Abstract
Objectives:
To estimate the patient burden in terms of the time spent on outpatient red blood cell (RBC) transfusions indicated for chemotherapy induced-anaemia (CIA) in patients with cancer in France.
Methods:
A retrospective chart review of patients with cancer receiving an outpatient RBC transfusion was conducted at seven treatment centres in France. Total treatment time for one transfusion visit per patient was measured as the elapsed time between pre- and post-transfusion vital sign assessment, including time from transfusion start to stop. Elapsed time from haemoglobin (Hb) level testing to transfusion start and from blood draw for compatibility testing to transfusion start were recorded. In addition, estimated travel time and distance to the transfusion centre, and clinical and demographic information were collected.
Results:
A total of 103 patients [63.1% men; mean age 66.2 years, standard deviation (SD) 11.9] were enrolled in the study (1 August 2010–31 October 2010). The four most frequent diagnoses were lung cancer (31.1%), urological cancer (15.5%), gynecological cancer (14.6%) and gastrointestinal/colorectal cancer (14.6%). Mean elapsed time between prevital and postvital sign assessment was 4.0 h [95% confidence interval (CI) 1.9–6.1], including a mean of 3.4 h (95% CI 2.5–4.2) for the transfusion itself. Hb level testing (mean pre-transfusion Hb level 8.0 g/dl, SD 0.8) and blood draw for compatibility testing were completed in a mean of 28.8 h (95% CI 1.3–56.2) and 9.4 h (95% CI 0–21.4) prior to transfusion respectively. Patients’ one-way mean travel time to the transfusion centre was 32.9 min (95% CI 28.5–37.4) and mean distance travelled was 25.4 km (95% CI 11.6–39.3).
Conclusion:
In France, CIA treatment with RBC transfusion is a time-consuming activity for patients that includes multiple trips to a medical facility, blood testing and the transfusion procedure itself. This burden is important to consider in the context of optimizing proactive monitoring and planning for supportive oncology care.
Keywords: anaemia, chemotherapy, patient burden, red blood cell transfusion
Introduction
Anaemia is a prevalent complication in patients with cancer who are treated with chemotherapy. The European Cancer Anaemia Survey, a large prospective survey conducted in 24 European countries that included more than 15,000 patients with various forms of cancer, demonstrated that the prevalence of anaemia was 51% for patients receiving chemotherapy and 44% for patients receiving concomitant chemotherapy/radiotherapy compared with only 32% of newly diagnosed patients receiving no cancer treatment [Ludwig et al. 2004]. Despite the high incidence and prevalence of chemotherapy induced-anaemia (CIA), the anticipated occurrence of this condition is often underestimated, and evaluation and treatment of CIA by healthcare providers is often delayed [Ludwig et al. 2004; Barrett-Lee et al. 2005; Steegmann et al. 2013]. If left untreated, CIA may cause a rapid decline in haemoglobin (Hb) levels, resulting in increased fatigue, which in consequence has a negative effect on the prognosis, quality of life and functional/psychological wellbeing of patients with cancer [Pirker et al. 2013; Cella, 1998; Cella et al. 2004]. As such, proactive identification and timely treatment of CIA is important to achieve optimal anaemia management outcomes. Current therapeutic options in CIA include erythropoiesis stimulating agent (ESA) therapy and red blood cell (RBC) transfusions [Scialdone, 2012]. In selecting a treatment for CIA, physicians consider the different associated risks and benefits in relation to clinical outcomes and health-related quality of life. For example, although ESA treatment can raise Hb levels and reduce RBC transfusion requirements [Hedenus et al. 2003; Vansteenkiste et al. 2002], there is some controversial evidence that ESA treatment in some studies may have been associated with an increase in the risk of death and disease progression [Tonia et al. 2012; Glaspy et al. 2010]. These risks should be balanced against the potential benefits of ESA treatment, taking into account each patient’s clinical circumstances and preferences.
