See Martinez-Martinez et al. (doi:10.1093/brain/awu153) for a scientific commentary on this article.
Carvajal-González et al. describe the first prospective cohort of patients with glycine receptor antibodies. The majority have progressive encephalomyelitis with rigidity and myoclonus. The antibodies bind to extracellular determinants on glycine receptor-α1 and to glycine receptors on spinal cord and brainstem neurons. The patients make a good recovery with immunotherapies.
Keywords: stiff person syndrome, progressive encephalomyelitis with rigidity and myoclonus, autoimmune encephalitis, glycine receptor, autoantibody
Abstract
The clinical associations of glycine receptor antibodies have not yet been described fully. We identified prospectively 52 antibody-positive patients and collated their clinical features, investigations and immunotherapy responses. Serum glycine receptor antibody endpoint titres ranged from 1:20 to 1:60 000. In 11 paired samples, serum levels were higher than (n = 10) or equal to (n = 1) cerebrospinal fluid levels; there was intrathecal synthesis of glycine receptor antibodies in each of the six pairs available for detailed study. Four patients also had high glutamic acid decarboxylase antibodies (>1000 U/ml), and one had high voltage-gated potassium channel-complex antibody (2442 pM). Seven patients with very low titres (<1:50) and unknown or alternative diagnoses were excluded from further study. Three of the remaining 45 patients had newly-identified thymomas and one had a lymphoma. Thirty-three patients were classified as progressive encephalomyelitis with rigidity and myoclonus, and two as stiff person syndrome; five had a limbic encephalitis or epileptic encephalopathy, two had brainstem features mainly, two had demyelinating optic neuropathies and one had an unclear diagnosis. Four patients (9%) died during the acute disease, but most showed marked improvement with immunotherapies. At most recent follow-up, (2–7 years, median 3 years, since first antibody detection), the median modified Rankin scale scores (excluding the four deaths) decreased from 5 at maximal severity to 1 (P < 0.0001), but relapses have occurred in five patients and a proportion are on reducing steroids or other maintenance immunotherapies as well as symptomatic treatments. The glycine receptor antibodies activated complement on glycine receptor-transfected human embryonic kidney cells at room temperature, and caused internalization and lysosomal degradation of the glycine receptors at 37°C. Immunoglobulin G antibodies bound to rodent spinal cord and brainstem co-localizing with monoclonal antibodies to glycine receptor-α1. Ten glycine receptor antibody positive samples were also identified in a retrospective cohort of 56 patients with stiff person syndrome and related syndromes. Glycine receptor antibodies are strongly associated with spinal and brainstem disorders, and the majority of patients have progressive encephalomyelitis with rigidity and myoclonus. The antibodies demonstrate in vitro evidence of pathogenicity and the patients respond well to immunotherapies, contrasting with earlier studies of this syndrome, which indicated a poor prognosis. The presence of glycine receptor antibodies should help to identify a disease that responds to immunotherapies, but these treatments may need to be sustained, relapses can occur and maintenance immunosuppression may be required.
Introduction
Antibodies to receptors, ion channels or related proteins have become important markers for diseases that often improve substantially with immunotherapies (Vincent et al., 2011; Lancaster and Dalmau, 2012; Zuliani et al., 2012). N-methyl-D-aspartate antibodies are associated with anti-NMDA receptor (NMDAR) encephalitis and antibodies to leucine-rich, glioma inactivated 1 protein and contactin-associated protein 2 [components of the voltage-gated potassium channel (VGKC)-complex], with limbic encephalitis, Morvan’s syndrome or peripheral nerve hyperexcitability. Antibodies to other CNS receptors have also been found in limbic encephalitis (Lancaster and Dalmau, 2012), and to dopamine receptors in children with a rare form of basal ganglia encephalitis (Dale et al., 2012). In addition, antibodies to the glial protein aquaporin 4 are an established cause of neuromyelitis optica (Jarius and Wildemann, 2010). Because each of these antibodies can bind to antigenic epitopes that are exposed on the surface of the cell they are likely to be pathogenic.
Stiff person syndrome is another antibody-associated syndrome, often with antibodies to glutamic acid decarboxylase (GAD; reviewed by Brown and Mardsen, 1999; Meinck and Thompson, 2002; Alexopoulos and Dalakas, 2010). This enzyme, however, is intracellular rather than on the cell surface. Many patients do not respond well to immunotherapies but there are reports of clinical improvement with intravenous immunoglobulins or plasma exchange (reviewed by Alexopoulos and Dalakas, 2010), and recent reports of experimental transfer of disease to experimental animals with GAD or amphiphysin antibodies (Geis et al., 2010, 2012; Hansen et al., 2013).
A proportion of patients with GAD antibodies have a syndrome called stiff person syndrome plus or progressive encephalomyelitis with rigidity and myoclonus (PERM). PERM is similar to stiff person syndrome with rigidity, stimulus-sensitive spasms, myoclonus, hyperekplexia and autonomic disturbance, but with additional brainstem or other neurological defects. In general, PERM has been described as more severe, progressive and more often fatal compared with stiff person syndrome (Brown and Marsden, 1999; Meinck and Thompson, 2002). In a few cases, pathological findings on post-mortem have confirmed inflammation in PERM (Whiteley et al., 1976; Turner et al., 2011), suggesting that there may be T cell-mediated damage in both diseases.
In 2008, however, we described a patient with PERM, without GAD antibodies, who made a delayed but substantial recovery after receiving steroids, plasma exchange, intravenous immunoglobulin and cyclophosphamide. Retrospectively, the patient’s sera, at onset and peak of disease, contained antibodies that bound to GlyRα1 subunits (now known as GLRA1) expressed on the surface of transfected human embryonic kidney cells, and also immunoprecipitated GlyRα1 (Hutchinson et al., 2008). Since then, further patients with glycine receptor (GlyR) antibodies have been described (Clerinx et al., 2011; Mas et al., 2011; Turner et al., 2011; Iizuka et al., 2012; Peeters et al., 2013; Piotrowicz et al., 2011; Damasio et al., 2013; Stern et al., 2014; Bourke et al., 2014) with combinations of stiffness, rigidity, excessive stimulus-evoked startle, brainstem and autonomic signs. GlyR antibodies have also been found in retrospective cohorts of adults or children (Alexopoulos et al., 2013; Clardy et al., 2013; McKeon et al., 2013), with or without GAD antibodies. Here we describe the clinical spectrum of 52 GlyR antibody-positive patients (including those reported previously, see above), and the treatment responses, antibody characteristics and immunohistochemical localization of target antigens in rodent brain tissue.
Materials and methods
Patients
We identified 52 patients prospectively from 779 samples (including 55 serum/CSF pairs) referred to the Oxford Neuroimmunology service for GlyR antibody screening from 2008 to March 2012. Consent forms and questionnaires were sent to the neurologists (the glycine receptor antibody study group, see Appendix 1) who referred positive samples, and the data collated and analysed in Oxford. The study was performed with consent from the Regional Ethics Committee Ref: 07/Q1604/28. Sera positive for NMDAR or aquaporin 4 antibodies were used as serum controls, and six archived multiple sclerosis CSFs were used as CSF controls. We also examined GlyR antibodies in a retrospective cohort of 56 patients (35 females, 21 males) with stiff person syndrome, PERM or related disorders that had been collected in Heidelberg, Germany.
Detection of glycine receptor-bound antibodies for diagnosis
For screening we used immunofluorescence cell-based assays on human embryonic kidney (HEK) 293 cells transfected to express homo-pentamers of GlyRα1 tagged with enhanced green fluorescent protein, as previously described (Hutchinson et al., 2008) and used in the Oxford clinical diagnostic service (see Supplementary material for details). Serum was tested at 1:20 and CSF at 1:2. The intensity of the staining was assessed visually by two independent observers, using a semiquantitative score (0 = no binding; 1–2 = low level binding; 2–4 = increasing strength of binding) as described previously (Leite et al., 2008; Waters et al., 2008). All sera and CSFs with positive binding were retested and checked for negativity against a second antigen to confirm specificity. When sufficient sample was available, GlyR antibody-positive sera and CSFs were also tested at serial dilutions to identify the concentration at which binding was scored as 1 (‘endpoint’ dilution). All of the available first samples were also tested for NMDAR and GAD antibodies by routine tests; 28 had VGKC-complex antibodies requested previously by the referring neurologists.
