et al. (2013) ODM-201, a new generation androgen receptor inhibitor for castration-resistant prostate cancer: preclinical and phase i data. ASCO Meeting Abstracts 31: 65 [Google Scholar]
- Fizazi K., Scher H., Molina A., Logothetis C., Chi K., Jones R., et al. (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13: e464. [DOI] [PubMed] [Google Scholar]
- George D., Halabi S., Shepard T., Vogelzang N., Hayes D., Small E., et al. (2001) Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 7: 1932–1936 [PubMed] [Google Scholar]
- Gerritsen W., Kwon E., Fizazi K., Bossi A., Van Den Eertwegh A., Logothetis C., et al. (2013) CA184-043: a randomized, multicenter, double-blind phase 3 trial comparing overall survival (OS) in patients (PTS) with post-docetaxel castration-resistant prostate cancer (CRPC) and bone metastases treated with ipilimumab (IPI) versus placebo (PBO), each following single-dose radiotherapy (RT). European Journal of Cancer. 49: S678–S679 [Google Scholar]
- Goyal J., Antonarakis E. (2012) Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett 323: 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J., Antony L., Dalrymple S., Brennen W., Gerber S., Hammers H., et al. (2013) Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res 73: 1386–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Lu N., Qian J., Sensintaffar J., Shao G., Brigham D., et al. (2013) A clinically relevant androgen receptor mutation confers resistance to 2nd generation anti-androgens enzalutamide and ARN-509. Cancer Discov 3: 1020–1029 [DOI] [PubMed] [Google Scholar]
- July L., Akbari M., Zellweger T., Jones E., Goldenberg S., Gleave M. (2002) Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate 50: 179–188 [DOI] [PubMed] [Google Scholar]
- Kantoff P., Higano C., Shore N., Berger E., Small E., Penson D., et al. (2010a) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422 [DOI] [PubMed] [Google Scholar]
- Kantoff P., Schuetz T., Blumenstein B., Glode L., Bilhartz D., Wyand M., et al. (2010b) Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28: 1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiniwa Y., Miyahara Y., Wang H., Peng W., Peng G., Wheeler T., et al. (2007) CD8+ FOXP3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 13: 6947–6958 [DOI] [PubMed] [Google Scholar]
- Knudsen B., Gmyrek G., Scherr D., Vaughan E., Nanus D., Kattan M., et al. (2002) High expression of the Met receptor in prostate cancer metastasis to bone. Urology 60: 1113–1117 [DOI] [PubMed] [Google Scholar]
- Kwon E., Hurwitz A., Foster B., Madias C., Feldhaus A., Greenberg N., et al. (1997) Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer, Proc Nat; Acad Sci U S A 94: 8099–8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick S., Bradbury R., Broadbent N., Campbell H., Gaughan L., Growcott J., et al. (2012) Preclinical profile of AZD3514: a small molecule-targeting androgen receptor function with a novel mechanism of action and the potential to treat castration-resistant prostate cancer. Cancer Res 72(8 Suppl.): 3848 [Google Scholar]
- Montgomery R., Eisenberger M., Rettig M., Chu F., Pili R., Stephenson J., et al. Phase I clinical trial of galeterone (TOK-001), a multifunctional antiandrogen and CYP17 inhibitor in castration resistant prostate cancer (CRPC), J Clin Oncol 30(15 Suppl.): 4665 [Google Scholar]
- Morris M., Hammers H., Sweeney C., Antonarakis E., Cho S., Pandit-Taskar N., et al. (2013) Safety of radium-223 dichloride (Ra-223) with docetaxel (D) in patients with bone metastases from castration-resistant prostate cancer (Crpc): a phase I Prostate Cancer Clinical Trials Consortium Study ASCO Meeting Abstracts 31: 5021 [Google Scholar]
- Olsson A., Björk A., Vallon-Christersson J., Isaacs J., Leanderson T. (2010) Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer 9: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin A., Jones R., Van Der Noll R., Graham J., Ong M., Finkelman R., et al. (2013) A first-in-human study of the oral selective androgen receptor down-regulating drug (SARD) AZD3514 in patients with castration-resistant prostate cancer (CRPC). ASCO Meeting Abstracts 31: 4511 [Google Scholar]