Physicians should also consider how the treatment fits into providing patient-centred care. Patient-centred care, one of the six key elements of high-quality care according to the Institute of Medicine, is defined as ‘Providing care that is respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions’ [Committee on Quality of Health Care in America, Institute of Medicine, 2001]. Consideration of patient burden in terms of the time spent being treated for CIA during treatment selection is a potentially important component of providing patient-centred care. Although detailed assessments of the time spent on healthcare activities associated with ESA therapy are available from multiple sources [Fortner et al. 2005; Houts et al. 2006; Meehan et al. 2006], only a few studies have explored the care time associated with RBC transfusion, of which only one reported these outcomes from the patient perspective [Minuk et al. 2008; Ueno et al. 2006; Shreay et al. 2013]. Two single-centre studies have reported data estimating time-based outcomes from the site perspective. One single-centre study conducted in Canada prospectively examined the ‘chair time’ for 44 RBC transfusions and reported an average chair time of 231 min [standard deviation (SD) 47 min; 3.9 h; mean 2.2 RBC units transfused] [Minuk et al. 2008], while the other single-centre retrospective chart review study in the USA assessed the transfusion time for 100 transfusions and reported a mean total time of 223 min (SD 54 min; 3.7 h) for a two-unit transfusion [Ueno et al. 2006]. Another recent US study estimated the care time associated with RBC transfusion to treat CIA from the patient’s perspective [Shreay et al. 2013]. This study of 110 patients with CIA found that patients spent on average 4.2 h receiving treatment between prevital and postvital sign assessment. This time spent included 3.6 h to receive the actual RBC transfusion (mean 1.9 ± 0.5 RBC units transfused).
As national CIA treatment and management guidelines differ markedly, results from the USA may not be generalizable to other countries. Therefore, to better understand patient burden in relation to time spent receiving treatment for CIA, study extensions outside of the USA are warranted. The aim of this study is to estimate the patient burden in terms of time spent on outpatient RBC transfusion treatment indicated for CIA in patients with cancer in France.
Methods
Study design
This multicentre retrospective chart review study conducted in France is an extension of a recently published US study. Consequently, the chart review methodology employed has also been reported elsewhere [Shreay et al. 2013]. In this French cohort, 103 patients with CIA undergoing RBC transfusion were enrolled from seven treatment centres administering RBC transfusions and selected to represent the standard of care in France. Data on clinical tests and RBC treatment characteristics were collected from the patient medical charts and time spent in the medical facility during a RBC transfusion visit, which was used to quantify the outpatient RBC transfusion treatment time. Anonymized data were abstracted by site study staff, recorded on paper case report forms and entered into an electronic data capture (EDC) system. This study was approved by ethic committees in France. All data collected were deidentified and compliant with the European Union Data Protection Directive in France.
Patient identification and recruitment
Study sites identified all patients with a diagnosis of nonmyeloid malignancy who received an outpatient RBC transfusion indicated for CIA between 1 August 2010 and 31 October 2010. Each patient was assigned a study identification number and their details uploaded to a secure EDC system. All patients were randomly selected using the EDC system; patient medical charts were then reviewed in order of randomization to confirm study eligibility until a target cohort of 15 eligible patients was identified per site. The sample size of the final study cohort varied from 10 to 22 patients among all sites.
Eligible patients were 18 years or older at the time of RBC transfusion, had a diagnosis of nonmyeloid malignancy (including all solid tumours, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, chronic lymphocytic leukaemia and multiple myeloma), received an RBC transfusion indicated for CIA in an outpatient setting and had a medical chart (i.e. paper or electronic medical records) available for review. Patients with a diagnosis of acute leukaemia, chronic myelogenous leukaemia, myelodysplastic syndromes or marrow fibrosis, and patients who had received other therapies besides their RBC transfusion [excluding diphenhydramine (Benadryl, McNeil-PPC, Inc. (Subsidiary of Johnson & Johnson), Fort Washington, Pennsylvania), corticosteroids, ESA or acetaminophen] during their RBC transfusion visit were excluded from the study.
Data collection
Each site completed a site questionnaire which included questions pertaining to study site location, geographic setting, facility type, profit status, teaching status, number of outpatient transfusion beds/chairs and maximum number of transfusions performed at one time at the site.
For all enrolled patients, demographic and clinical data were collected. Primary study endpoints were total treatment time associated with one RBC transfusion visit (time from pre-transfusion to post-transfusion vital signs assessment) and RBC transfusion duration (transfusion start and stop time). Secondary study endpoints included elapsed time (i.e. measured duration) between pre-transfusion Hb level testing and start of RBC transfusion (derived from date and time of Hb level testing and start time of RBC transfusion), elapsed time between blood draw for compatibility testing and start of RBC transfusion (derived from date and time of blood draw and start of RBC transfusion), and travel time and distance between patient’s residence and the medical facility on the day of the RBC transfusion visit [time was calculated based on distance using MapQuest software (available at http://www.mapquest.com) and patient address information; the fastest route was assumed for all patients]. The number of RBC transfusion visits made by patients in the most recent year was also recorded.