The subclass of the GlyR antibodies was determined by use of specific anti-subclass antibodies to IgG1, IgG2, IgG3, IgG4 and IgM (Invitrogen). Their ability to fix complement was demonstrated on the transfected HEK293 cells. After the addition of the patient serum (1:20) and washing, fresh human complement was added at 37°C for 1 h. Surface-bound C3b was detected with a fluorescent commercial antibody (Dako) as previously described (Leite et al., 2008; Waters et al., 2008). To determine subunit specificity, all positive samples were also screened against the other α subunits of the GlyR, α2 and α3 (see Supplementary material for details).
Blood–brain barrier integrity and antibody index
Full details are given in the Supplementary material. The six serum/CSF pairs with sufficient volumes were used to determine the integrity of the blood–brain barrier and the intrathecal synthesis of specific GlyR antibodies. Western blotting was used to measure total levels of IgG in paired sera (1:300) and undiluted CSF and for the albumin content of the sera (1:300) and CSFs, comparing with IgG and albumin standard curves. The IgG index was calculated according to:
 |
Effects of antibody binding to glycine receptor expressed on HEK cells
Full details are given in Supplementary material. To see whether GlyR antibodies were associated with internalization of the GlyRs, sera (1:80 to 1:160) were incubated with GlyRα1 expressing HEK cells for 1 h at 4°C and washed. Fixed or live cells were then incubated at either 4°C, or 37°C for 5 min to 16 h. After fixation of all cells, surface-bound human IgG was detected and the coverslips scored as for the diagnostic assay (see above). To see if the loss of surface GlyR antibody IgG was due to internalization, surface bound GlyR antibody was visualized (green) and scored at different times and compared with cells fixed and permeabilized in 0.3% Triton™ X-100 so that internal IgG could be visualized with Alexa Fluor® goat anti-human IgG (red). The results were quantified and data analysed with ImageJ software.
To examine the levels of surface GlyRα1, rather than bound IgG, the same procedure was carried out using GlyR-EGFP (enhanced green fluorescent protein) transfected cells, and cyclohexamide to prevent new GlyR-EGFP synthesis. The percentage of cells with GlyR-EGFP on the surface or internalized was analysed. Finally to look at the endosomal location of internalized GlyR-EGFP, mouse antibodies to the late endosomal antigen (1:250; SantaCruz) were used, detecting with Alexa Fluor® 568 goat anti-mouse IgG (Invitrogen).
Binding to rat brain tissue sections
Full details are given in the Supplementary material. Indirect single and double immunofluorescence staining was performed on 11-µm cryostat sections of fresh frozen adult rat brain. The sections were fixed, blocked and incubated overnight at 4°C with human sera (1:200 to 1:800) and mouse monoclonal antibodies to the GlyRα1 subunit (1:500; Synaptic Systems) or rabbit polyclonal antibody to GAD (1:500; Sigma). The sections were washed, and bound patient or commercial antibodies detected with the appropriate secondary antibodies. Slides were photographed under a Leica fluorescence microscope (DM 2500) with a digital camera (QImaging, Rolera XR, Fast 1394). Four sera were pre-adsorbed against HEK cells expressing GlyRα1, GlyRα2, Glyα3 or untransfected HEK cells, or with recombinant GAD (12.5 µg/ml; RSR Ltd), overnight at 4°C before applying to the rat brain sections. To identify the neurons by confocal microscopy, sections were incubated with human sera (1:200 to 1:800), mouse monoclonal antibody to glycine receptor (as above) and either rabbit polyclonal antibody to microtubule-associated protein 2 (MAP2; 1:1000; Sigma) or rabbit polyclonal antibody to choline acetyltransferase (ChAT; 1:100; Millipore). Images were taken with a Zeiss confocal microscope (LSM 710).
Statistics
Kruskal-Wallis test and Dunn’s multiple comparisons were used to assess the immunotherapy responses. Two-way ANOVA and two-sided Students t-tests were used to analyse the internalization experiments.
Results
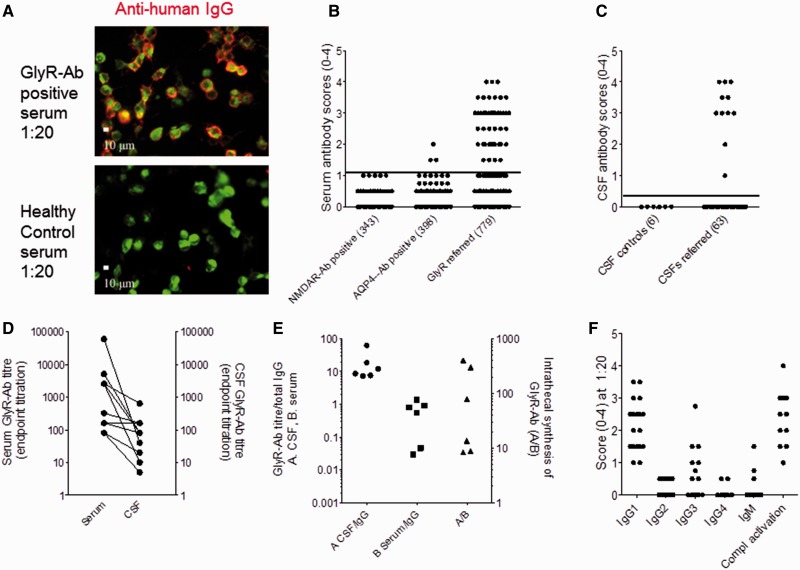
The prospective cohort was identified from samples referred for testing from 2008 to March 2012. Images of patient and healthy control sera binding to GlyRs expressed on HEK cells are shown in Fig. 1A and the initial scores for each patients’ serum and, when provided, CSF in Fig. 1B and C. For disease controls, we used samples identified as positive for NMDAR or aquaporin 4 antibodies, and multiple sclerosis CSFs. Only 1% of sera with aquaporin 4 antibodies, and none of those with NMDAR antibodies, bound to the GlyR cells at a score of >1 (Fig. 1B), and none of the control CSFs bound detectably (Fig. 1C). Therefore, we studied consecutive patients with GlyR antibody scores of >1 in serum and >0 in CSF. Forty-one sera and 11 serum/CSF pairs were positive. The remaining 727 patients, including 52 with serum/CSF pairs and five unpaired CSF samples were negative (median 0).
Figure 1.
GlyR antibodies (Ab) in PERM and related disorders. (A) A patient’s serum IgG binding to HEK293 cells expressing GlyRα1-EGFP (green); the IgG binding is detected with anti-human IgG (red). No IgG binding is observed with serum from a healthy individual. (B) Visual scores of sera (diluted 1:20) referred for routine GlyR antibody testing compared with scores in sera positive for NMDAR or aquaporin 4 (AQP4) antibodies studied as neurological controls. (C) Scores of referred CSF samples (diluted 1:1) compared with multiple sclerosis control CSFs. (D) Endpoint titrations for sera and paired CSFs; the CSF titres are generally lower than serum titres but in three patients are equal or only a dilution lower. (E) Calculation of intrathecal synthesis in six paired CSFs. GlyR antibody titres (from D) divided by IgG concentration for sera (serum/IgG) or for CSFs (CSF/IgG). Intrathecal synthesis rates are given as the ratio between these values as described above, and are raised in all patients, particularly in three. (F) Scores for each IgG subtype and for the deposition of complement C3 on the GlyRα1-expressing HEK cells. The antibodies are mainly IgG1 and IgG3 subtypes and activate complement on the surface of the GlyRα1-transfected cells.