- Parker C., Nilsson S., Heinrich D., Helle S., O’sullivan J., Fosså S., et al. (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369: 213–223 [DOI] [PubMed] [Google Scholar]
- Pili R., Häggman M., Stadler W., Gingrich J., Assikis V., Björk A., et al. (2011) Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 29: 4022–4028 [DOI] [PubMed] [Google Scholar]
- Postow M., Callahan M., Barker C., Yamada Y., Yuan J., Kitano S., et al. (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkopf D., Antonarakis E., Shore N., Tutrone R., Alumkal J., Ryan C., et al. (2012) ARN-509 in men with metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 23: ix317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Smith M., De Bono J., Molina A., Logothetis C., De Souza P., et al. (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368: 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor A., Cooperberg M., Vogelzang N., Scholz M., Concepcion R., Berry W., et al. (2013) Real-world experience with sipuleucel-T in patients (PTS) with metastatic castration-resistant prostate cancer (MCRPC) who received prior docetaxel (D): data from PROCEED. ASCO Meeting Abstracts 31: 30 [Google Scholar]
- Scher H., Fizazi K., Saad F., Taplin M., Sternberg C., Miller K., et al. (2012) Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: results from the phase iii affirm study. J Clin Oncol 30: 122105825 [Google Scholar]
- Schiewer M., Goodwin J., Han S., Brenner J., Augello M., Dean J., et al. (2012) Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2: 1134–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M., Engelhardt J., Quigley M., Henning K., Chen T., Srinivasan M., et al. (2013) Anti-CTLA-4 antibodies of IGG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res epub ahead of print [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2013) Cancer statistics, 2013. CA-Cancer J Clin 63: 11–30 [DOI] [PubMed] [Google Scholar]
- Slovin S., Higano C., Hamid O., Tejwani S., Harzstark A., Alumkal J., et al. (2013) Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 24: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E., Halabi S., Dawson N., Stadler W., Rini B., Picus J., et al. (2004) Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 22: 1025–1033 [DOI] [PubMed] [Google Scholar]
- Small E., Lance R., Redfern C., Millard F., Gardner T., Karsh L., et al. (2013) A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (MCRPC). ASCO Meeting Abstracts 31: 5047 [Google Scholar]
- Smith D., Smith M., Sweeney C., Elfiky A., Logothetis C., Corn P., et al. (2013a) Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 31: 412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Antonarakis E., Ryan C., Berry W., Shore N., Liu G., et al. (2012) ARN-509 in men with high risk non-metastatic castration-resistant prostate cancer. ASCO Meeting Abstracts 31: 7 [Google Scholar]
- Smith M., Borre M., Rathenborg P., Werbrouck P., Van Poppel H., Heidenreich A., et al. (2013b) Efficacy and safety of enzalutamide (ENZA) monotherapy in hormone-naive prostate cancer (HNPC). ASCO Meeting Abstracts 31: 5001 [Google Scholar]
- Steinberg J., Oyasu R., Lang S., Sintich S., Rademaker A., Lee C., et al. (1997) Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res 3: 1707–1711 [PubMed] [Google Scholar]
- Tannock I., De Wit R., Berry W., Horti J., Pluzanska A., Chi K., et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., Mcdermott D., et al. (2012) Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 366: 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J., Kluger H., Callahan M., Postow M., Rizvi N., Lesokhin A., et al. (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hughes M., Kammula U., Royal R., Sherry R., Topalian S., et al. (2007) Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kim J., Edwards C., Xu Z., Taichman R., Wang C. (2005) Clusterin inhibits apoptosis by interacting with activated Bax. Nature Cell Biol 7: 909–915 [DOI] [PubMed] [Google Scholar]