Exploratory endpoints considered in relation to RBC transfusion treatment were pre-transfusion Hb levels (level at time of oncologist’s transfusion order), site of Hb level testing and blood draw for compatibility testing, number of blood units ordered and transfused, and transfusion reactions and hospitalization on transfusion day (during or after the RBC transfusion).
Data analyses
Patients who met study eligibility criteria were included in the analysis. Descriptive statistics were performed to evaluate demographic and clinical characteristics (e.g. age, sex, type of malignancy), and primary and secondary endpoints. For continuous variables, mean ± SD, median and range were examined. For categorical variables, percentages were reported. If appropriate, 95% confidence intervals (CIs) were calculated.
Results
Study sites and patient characteristics
A total of seven French sites from different regions (Picardie, Limousin, Haute Normandie, Aquitaine, Poitou-Charentes, Provence-Alpes-Côte d’Azur) were recruited to participate in the study. From the seven participating centres, four sites were part of an inpatient hospital, two were private clinics (hospital environment) and one was an outpatient hospital. Most of the sites (six sites) were nonprofit organizations (associations) located in urban areas (representative distribution of French oncology hospitals) and five sites were considered nonteaching centres (one centre reported their teaching status as unknown). The mean number of outpatient transfusion beds/chairs at the sites was 6.5 ± 6.3 beds/chairs (one site was excluded from this mean as it did not have beds/chairs dedicated to transfusion but shared beds from another unit) and the maximum number of transfusions that could be performed at one time was 3.7 ± 1.4 transfusions.
The demographic and RBC treatment history data of the 103 patients included in the final analysis are summarized in Table 1. Patients were mostly men (63.1%) and the mean age was 66.2 ± 11.9 years. The mean number of RBC transfusion visits experienced by patients over the course of the most recent year was 2.9 ± 2.8 transfusion visits. While the majority of patients (37.6%) had one transfusion visit, 16.5% patients had more than five transfusion visits (Table 1). The most common cancer diagnoses were lung cancer (31.1%), urological cancer (15.5%), gynaecological cancer (14.6%) and gastrointestinal/colorectal cancer (14.6%) (Figure 1).
Table 1.
Patient demographics and red blood cell treatment history.
| Demographics | N = 103 |
|---|---|
| Mean age (SD), years | 66.2 (11.9) |
| Men, n (%) | 65 (63.1) |
| RBC treatment history | N = 103 |
| RBC transfusion visits (past 12 months)*, n (%) | |
| 1 transfusion visit | 32 (37.6) |
| 2 transfusion visits | 25 (29.4) |
| 3–5 transfusion visits | 14 (16.5) |
| >5 transfusion visits | 14 (16.5) |
The number of RBC transfusion visits over the most recent year was reported for 85 patients (83%); RBC transfusion visit history was reported to be missing from the medical charts for 18 patients (17%).
RBC, red blood cell; SD, standard deviation.
Figure 1.
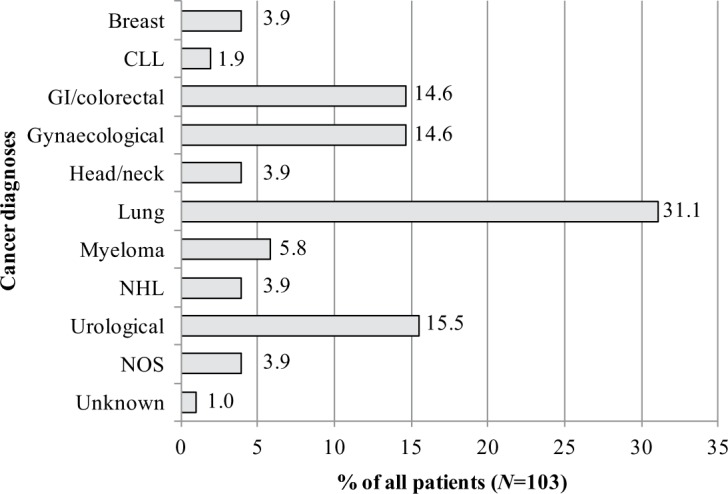
Cancer diagnoses of study population. CLL, chronic lymphocytic leukemia; GI, gastrointestinal; NHL, non-Hodgkin’s lymphoma; NOS, not otherwise specified.