The endpoint dilution titres in sera (1:40 to 1:60 000) and CSFs (1:5 to 1:640) varied widely (eg. Fig. 1D), but some patients had CSF levels equal to (n = 1) or very similar to serum levels suggesting marked intrathecal synthesis of the specific GlyR antibody. To assess this quantitatively, we measured serum and CSF IgG and albumin in each of six patients with sufficient remaining samples. The ratios of serum:CSF IgG (138–592) and albumin (187 to 403) were within normal limits with QAlb of 0.6 to 1.4 (normal 0.7 to 1.3), indicating no general blood–brain barrier breakdown. By contrast, the antibody index for the GlyR antibody ranged from 8 to 400 (normal <1.4). These values represent substantial intrathecal synthesis of the specific GlyR antibodies, particularly in the three patients with values >50 (Fig. 1E).
Clinical data of patients with glycine receptor antibody-positive referred samples
For the 52 prospectively-referred GlyR antibody-positive patients, questionnaires were sent to the referring neurologists. Seven patients with very low GlyR antibodies (not titrating beyond 1:40) were excluded from the cohort because they had minimal symptoms and were lost to follow-up (n = 2), the clinician did not respond (n = 1) or the patients received a different diagnosis: one with Creutzfeldt-Jakob disease (Angus-Leppan et al., 2013), one with confirmed hereditary myoclonus dystonia (Patient DYT_11), one with a motor polyneuropathy and one with possible psychogenic movement disorder.
Eighteen (40%) of the remaining 45 patients came from the UK and the rest from elsewhere (Table 1). There were 24 males and 21 females, aged 1 to 75 years (median 50 years). Four of the females were under 15 years of age at onset. At the time of first GlyR antibody-positive test, the duration of symptoms ranged from <1 to 96 months, but the majority (71%) of presentations were described as acute (20%), subacute (44%) or subacute with acute exacerbation (7%). In the others, the onset was more chronic or insidious and 18% presented with exacerbations of pre-existing disease.
Table 1.
General features of patients with GlyR antibodies referred from 2008–12
| Patients and clinical characteristics | Total patients |
|---|---|
| Total number of patients | 45 |
| Age at onset (years) | 1–75 |
| Gender F:M | 21:24 |
| Children F:M | 4:0 |
| Disease presentation | |
| Duration at time of positive serum GlyR antibody | 0.1–96 (2.5) (only provided in 18 cases) |
| Acute | 9 (20%) |
| Subacute | 20 (44%) |
| Subacute with acute exacerbation | 3 (7%) |
| Chronic/insidious | 5 (11%) |
| Chronic with acute exacerbation | 8 (18%) |
| Preceding events/associated conditions at any time | |
| Autoimmune/inflammatory diseases (known at onset) | 13 (n = 1 psoriasis; n = 6 thyroid disease; n = 3 diabetes; n = 1 rheumatoid arthritis; n = 1 sarcoid; n = 1 mixed connective disease) (29%) |
| Tumours in past medical history and successfully treated | 5 (n = 1 breast cancer; n = 1 breast cancer and thymoma; n = 1 thymoma and lymphoma; n = 1 Hodgkin lymphoma; n = 1 malignant melanoma (11%) |
| Tumours identified during the current neurological illness | 4 (n = 3 thymoma; n = 1 B cell marginal zone lymphoma associated with monoclonal gammapathy IgM (10%). n = 1 metastases from previous treated breast cancer |
The 45 patients were from UK/Northern Ireland (n = 18), Belgium (n = 4), Cyprus (n = 1), France (n = 2), Germany (n = 3), Ireland (n = 1), Italy (n = 2), Netherlands (n = 1), Norway (n = 1), Portugal (n = 1), Spain (n = 3), Sweden (n = 1), Japan (n = 3), Korea (n = 1), New Zealand (n = 2), and Canada (n = 1).
Autoimmune and paraneoplastic associations
Comorbid autoimmune diseases were not uncommon (13/45, 29%, Table 1). Five patients had previous successfully-treated tumours (Table 1), and four tumours were first identified during the neurological illness (three thymomas, one B cell marginal zone lymphoma with IgM monoclonal gammopathy). In addition, one female patient with a previously treated breast cancer had metastases at presentation.
Clinical features
The presenting symptoms are summarized in Table 2. The commonest features (69%) were spasms, often painful, and stiffness and rigidity of the neck, trunk or limb muscles; these were associated with walking difficulties and frequent falls. Excessive startle (42%), and eye movement disorders (diplopia, ptosis, nystagmus, 40%) or difficulty opening the mouth, swallowing, or speech problems, were all frequent. Non-specific sensory symptoms (pruritus, dysasthesias, hyperaesthesias) in the limbs including pain unrelated to muscle spasms, were reported in 22% of patients. In addition, 29% of patients had cognitive disturbance or seizures (13%).
Table 2.
Clinical features of GlyR antibody-positive patients referred from 2008–12
| Clinical features | Clinical features at onset n (%) | Clinical features at peak n (%) |
|---|---|---|
| Total number of patients | 45 | 30 |
| Spasms/stiffness/rigidity/myoclonus (neck, trunk or limb muscles) | 31 (69%) | 24 (80%) |
| Oculomotor disturbance: nerve or gaze palsy (eyelid ptosis, diplopia, nystagmus, slow/jerky movements) | 18 (40%) | 16 (53%) |
| Trigeminal, facial and bulbar disturbance (dysphagia, dysarthria, difficulty chewing, facial numbness, trismus) | 21 (47%) | 17 (57%) |
| Excessive startle (spontaneous or triggered by noise or touch) | 19 (42%) | 17 (57%) |
| Walking difficulties/falls, mostly related to stiffness/rigidity/spasms | 19 (42%) | 24 (80%) |
| Limb paresis/pyramidal signs | 10 (22%) | 18 (60%) |
| Limb or gait cerebellar ataxia | 6 (13%) | 6 (20%) |
| Autonomic disturbance (hyper/hypohidrosis, dry mouth, brady/tachycardia, hypo/hypertension, bladder, bowel or sexual dysfunction) | 13 (29%) | 13 (43%) |
| Cognitive impairment/encephalopathy/seizures | 16 (36%) | 15 (50%) |
| Sensory symptoms/pain | 10 (22%) | 14 (47%) |
| Respiratory failure (admission in ICU/ventilation) | 8 (18%) | 8 (27%) |
ICU = intensive care unit.
The clinical features were also documented at peak of illness in 30 of the patients. At this stage, spasms, stiffness and rigidity were prominent (80%), with eye movement disturbance and facial/bulbar motor disturbance in ∼60%. Hyperekplexia and muscle weakness were more frequent by this stage, as were cognitive deficits, encephalopathy, and seizures. Autonomic disturbance was evident in 43%, with urinary retention particularly common, and respiratory failure was noted in 27%. Videos of representative patients are available with the published case reports (Hutchinson et al., 2008; Clerinx et al., 2011; Iizuka et al., 2012; Peeters et al., 2013; Damasio et al., 2013).
Investigations
Investigations performed were variable and the results are summarized in Table 3. In general MRI was uninformative, with no abnormality detected in 26/36, but in two patients there was evidence of inflammation in the temporal lobes, and two had other FLAIR lesions. Spinal cord MRI was uniformly negative in 18/23 patients but showed short or patchy lesions in four; one had longitudinally-extensive lesions. EMG was performed in 29; eight had continuous motor unit activity, six had spontaneous or stimulus-induced activity, two had evidence of sensory neuropathy and one had neuromyotonia, but 12 had no abnormality detected. EEG showed slow activity in 11/21, three with focal temporal lobe epileptiform activity and one with cortical disturbance; six patients had no EEG changes. CSF examination in 30 patients showed a pleocytosis in 13, a raised protein in four and six with oligoclonal bands. Serum GAD antibodies were reported in nine, but were only confirmed on GlyR antibody-positive samples as high titre (>1000 U/ml) in four. One patient had high VGKC-complex antibodies and two patients had low VGKC-complex antibodies, three had NMDA receptor antibodies (one reported in Turner et al., 2011; two low positive) and six had thyroid antibodies. No onconeural antibodies were reported.
Table 3.
Clinical investigations of GlyR antibody patients referred from 2008–12
| Total number of patients | 45 | |
|---|---|---|
| Investigations (number performed) | Results | Number of abnormal results/number tested |
| Brain MRI (n = 36) | n = 3 with white matter lesions; n = 1 atrophy; n = 2 temporal lobe inflammation; n = 2 other FLAIR lesions; n = 2 small vessel disease; n = 26 normal | 10/36 |
| Spinal cord MRI (n = 23) | n = 4 short or patchy lesions; n = 1 longitudinally-extensive lesion; n = 18 normal | 5/23 |
| EMG (n = 29) | n = 8 continuous motor unit activity; n = 6 spontaneous or stimulus-induced activity; n = 2 sensory neuropathy; n = 1 neuromyotonic discharges; n = 12 normal | 17/29 |
| EEG (n = 21) | n = 11 slow activity; n = 3 focal epileptic; n = 1 cortical disturbance; 6 normal | 15/21 |
| CSF (n = 30) | Any abnormality: n = 18 | 18/30 |
| n = 13 pleocytosis; n = 4 prot (>1 g/l); n = 6 OCB; normal n = 12 | ||
| GAD antibodies (n = 43) | 4 >1000 U/ml (∼25 000 IU/ml); 1 100 U/ml; 40 negative | 4/45 |
| Chest CT/whole body PET scan (n = 20) | n = 3 thymoma; n = 1 abdominal lymph nodes; n = 1 breast metastases; n = 15 normal | 5/20 |
| Other autoantibodies | n = 4 high GAD (>1000 U/ml); n = 1 high VGKC (2248 pM); n = 2 low VGKC (<200 pM); n = 3 low NMDAR; n = 6 thyroid; n = 1 ANA; n = 1 Sjogrens Syndrome; 15 normal; no paraneoplastic antibodies reported | 13/28 |
Onconeural antibodies were reported negative by the referring neurologists and were not retested in this study.
ANA = antinuclear antibodies; OCB = oligoclonal bands.
Classification
We asked the referring neurologists for their diagnoses but our final classification was based on Meinck and Thompson (2002) defining stiff person syndrome as axial stiffness with or without hypereflexia and/or autonomic disturbance, but no other apparent brainstem involvement, and PERM as stiff person syndrome but with clear brainstem involvement, sometimes with additional ataxia or seizures. We classified those with only brainstem features, epilepsy or limbic encephalitis as separate categories even though in some patients immunotherapies might have prevented them progressing to a diagnosis of stiff person syndrome or PERM.
The physicians’ diagnoses and our classification, with GlyR antibody titres in serum, and CSF when available, are shown for each patient in Table 4. Thirty-three patients were classified as PERM, two as stiff person syndrome (one with seizures), two with brainstem involvement (one respiratory, one auditory and balance problems), five with limbic or other encephalopathy without brainstem or spinal cord features, and three as ‘other’; the latter included two with probable demyelinating syndromes, and one with slowly progressive cognitive impairment and dizziness of unclear origin. In the majority of the patients the diagnosis of the referring neurologist was similar to our classification. However, there were two PERM patients who presented with myasthenia gravis-like weakness and stimulus-sensitive myoclonus, one with a history of Hodgkin’s lymphoma, the other with a thymoma; neither had acetylcholine receptor or MUSK antibodies. One PERM patient with co-existing high VGKC-complex antibodies was originally diagnosed as Morvan’s syndrome; another PERM patient had a diagnosis of post-encephalitic Parkinson’s disease but made a striking recovery with immunotherapies. Although the higher GlyR antibodies tended to be found in the patients with PERM or brainstem encephalitis, there was no clear relationship between GlyR antibody titre and clinical picture (Table 4).
Table 4.
Diagnoses, tumours, immunotherapies, outcomes and relapses in 45 patients with GlyR-Abs
| Sex, age | Physicians diagnosis | Our classification | Serum titre | CSF titre | Tumours newly identified or in past history | mRS maximum | Immuno-therapies | mRS final | Second line or ongoing treatments | Relapses after GlyR antibody identification and immuno-therapies |
|---|---|---|---|---|---|---|---|---|---|---|
| M,28 | PERM + seizures Turner et al. (2011) | PERM | 1280 | No tumour identified | 5 | None | 6 | Died | ||
| M,68 | PERM | PERM | 640 | No tumour identified | 5 | St, PEx, IvIg | 6 | Died | ||
| M,56 | PERM | PERM | Not titrated | No tumour identified | 5 | St, PEx, CyP | 6 | Died | ||
| M,34 | PERM/MG-like plus myoclonus | PERM | 160 | 80 | Thymoma | 5 | Not known | 6 | Died after PE before thymectomy | |
| M,75 | PERM | PERM | 1280, high GAD-Ab | 80 | Thymoma | 5 | PEx | 1 | Thymectomy | No relapse, died subsequently unclear cause |
| M,49 | PERM Clerinx et al. (2011) | PERM | 20 (post PEx) | Thymoma | 5 | St, PEx | 0 | Thymectomy | No | |
| M,72 | PERM/Morvan’s syndrome | PERM | 640 | Marginal zone B cell lymphoma | 5 | St, PEx | 1 | Rtx, CyP with lymphoma treatment | No | |
| F,65 | PERM | PERM | Not titrated | Metastases from PH breast cancer | 5 | St, PEx | 1 | No relapse, died later from breast cancer metastases | ||
| F,50 | Brainstem syndrome (1999), seizures (2007), atypical SPS | PERM | 80, high GAD-Ab | PH breast cancer 1999, thymoma 2007 | 4 | PEx | 1 | Rtx, CyP | No further relapses | |
| M,51 | PERM/MG-like plus myoclonus | PERM | 320 | 80 | PH Hodgkin’s lymphoma | 3 | St, IvIg | 4 | St | Relapsing-remitting course |
| F,37 | PERM Peeters et al. (2013) | PERM | 1280 | 640 | No tumour | 5 | St, PEx | 1 | Aza | One relapse |
| M,29 | PERM Supplementary Case 1 | PERM | 160 | 160 | No tumour | 5 | St | 1 | St/Aza | No subsequent relapse |
| M,58 | PERM Piotrowicz et al. (2011) | PERM | 2560 | 80 | No tumour | 4 | St, PEx | 1 | Aza then Myc | One relapse |
| F,14 | PERM | PERM | 640 | No tumour | 4 | St, PEx, IvIg | 1 | One relapse | ||
| M,54 | PERM Hutchinson et al. (2008), Sample from relapse | PERM | 2560 | 5 | No tumour | 5 | St, PEx, IvIg | 2 | Myc, IvIg post relapse | One relapse |
| M,40 | PERM Stern et al. (2014) | PERM | 1280 | No tumour | 5 | St, PEx, IvIg | 3 | One relapse | ||
| M,48 | PERM Mas et al. (2011) | PERM | 640 | No tumour | 4 | St, IvIg | 2 | No relapse but chronic course | ||
| M,54 | PERM/rhomboencephatis Supplementary Case 2 | PERM | 80 | 20 | No tumour | 5 | St, PEx, CyP | 2 | CyP, reducing St | No relapse, still improving (Supplementary Case 2) |