Clinical tests and RBC transfusion treatment
The site location of patient clinical tests performed prior to RBC transfusion and characteristics of RBC transfusion treatment are presented in Figure 2 and Table 2. For the Hb level test, 27 patients (26.2%) went to an outpatient hospital, 20 (19.4%) went to an inpatient hospital and 3 (2.9%) went to an oncologist office/private practice. For blood draw for compatibility testing, 33 patients (32.0%) went to an outpatient hospital, 29 (28.2%) went to an inpatient hospital and 3 (2.9%) went to a transfusion centre laboratory (Figure 2).
Figure 2.
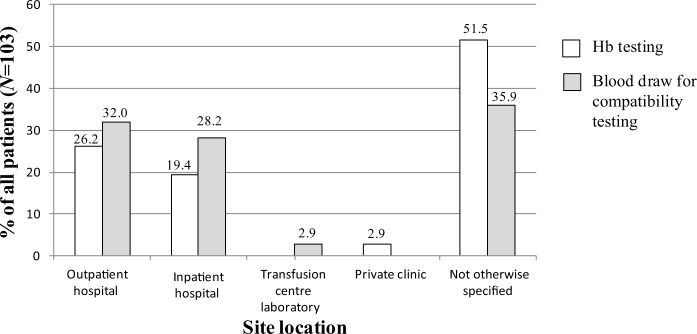
Site location for clinical tests. Hb, haemoglobin.
Table 2.
Red blood cell transfusion treatment.
| Pre-transfusion Hb level | N = 98* |
|---|---|
| Mean g/dl (SD) | 8.0 ± 0.8 |
| Hb level category, n (%) | |
| 9.5 to <13.0 g/dl | 5 (5.1) |
| 8.0 to <9.5 g/dl | 45 (45.9) |
| < 8.0 g/dl | 48 (49) |
| RBC transfusion, units ordered/transfused | N = 103 |
| RBC units ordered | |
| Number of units, mean (SD) | 2.1 ± 0.3 |
| RBC units, n (%) | |
| 2 units | 96 (93.2) |
| 3 units | 7 (6.8) |
| RBC units transfused | |
| Number of units, mean (SD) | 2.1 ± 0.3 |
| RBC units, n (%) | |
| 1 unit | 1 (1.0) |
| 2 units | 95 (92.2) |
| 3 units | 7 (6.8) |
Among all patients (N=103), pre-transfusion Hb level was reported for 98 patients (95.1%). Data on Hb levels were reported to be missing from the medical charts of five patients (5.3%).
HB, haemoglobin; RBC, red blood cell; SD, standard deviation.
At the time the oncologist’s RBC transfusion order was made (mean 2.1 ± 0.3 RBC units ordered), the mean patient Hb level was 8.0 ± 0.8 g/dl; 48 patients (49.0%) had an Hb level below 8g/dl, 45 (45.9%) had an Hb level between 8.0 and 9.5 g/dl and 5 (5.1%) had an Hb level between 9.5 and 13 g/dl. For the RBC transfusion treatment, a mean number of 2.1 ± 0.3 RBC units were transfused; 1 patient (1.0%) had one unit transfused, 95 (92.2%) had two units transfused and 7 (6.8%) had three units transfused.
Burden associated with RBC transfusion
The patient burden in terms of time spent on the RBC transfusion is presented in Table 3. The mean elapsed time between pre-transfusion vital signs assessment and post-transfusion vital signs assessment was 4.0 h (95% CI 1.9–6.1), including a mean of 3.4 h (95% CI 2.5–4.2) to receive the actual RBC transfusion treatment. Considering that patients had a mean of 2.1 ± 0.3 blood units transfused, this suggests that patients spent 1.7 h on average per blood unit transfused per transfusion.
Table 3.