| F,61 | Post-encephalitic parkinsonism | PERM | 640 | No tumour | 5 | St, IvIg | 2 | No relapse but not complete recovery | ||
| F,61 | PERM | PERM | 20 | No tumour | 5 | IvIg | 0 | St, IvIg continuing | No | |
| F,28 | Brainstem encephalomyelitis Supplementary Case 3 | PERM | 1280 | No tumour | 4 | None | 0 | No | ||
| F,61 | PERM without startle Iizuka et al. (2012) | PERM | 1280 | 640 | No tumour | 5 | St, IvIg, CySp | 0 | CySp | No |
| F,47 | PERM with seizures | PERM | 80 | No tumour | 5 | IvIg | 1 | No | ||
| M,69 | PERM | PERM | 160 | No tumour | 5 | St, IvIg | 1 | No | ||
| F,1 | PERM Damasio et al. (2013) | PERM | 320 | No tumour | 5 | St, IvIg | 1 | Myc + IvIgreducing IvIg | No | |
| F,40 | PERMS/Jerking SPS | PERM | 160 | No tumour | 3 | St | 1 | No | ||
| M,39 | SPS | PERM | 640 | No tumour | 3 | PEx, IvIg | 1 | No | ||
| F,33 | PERM Mas et al. (2011) | PERM | Not titrated | No tumour | 4 | St, IvIg | 1 | No | ||
| M,50 | PERM | PERM | 80 | No tumour | 5 | St, IvIg | 2 | No | ||
| M,56 | PERM Bourke et al., (2014) | PERM | 60000 | 80 | No tumour | 5 | St, IvIg | 2 | Reducing St | No |
| M,49 | SPS atypical | PERM | 80 | No tumour | 4 | PEx | 2 | Reducing St | No | |
| M,53 | SPS | PERM | 80 | No tumour identified | 5 | St | 1 | LFU | ||
| M,60 | PERM plus Mas et al. (2011) | PERM | 200 | No tumour identified | 5 | None | 5 | LFU | ||
| F,22 | SPS | SPS | 320 | PH thymoma, lymphoma | 4 | St | 0 | No | ||
| F,53 | SPS | SPS | 80, high GAD-Ab | 5 | No tumour | 4 | St, PEx | 3 | Chronic course | |
| F,5 | Epileptic encephalopathy | Epileptic encephalopathy | 2560 | No tumour | 5 | St, PEx, IvIg | 3 | No | ||
| M,25 | Limbic encephalitis with status epilepticus | Epileptic encephalopathy | 640 | No tumour | 5 | St, IvIg | 1 | No | ||
| F,55 | Limbic encephalitis | Epileptic encephalopathy | Not titrated | No tumour | 4 | IvIg | LFU | No | ||
| F,31 | Meningo-encephalitis | Epileptic encephalopathy | 40 | No tumour | 4 | None | 3 | No known relapse | ||
| F,48 | Recurrent encephalopathy | Encephalopathy recurrent | 80 | No tumour | 3.0 | None | 2 | No known relapse | ||
| F,58 | Mild PERM with hypoventilation episodes Bourke et al. (2014) | Brainstem encephalitis with respiratory failure | 1280 | PH malignant melanoma | 4 | None | 0 | No | ||
| M,68 | Steroid-responsive deafness predominantly | Brainstem encephalitis with autoimmune deafness | 10240 | No tumour | 2.0 | St | 1 | Aza | No | |
| F,8 | ADEM with optic neuritis | Other | 320 | No tumour | 5 | St, IvIg | 1 | No | ||
| M,28 | Chronic relapsing inflammatory optic neuritis | Other | 640 | No tumour | 2.0 | St | 0 | No | ||
| F,70 | Slow cognitive decline, not Alzhiemer’s disease | Other | 100 | No tumour | 2.0 | None | 1 | No |
ADEM = acute demyelinating encephalomyelitis; St = steroids; IvIg = intravenous immunoglobulins; PEx = plasma exchange; Myc = mycophenolate mofetil; Aza = azathioprine; CyP = cyclophosphamide; CySp = cyclosporine; PH = past history; mRS = modified Rankin scale; SPS = stiff person syndrome; MG = myasthenia gravis; PE = plasma exchange; Rtx = rituximab; LFU = lost to follow-up.
Some samples were insufficient for titration.
The four children were female, and although two had PERM (Damasio et al., 2013; Chan et al., in press), one had an epileptic encephalopathy (Hacohen et al., 2013) and the other optic neuritis/acute demyelinating encephalomyelitis (ADEM).
Severity at onset, treatments and outcomes
Modified Rankin scores were provided by the neurologists on the questionnaires and by e-mail after subsequent follow-up. The scores and immunotherapies are given in Table 4. Although at maximum severity three patients had mild (score 2), and four had only moderate (score 3) disability, the remaining 38 patients were graded as 4 (n = 12) or 5 (n = 26). Twenty-four of thirty-three (72%) patients with PERM were graded as modified Rankin scale 5.
Most patients were given symptomatic treatments that were often helpful. The patients with neoplastic disease at presentation were treated with surgery (two removal of thymoma), or chemotherapy (one B cell lymphoma) and improved dramatically (immunotherapies also given). The remaining patient (myasthenia gravis-like presentation) with new radiological diagnosis of thymoma died before surgery as a result of a pulmonary embolism.
Immunotherapies were given in 37 patients (none given in seven, information unavailable in one; Table 4). Approaches to immunotherapy were variable but typically started with high dose (e.g. 1 mg/kg) prednisolone, often preceded by intravenous methyl-prednisolone, and followed by plasma exchange or intravenous immunoglobulin, or both; the latter were often repeated and the steroids generally weaned slowly. Three had additional cyclophosphamide, one received cyclosporine and two rituximab (one as part of his lymphoma treatment) before discharge (Table 4). A few are on azathioprine or mycophenolate.
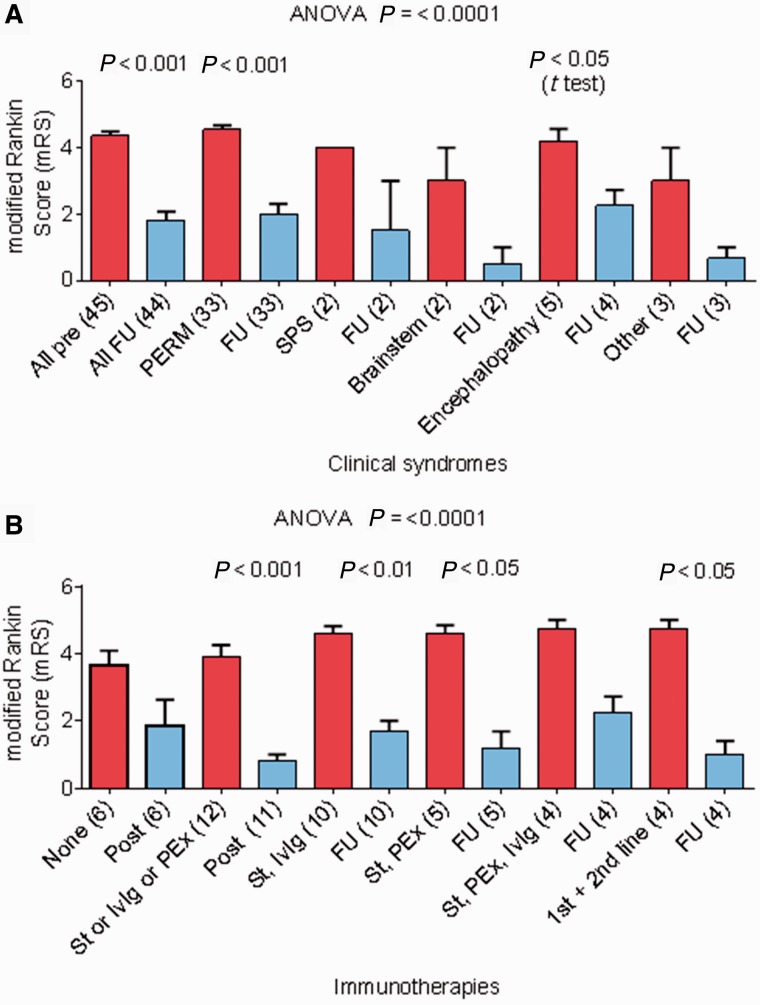
Duration of follow-up in surviving patients was from 18 months to 7 years (median 3 years). The modified Rankin scale scores at latest follow-up are included in Table 4, and shown graphically stratified according to disease classification (Fig. 2A) or immunotherapies used (Fig. 2B). The outcomes were generally very good with the modified Rankin scale scores falling from a median of 5 at maximum severity to 1. However, four patients had died in hospital, two during the acute illness (one in Turner et al., 2011) and two from indirect causes (one pulmonary embolism while recovering from respiratory failure, one after a fall while on warfarin); these patients are included in Fig. 1A but not in Fig. 2B because long-term follow-up was not available. In addition, two who had made good responses, died subsequently of non-neurological conditions (one recurrence of breast cancer metastases, one systemic oedema with cardiac and renal failure of unknown cause). Another patient had a cardiac arrest during his illness (Mas et al., 2011) and was in a vegetative state when last known. Three case histories, not previously reported, describe some of the clinical features and the variable treatments required (Supplementary material).
Figure 2.
Modified Rankin scale (mRS) scores at maximum severity and at recent follow-up in patients with PERM or related syndromes. One patient was lost to long-term follow-up. (A) Modified Rankin scale scores according to the clinical syndrome compared between maximum severity and follow-up in all patients including the four who died (modified Rankin scale 6). (B) Modified Rankin scale scores before and after different immunotherapies for all patients, excluding the four who died. Further details are available in Table 4. FU = follow-up; PEx = plasma exchange; IvIg = intravenous immunoglobulins; St = steroids.
Relapses
Relapses following successful treatment have occurred in the index case (Hutchinson et al., 2008) 9 years after good recovery, presenting with worse mobility, some limb stiffness but no recurrence of ophthalmoplegia or hyperekplexia. The patient is on mycophenolate and intravenous immunoglobulin and has a baclofen pump. Another male patient had had two episodes treated with steroids before the third, when the GlyR antibodies were first detected (Peeters et al., 2013). A year after discharge, while receiving steroids (8 mg ad) and plasma exchange every 3–4 months, he had a relapse (fourth event) with dysphagia, diplopia, bilateral ptosis, neck rigidity and severe slowing of vertical saccades. He was given intravenous methyl-prednisolone followed by tapering oral prednisolone, and azathioprine (100 mg daily) was commenced. Current modified Rankin scale is 1. Another patient with similar relapse history is described in the Supplementary material (Case 1). Three other patients have had relapses following discharge and good recovery. These and ongoing immunotherapies are summarized in Table 4.
Characterization of glycine receptor antibodies
Serum and CSF GlyR antibodies were found to be predominantly of the complement-fixing IgG1 subclass with some IgG3 and, as expected for this IgG subclass, the antibodies were able to activate complement on the cell surface of live GlyR-α1 expressing HEK cells, as shown by the deposition of C3b (Fig. 1F). We also looked for the ability of the antibodies to induce temperature-dependent internalization of the GlyRs. Incubation with PERM sera (1:80) at 37°C, but not at 4°C or when the cells were fixed before serum incubation at 37°C, resulted in a reduction in surface IgG binding (Fig. 3A and B) and internalization of IgG (Fig. 3C and D). This was associated with internalization and loss of GlyR-EGFP expression (Fig. 3E and F), which appeared within the HEK cells co-localizing with the late endosomal marker LAMP2 (Fig. 3G and H).
Figure 3.
GlyR antibodies and GlyRα1s are internalized in HEK293 cells and GlyRα1 is targeted to the lysosomal pathway. (A) GlyR antibodies (Ab; red) bound to the surface of GlyRα1-EGFP (green) transfected HEK cells (shown at 0 h and 16 h). Loss of surface bound GlyR antibody is seen by 16 h when the cells are incubated at 37°C, but not when incubated at 4°C, or when fixed before incubation. (B) Results from four GlyR antibody-positive sera compared with four healthy individuals’ (HC) sera with the surface IgG binding scored after different incubation times. Two-way ANOVA (F = 7.832, df = 20, P < 0.0001). Significant differences are marked with asterisks. (C) By 2 h of incubation at 37°C, the surface bound GlyR antibodies (green) are partly lost but can be detected after fixing and permeabilization (perm) with anti-human IgG (red). Unpermeabilized (Not perm) cells did not show internalized IgG. (D) The percentage of cells with internalized GlyR antibody at 0 and 2 h. Results from two sera were compared between extracellular (green) and intracellular (red) IgG using a two-sided students t-test (*t = 9.448, df = 18, P < 0.0001; **t = 8.707, df = 18, P < 0.0001). (E) GlyR antibody binding causes internalization of the GlyRα1. After 1 h treatment with cyclohexamide, HEK cells expressing GlyRα1-EGFP were incubated at 37°C with patient or healthy control sera for 2 h. Incubation with patient serum led to a decrease in the percentage of cells expressing GlyRα1-EGFP (upper row), but not with control serum (lower row), as shown also at higher resolution on the right in cells post-labelled with DAPI (blue). (F) Quantification of GlyRα1-EGFP internalization from two experiments with a single serum. Groups were compared using a two-sided Student’s t-test (**t = 10.15, df = 18, P < 0.0001; *t = 15.01, df = 18, P < 0.0001). (G) Internalized GlyRα1-EGFP is targeted to the lysosome. The GlyRα1-EGFP expressing HEK cells were labelled with antibodies to the late endosomal marker (LAMP2). Internalized GlyRα1-EGFP (green) co-localized with LAMP2 (red) in cells exposed to patients’ serum but co-localization was much less in cells exposed to healthy control serum. (H) The percentage of cells with co-localization of GlyRα1-EGFP and LAMP2 from two experiments with one serum from GlyR antibody and one healthy control. Groups were compared using a two-sided Students t-test (*t = 8.515, df = 18, P < 0.0001; **t = 13.73, df = 18, P < 0.0001).
Binding of the sera to other glycine receptor α subunits
Twenty-five sera were tested for binding to GlyRα1, 2 and 3 subunits as homomers. At 1:80 serum dilution, eight patients had antibodies only to the GlyRα1 subunits, four had antibodies that bound to GlyRα1 and GlyRα3 subunits, and the remaining 13 had antibodies that bound to all three GlyR α subunits (Supplementary Fig. 1A). These different reactivities did not relate to the overall GlyRα1 antibody titres (Supplementary Fig. 1B). Although expression of all three GlyRα subunits in the HEK cells slightly increased antibody binding in a few samples, pre-adsorption of the GlyR antibodies against HEK cells expressing GlyRα1, GlyRα2 or GlyRα3 subunits, eliminated or substantially reduced binding to GlyRα1 indicating that the majority of the GlyR antibodies bound to an epitope common to all three α subunits (data not shown).
Binding of sera to rodent brain and spinal cord sections
GlyRα subunit expression in rodent brain was first examined using monoclonal antibodies to GlyRα1, GlyRα2 and GlyRα3 (Supplementary Table 1). The GlyRα1 was strongly expressed in the neuropil and on the cell surface of specific neurons in the brainstem and spinal cord, with no detectable expression in the hippocampus, cerebral or cerebellar cortex. GlyRα2 and GlyRα3 binding was mainly intracellular and in additional areas such as the hippocampus, striatum, cerebellar cortex and layers III–VI of the cerebral cortex. GlyRα3 was also found on dendrites in the hippocampus, granular cell layer of the cerebellum, brainstem and spinal cord (Supplementary Fig. 2).