Patient time spent on red blood cell transfusion treatment.
| n (%) | Mean | SE | Range | 95% CI | |
|---|---|---|---|---|---|
| RBC transfusion (h) | |||||
| Prevital to postvital signs assessment | 90 (87.4) | 4.0 | 0.9 | 1.0–10.8 | 1.9–6.1 |
| Transfusion start time to stop time | 89 (86.4) | 3.4 | 0.4 | 0.2–6.3 | 2.5–4.2 |
| Laboratory testing (h) | |||||
| Hb level testing to start of transfusion | 39 (37.9) | 28.8 | 9.9 | 0.0–99.0 | 1.3–56.2 |
| Blood draw for compatibility testing to transfusion | 59 (57.3) | 9.4 | 4.3 | 0.03–67.3 | 0–21.4 |
| Travel | |||||
| Distance (km) | 103 (100) | 25.4 | 5.7 | 0.8–136.0 | 11.6–39.3 |
| Time (min) | 103 (100) | 32.9 | 2.2 | 5.0–120.0 | 28.5–37.4 |
The mean elapsed time between pre-transfusion Hb test and start of RBC transfusion was 28.8 h (95% CI 1.3–56.2).The results indicated that patients had to wait for up to 4 days (99 h) between pre-transfusion Hb testing and start of RBC transfusion. The mean elapsed time between blood draw for compatibility testing and start of RBC transfusion was 9.4 h (95% CI 0–21.4). The results indicated that patients had to wait for up to 3 days (67.3 h) between blood draw for compatibility testing and the start of their RBC transfusion. It should be noted that this time overlapped the elapsed time between Hb test and start of RBC transfusion.
Details about patients’ one-way journey between their residence and the medical facility on the day of their RBC transfusion visit were also collected (Table 3). For a one-way trip, patients had to travel a mean distance of 25.4 km (95% CI 11.6–39.3); the largest category of study patients (21.4%) travelled between 10 and 15 km, and 19.4% had to travel more than 50 km. The mean travelling time was 32.9 min (95% CI 28.5–37.4); the largest category of study patients (30.1%) travelled between 25 and 50 min and 22.3% travelled for more than 50 min (data not shown).
Finally, transfusion reactions were reported for two patients: hives, itching and flushing were reported for one patient and fever was reported for the other patient. On transfusion visit day, a total of 26 patients (25.2%) were hospitalized during or post RBC transfusion (mean hospitalization duration 11 ± 31.1 days). Patients were hospitalized for reasons not related to the RBC transfusion: disease complications (42.3%), chemotherapy administration (11.5%), surgery (3.9%) and reasons not otherwise specified (34.6%). Reasons for hospitalizations were reported to be unknown for 7.7% of patients.
Conclusion
Consistent with the results of the US implementation of this study, CIA treatment with RBC transfusion is a time-consuming activity for patients with cancer receiving chemotherapy. Specifically, this study has shown that patients with CIA are required to spend an average of 4.0 h from prevital to postvital sign assessment, including a mean of 3.4 h for the transfusion itself. Considering that patients, on average, received about three transfusions during the previous 12 months, this translates to almost 12 h per patient per year spent in treatment for CIA. These results are similar to results obtained in the US implementation [Shreay et al. 2013], which estimated that patients had to spend a mean of 4.2 h (95% CI 3.64–4.81) between prevital and postvital sign assessment, including a mean of 3.6 h to receive their actual RBC transfusion (mean 1.9 ± 0.5 blood units transfused). The transfusion time of 3.4 h reported here is also comparable to the results obtained in the two single-centre studies [Minuk et al. 2008; Ueno et al. 2006], in which an average chair time of 231 ± 47 min (3.9 h; for 2.2 units on average) and mean transfusion duration of 223 ± 54 min (3.7 h; for two units on average) were reported. In addition to the burden in terms of time spent on RBC transfusion, our study has also taken into account the elapsed time between the Hb testing and blood draw for compatibility testing and the start of the RBC transfusion. Though optimal care for CIA includes timely treatment, this study indicates that patients have to wait about 29 h on average after their Hb level testing before receiving a RBC transfusion.
In this study it is likely that time spent on outpatient RBC transfusion treatment indicated for CIA is an underestimate of patient burden. For example, this study presents the time estimates for one RBC transfusion visit while on average patients had three transfusion visits per year and 16.5% patients had more than five transfusion visits per year, which almost doubles the estimated mean total time spent per year. In addition, contributing to a likely underestimate of time spent, these results do not take into account the time spent by the patient travelling to the clinic for their laboratory tests, the time spent by patients getting prepared for the visit, rescheduling activities, the waiting time spent at the clinic for patient registration, insurance verification, and waiting time due to healthcare staff schedule delays at the clinic. For example, a patient survey by Fortner and colleagues reported that total time affected by laboratory visits for having blood drawn was 1.93 ± 2.22 h on average [Fortner et al. 2005]. This affected time included time spent at the medical office, time spent rearranging work activities, time getting ready for the visit, and time travelling to and from the visit.