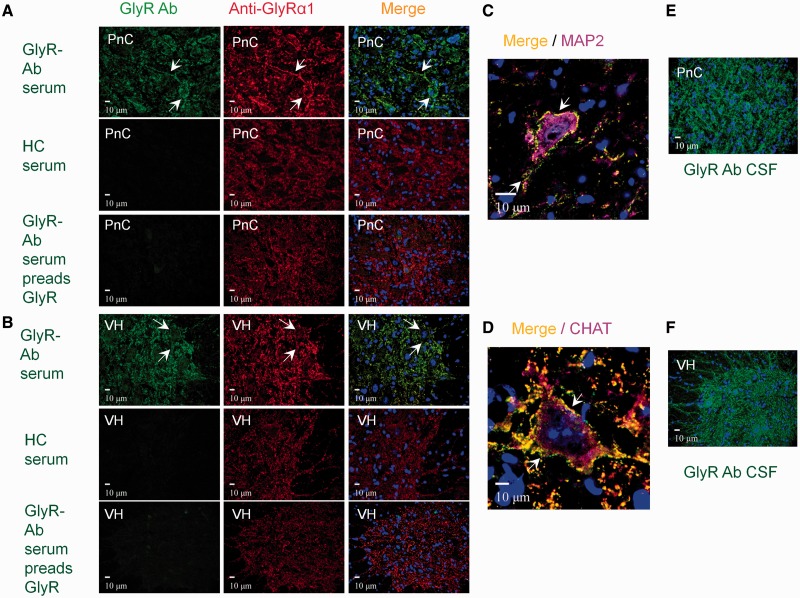
Patient’s sera were then examined for binding to rat brain sections (at 1:200 to 1:800 dilution) and co-localization with the commercial antibodies. All the sera bound in a punctate manner to the cell bodies and neuropil of the brainstem and both ventral and dorsal horns of the spinal cord, and co-localized with monoclonal antibodies to GlyRα1 (Fig. 4A and B). This was clearly seen on large neurons, co-stained with antibodies to MAP2, in the pedunculo reticular complex in the brainstem (Fig. 4C), and on motor neurons co-stained with antibodies to choline acetyltransferase in the spinal cord (Fig. 4D). Dorsal horn neurons were also clearly stained (data not shown). The CSF staining patterns were similar to those observed with sera (Fig. 4E and F). In addition, the four AD antibody-positive sera bound in an uneven punctate distribution to the molecular and granular cell layer of the cerebellum and in the molecular and pyramidal striatum in the cornu ammonis and dentate gyrus of the hippocampus (Supplementary Fig. 3). This intracellular binding co-localized with GAD. Pre-adsorption experiments with mock-transfected or GlyRα1-expressing HEK cells, or with soluble GAD, confirmed the specificity of each antibody (Supplementary Fig. 3). Other sera, in addition to GlyRα1 co-localization, also bound to intracellular antigens that were widely distributed and were not adsorbed by GlyRα1-HEK cells or soluble GAD (data not shown). Representative results of seven sera are summarized in Supplementary Table 2.
Figure 4.
GlyR antibody-positive patient serum from a typical PERM patient binds to CNS regions involved in motor regulation. (A) Top row: Double labelling of neurons in the pontine reticular nucleus (PnC) of the brainstem with patient serum (green) and monoclonal antibody to the GlyRα1 (red) show colocalization on the neuronal soma and in the neuropil (arrows); nuclei are stained with DAPI (blue). Middle row: Healthy control serum did not bind to the pontine reticular nucleus. Lower row: After pre-adsorption against HEK-GlyRα1 cells there was a marked decrease in pontine reticular nucleus staining. (B) Similar results are shown for binding to the ventral horn of the spinal cord. (C and D) Confocal images show the patient’s serum (green) colocalizing with GlyRα1 monoclonal antibody (red) on the surface of a large neuron in the pontine reticular nucleus, which is labelled with the cytoplasmic neuronal marker anti-MAP2 antibody (purple), or on a motor neuron in the ventral horn of the spinal cord labelled with motor neuron marker anti-choline acetyltranferase antibody [CHAT (purple)]. (E and F) CSF from the same patient binds in a similar pattern. Results of seven representative patients are summarized in Supplementary Table 2. HC = healthy control.
Retrospective cohort of patients with stiff person syndrome and related syndromes
We also analysed sera and/or CSFs archived over many years from patients (17 males, 34 females; ages 13–72 years) with diagnoses of stiff person syndrome (n = 21, including two with stiff leg syndrome), PERM (n = 24) or suspected acquired hyperekplexia (n = 6). Thirty of the 47 tested were positive for GAD antibodies. GlyR antibodies were found in six sera and four CSFs. Only one of the seven patients with GlyRα antibodies also had raised GAD antibodies at a high level (>1000 U/ml). The clinical details of these patients and treatment responses are given in Supplementary Table 3.
Discussion
GlyR antibodies have only recently been recognized. Here we document the first prospectively-diagnosed cohort of 45 patients, classifying their clinical syndromes and characterizing their antibodies. Thirty-three patients were classified as PERM, but the disorders of eye movements, speech or swallowing required exclusion of myasthenia in two patients. Autonomic disturbance was marked in many patients and respiratory failure may have contributed to two of the four hospital deaths. A few of the patients had seizures or other supratentorial involvement but in addition there were five patients who only had encephalopathies with seizures and two with predominant brainstem involvement; three had unclear or other diagnoses. Despite the delay in a specific diagnosis in some cases, and the variable immunotherapies used, the majority of patients showed a good or very good response to these treatments, contrasting with previous studies of PERM, but relapses have occurred subsequently in six.
The patients were identified by the presence of a positive GlyR antibody but the final clinical classification was based principally on Meinck and Thompson (2002) and Espay and Chen (2006) defining PERM as brainstem involvement in addition to the axial or limb rigidity typical of stiff person syndrome in its varied forms (Brown and Marsden, 1999). Stiff person syndrome may evolve into PERM (Meinck and Thompson, 2002; Espay and Chen 2006) and we did find very high GAD antibody levels, typically associated with stiff person syndrome, in three patients with diagnoses of PERM (one in Iizuka et al., 2012), one of whom had a previous diagnosis of stiff person syndrome. The presence of both antibodies has also been reported in a proportion of archived adult and paediatric patients (Clardy et al., 2013; McKeon et al., 2013) similar to those in the historical cohort that we tested here (Supplementary Table 3). The coexistence of GlyR and GAD antibodies suggests that neuronal surface antibodies, perhaps to different targets, could determine the clinical phenotypes of patients with GAD antibody associated diseases, which include cerebellar ataxia and a seizure-dominant form of limbic encephalitis (Malter et al., 2010; Saiz et al., 2008). Indeed in an earlier report we showed that some patients with stiff person syndrome have additional antibodies to unidentified antigen(s) on the surface of GABAergic neurons (Chang et al., 2013). Overall, our prospective cohort suggests that, although GAD antibodies can co-exist with GlyR antibodies, the latter usually appear independently and signify a good clinical response to immunosuppressive therapies. This is better than that expected in patients with typical stiff person syndrome, who show a variable response to immunotherapy with infrequent recovery (Dalakas et al., 2001; McKeon et al., 2012), possibly due to T cell-mediated cytotoxicity as observed in some post-mortem studies (Costa et al., 2002; Warren et al., 2002; Holmøy et al., 2009).
The GlyR antibody levels varied widely between individuals in sera and CSFs and there was exceptionally high intrathecal synthesis of the specific GlyR antibodies in three of the six available for detailed study (Fig. 1E). It seems that high CSF levels are common in this condition, and may even be associated with undetectable serum antibody in retrospective cohorts (Supplementary Table 3; Clardy et al., 2013; McKeon et al., 2013), but we did not encounter this in our prospective patients. Perhaps in some patients with PERM or related syndromes there is an insidious onset with a maturing immune response that localizes to the CNS and continues to produce GlyR antibodies there even if the peripheral immune response decreases spontaneously over time. Nevertheless, the original case (Hutchinson et al., 2008) examined at relapse in 2011 had low CSF GlyR antibodies (1:5) compared with very high serum levels (1:2560; Table 4). The relationship between serum and CSF levels of this antibody and clinical status is complex as for other cell surface antibodies.
Because the antibodies were predominantly IgG1 and deposited complement on GlyR-transfected cells, it is possible that complement-mediated mechanisms contribute to the disease pathology in vivo. However, NMDAR antibodies are predominantly IgG1 (Irani et al., 2010), but do not seem to cause complement-mediated damage (Dalmau et al., 2008; Tuzun et al., 2009). Another potential mechanism would be loss of GlyRs by internalization following divalent antibody binding; this occurred in GlyR-transfected HEK cells, with a time course similar to that reported for antibodies to other cell surface antigens such as acetylcholine receptors (Drachman et al., 1978), although not muscle-specific kinase (Koneczny et al., 2013). Internalization has been shown for NMDAR antibodies in live neurons in culture (Dalmau et al., 2008; Hughes et al., 2010), but obtaining cultured inhibitory neurons in sufficient numbers for quantitative analysis of internalization of GlyRs is not easy. Regardless of the pathogenic mechanisms involved, which could include a direct inhibition of GlyR function, the normality in the MRI images and the substantial recovery after immunotherapies in the majority of patients argues against a destructive process and suggests direct inhibition or internalization of the GlyRs as likely causes of the dysfunction.