Regarding travel time to the care setting for treatment, the results of this study demonstrated that patients had to travel an average of 32.5 min for a one-way trip and 65 min including the return journey. This is consistent with other findings in which the average one-way trip was 30.0 min [Shreay et al. 2013] and the round trip from home to the clinic was 64 min [Fortner et al. 2005]. In our study, to be conservative, the fastest route was assumed for the purpose of calculating distance travelled and time spent for all patients, and different modes of transport were not considered. Travel and distance have been identified as potential stressors in terms of psychological adjustment or a barrier to patients’ compliance and healthcare professionals’ decisions in relation to prescribed treatment [Payne et al. 2000]. Finally, indirect financial costs associated with RBC transfusion treatment such as transportation costs, lost wages, food costs, expenditures for child care, hotel accommodation and parking costs may also have an impact on patient burden [Fortner, 2006].
Lost productivity and loss of quality of life resulting from time spent attending the RBC transfusion visits were not considered in this study but are also important factors when evaluating the overall burden of RBC transfusions. Patients attending a visit for RBC transfusion may lose time from work or from daily activities. Also, other personal activities, such as paid vacation, housework, caring for family, hobbies and social activities, may have to be deferred. A patient survey reported that almost all of the patients surveyed (99%) indicated that at least one life activity had been altered by a medical visit. Spending time with friends and family (60%) as well as housework activities (51%) were the most frequently reported activities affected by medical visits [Fortner et al. 2005]. All of these visits affect the patient’s normal life activities both in the time taken away from those activities and in their associated costs, such as lost work time. In addition, there can be logistical challenges in transportation and living arrangements for each visit.
In conclusion, data from this study provide evidence that RBC transfusion treatment is a burden in terms of time spent on RBC transfusion for patients with CIA in France. The burden on patients with cancer can be caused by different factors which must all be considered in providing optimal care. Patient burden is important to consider in the context of optimizing proactive monitoring and planning for supportive oncology care. To properly assess the important indicators of ‘value’ such as cost effectiveness, rigorous real-world evidence is required to populate health economics and other burden of illness evaluations. This study was designed to produce important data that can be used as inputs for these types of analyses.
Acknowledgments
The authors would like to thank the participating centres for their contribution to this study: Polyclinique Francheville, Centre Hospitalier de Chauny, Hôpital Sainte Musse, Polyclinique Bordeaux Rive Droite (Clinique des 4 Pavillons), Centre Hospitalier de Brive – Pôle Cancérologie, Centre Clinical and Centre Frédéric Joliot (Clinique Saint-Hilaire). Also the authors thank Elizabeth Donahue, Sonja Gross, Julie Barrett and Anne Cecile Touchot, members of UBC operations team, Noreen Lordan, UBC data manager and Ray Shieh, UBC statistician for their contribution to this study.
Footnotes
Funding: This research was supported by a financial grant from Amgen Inc.
Conflict of interest statement: Authors Marie-Pierre Desrosiers and Krista Payne are employees of United BioSource Corporation, a consultancy that has also received grants for other, unrelated research from Amgen Inc. Patricia Corey-Lisle is an employee and shareholder of Amgen Inc. Helen Collins and Margarita De la Orden were employees of Amgen Inc. at the time the study was conducted. Dr Levaché and Dr. Dumont were participating investigators in the study and their institutions were provided fair market compensation by Amgen for their staff’s time for data collection.
Contributor Information
Patricia K. Corey-Lisle, Amgen Inc., One Amgen Center Dr., MS: 28-3-A, Thousand Oaks, CA 91320-1799, USA
Marie-Pierre Desrosiers, United BioSource Corporation, Montreal, Quebec, Canada.
Helen Collins, Amgen Inc., Thousand Oaks, CA, USA.
Margarita De La Orden, Amgen Inc., Uxbridge, UK.
Krista A. Payne, United BioSource Corporation, Montreal, Quebec, Canada
Charles Briac Levaché, Polyclinique Francheville, Périgueux, France.
Patrick Dumont, Department of Pulmonology, Centre Hospitalier Chauny, Chauny, France.
References
- Barrett-Lee P., Bokemeyer C., Gascón P., Nortier J., Schneider M., Schrijvers D., et al. (2005) Management of cancer-related anemia in patients with breast or gynecological cancer: new insights based on results from the European Cancer Anemia Survey. Oncologist 10: 743–757 [DOI] [PubMed] [Google Scholar]
- Cella D. (1998) Factors influencing quality of life in cancer patients: anemia and fatigue Semin Oncol 25(3 Suppl. 7): 43–46 [PubMed] [Google Scholar]
- Cella D., Kallich J., McDermott A., Xu X. (2004) The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol 15: 979–986 [DOI] [PubMed] [Google Scholar]
- Committee on Quality of Health Care in America, Institute of Medicine (2001) Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press [Google Scholar]
- Fortner B. (2006) Impact of less frequent injections on patients, caregivers, and practices. Oncology (Williston Park) 20(8 Suppl. 6): 33–38 [PubMed] [Google Scholar]
- Fortner B., Tauer K., Zhu L., Okon T., Moore K., Templeton D., et al. (2005) The impact of medical visits for chemotherapy-induced anemia and neutropenia on the patient and caregiver: a national survey. Community Oncol 4: 211–217 [Google Scholar]
- Glaspy J., Crawford J., Vansteenkiste J., Henry D., Rao S., Bowers P., et al. (2010) Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 102: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenus M., Adriansson M., San Miguel J., Kramer M., Schipperus M., Juvonen E., et al. (2003) Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 122: 394–403 [DOI] [PubMed] [Google Scholar]
- Houts A., Loh G., Fortner B., Kallich J. (2006) Patient and caregiver time burden associated with anaemia treatment in different patient populations. Support Cancer Care 14: 1195–1204 [DOI] [PubMed] [Google Scholar]
- Ludwig H., Van Belle S., Barrett-Lee P., Birgegård G., Bokemeyer C., Gascón P., et al. (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40: 2293–2306 [DOI] [PubMed] [Google Scholar]
- Meehan K., Tchekmedyian N., Smith R., Kallich J. (2006) Resource utilisation and time commitment associated with correction of anaemia in cancer patients using epoetin alfa. Clin Drug Invest 26: 593–601 [DOI] [PubMed] [Google Scholar]
- Minuk L., Chin-Yee I., Hibbert A., Chan D., Xenocostas A., et al. (2008) Red blood cell transfusion and chemotherapy administration: a study of resource utilization. Common Oncol 5: 598–603 [Google Scholar]
- Payne S., Jarrett N., Jeffs D. (2000) The impact of travel on cancer patients’ experiences of treatment: a literature review. Eur J Cancer Care (Engl) 9: 197–203 [DOI] [PubMed] [Google Scholar]
- Pirker R., Pirolli M., Quigley J., Hulnick S., Legg J., Collins H., et al. (2013) Hemoglobine decline in cancer patients receiving chemotherapy without an erythropoeisis-stimulating agent. Support Care Cancer 21: 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialdone L. (2012) Overview of supportive care in patients receiving chemotherapy: antiemetics, pain management, anemia, and neutropenia. J Pharm Pract 25: 209–221 [DOI] [PubMed] [Google Scholar]
- Shreay S., Desrosiers M., Corey-Lisle P., Payne K. (2013) A retrospective study to evaluate the time burden associated with outpatient red blood transfusions indicated for anemia due to concomitantly administered chemotherapy in cancer patients. Support Care Cancer 21: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmann J., Sánchez Torres J., Colomer R., Vaz Á., López J., Jalón I., et al. (2013) Prevalence and management of anaemia in patients with non-myeloid cancer undergoing systemic therapy: a Spanish survey. Clin Transl Oncol 15: 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonia T., Mettler A., Robert N. (2012) Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 12: CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno W., Beveridge R., Kales A. (2006) Costs of outpatient red blood cell transfusions. J Support Oncol 4: 494–495 [PubMed] [Google Scholar]
- Vansteenkiste J., Pirker R., Massuti B., Barata F., Font A., Fiegl M., et al. (2002) Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 94: 1211–1220 [DOI] [PubMed] [Google Scholar]