GlyRs belong to the superfamily of ligand gated ion channels and are pentameric proteins composed of two α and three β subunits (although homomers are also expressed on the cell surface as used here for the study of subunit specificity). Glycine activation of the GlyR leads to an influx of Cl− into the neurons and results in hyperpolarization of the membrane potential and reduced excitation (Legendre, 2001; Lynch, 2004, 2009). GlyRα1, identified by a highly specific monoclonal antibody, was mainly expressed in the upper and lower brainstem, the spinal cord and in the thalamus, hypothalamus and the colliculus (Supplementary Table 1). This distribution was similar to that previously reported (Araki et al., 1988; Rampon et al., 1996). The distribution of the GlyRα2 and GlyRα3 in the brain tissue, by contrast, is less clear. GlyRs and/or GlyR-subunit messenger RNA transcripts have been detected in areas such as the cerebellum, basal ganglia, limbic system and cerebral cortex, but functional roles have only been identified in the cerebellum and hippocampus where inhibitory currents have been recorded (Dieudonne, 1995; Song et al., 2006; reviewed by Xu and Gong, 2010), and in the spinal cord (Harvey et al., 2004). Using the monoclonal antibodies available to us (which identified the appropriate subunits on transfected HEK cells, Supplementary Fig. 1), the GlyRα2 and GlyRα3 appeared principally intracellular and their roles in the clinical features are unclear.
None of the GlyR antibody sera (tested at a higher dilution than that used on the HEK cells) co-localized with the intracellular binding of monoclonal antibodies to GlyRα2 or α3, but the binding to the surface of brainstem and spinal cord neurons co-localized with monoclonal antibodies to GlyRα1, confirming the specificity and likely targets of the antibodies. A number of sera also bound to other brain regions, notably the cerebellum, basal ganglia and hippocampus, where GlyRα1 was not observed. In four patients this intracellular staining was due to co-existing GAD antibodies but in others there could be novel intracellular antigen(s), although antibodies against intracellular antigens are unlikely to be pathogenic. Overall, the patients’ serum binding was mainly directed against those regions, brainstem and spinal cord, where impaired GlyR expression is likely to underlie the key features of these diseases. Although mainly thought to regulate motor and sensory excitation in the spinal cord, GlyRs have also been shown to have a role in visual and auditory processing (Wässle et al., 2009; Dutertre et al., 2012); GlyR antibodies reducing the activity of GlyRs in these pathways could explain the dizziness or deafness in two patients and possibly the visual disturbance in two who were considered to have demyelinating syndromes (McKeon et al., 2012). GlyRs are also expressed in regions known to regulate autonomic function; for instance, the locus coeruleus, nucleus solitarius, and the rostral ventrolateral medulla (Cabot et al., 1992; Rampon et al., 1996; Waldvogel et al., 2010); in these sites a reduction in GlyR control of sympathetic activity by the GlyR antibodies could be responsible for the increased sympathetic tone found in the patients, e.g. tachycardia and urinary retention and, as the rostral ventrolateral medulla is involved in generating the rhythmic respiratory pattern, the respiratory failure observed in some patients (Paton and Ritcher, 1995).
In conclusion, detection of GlyR antibodies may prove helpful in the diagnosis of patients with symptoms and signs that include ocular motor and other brainstem dysfunction, hyperekplexia, stiffness, rigidity, myoclonus and spasms, and their detection will support the use of immunotherapies that are likely to be clinically effective. Investigations (e.g. CSF, MRI, EEG and EMG) may be normal, but neoplasia, particularly thymomas and lymphomas are not uncommon. Although in the majority of cases serum antibodies are sufficient, paired CSFs are valuable for comparison and can be helpful in rare cases when serum levels (after dilution to 1:20) are low (Supplementary material; Case 2). The detection of the antibodies in encephalopathic or demyelinating syndromes, or with brainstem features of auditory or vestibular dysfunction, suggests that there can be limited or more diverse phenotypes. Finally, involvement of autonomic and respiratory systems in many patients may be responsible for unexplained deaths, and relapses may occur following successful treatment, indicating that long-term follow-up and maintenance immunosuppression should be considered.
Supplementary Material
Acknowledgements
We are very grateful to Prof. Peter Brown for his helpful comments, to all members of the Glycine receptor antibody study group who provided clinical data, and Ms Linda Clover for help with immunohistology. The GlyR alpha 2 clone was the kind gift of Prof. R Harvey.
Glossary
Abbreviations
- GAD
glutamic acid decarboxylase
- GlyR
glycine receptor
- HEK
human embryonic kidney cells
- NMDAR
N-methyl-D-aspartate receptor
- PERM
progressive encephalomyelitis with rigidity and myoclonus
- VGKC
voltage-gated potassium channel
Appendix 1
Glycine receptor antibody study group
Mirdhu Wickremaratchi, Alberto Cifelli, Anu Jacob, Richard Perry, Robin Howard, Cannan Nithi, Martin Turner, John McConville, Stanley Hawkins, Michael Watt, Tom Esmonde, Jim Morrow (UK), Ludo Vanopdenbosch (Belgium), Kleopas Kleopa (Cyprus), Jean Gallard, Veronique Rogemond, Jerome Honnorat (France), Jens Reimann (Germany), Sean O’Reardon (Ireland), Luca Durelli, Luigi Zuliani (Italy), Peter Maat (Netherlands), Kristi Alvik (Norway), Joana Damásio (Porto), Francesc Graus (Spain), Christian Berger (Switzerland), Andre Mattman (Canada), Noriko Nishikawa, Takahiro Iizuka, Kokoro Ozaki (Japan), Se Hee Kim (Korea), Neil Anderson (New Zealand).
Funding
A.C.-G. thanks Colfuturo-Colciencias for fellowship support. Further support was provided by the Oxford NIHR Biomedical Research Centre and the NHS National Commissioning Group (M.I.L., P.W., M.W., A.V.) and the NIHR Biomedical Research Centre at UCLH NHS Foundation Trust (M.P.L.).
Conflict of interest
The University of Oxford holds patents and receives royalties and payments for antibody tests, and AV, PW and BL receive a share of royalties for VGKC-complex antibodies. There is no patent on glycine receptor antibodies.
Supplementary material
Supplementary material is available at Brain online.
References
- Alexopoulos H, Dalakas MC. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur J Clin Invest. 2010;40:1018–25. doi: 10.1111/j.1365-2362.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology. 2013;81:1962–4. doi: 10.1212/01.wnl.0000436617.40779.65. [DOI] [PubMed] [Google Scholar]
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol. 2013;70:919–22. doi: 10.1001/jamaneurol.2013.2077. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–24. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Bourke D, Roxburgh R, Vincent A, Cleland J, Jeffery O, Dugan N, et al. Hypoventilation in glycine-receptor antibody related progressive encephalomyelitis, rigidity and myoclonus. J Clin Neurosci. 2014;21:876–8. doi: 10.1016/j.jocn.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol. 1999;246:648–52. doi: 10.1007/s004150050425. [DOI] [PubMed] [Google Scholar]
- Cabot JB, Alessi V, Bushnell A. Glycine-like immunoreactive input to sympathetic preganglionic neurons. Brain Res. 1992;571:1–18. doi: 10.1016/0006-8993(92)90504-3. [DOI] [PubMed] [Google Scholar]
- Chan DWS, Thomas T, Lim M, Ling S, Woodhall M, Vincent A. Focal status epilepticus and progressive dyskinesia: a novel phenotype for glycine receptor antibody-mediated neurological disease in children. Dev Med Child Neurol. 2014 doi: 10.1016/j.ejpn.2016.08.013. in press. [DOI] [PubMed] [Google Scholar]
- Chang T, Alexopoulos H, McMenamin M, Carvajal-Gonzalez A, Alexander SK, Deacon R, et al. Neuronal surface and glutamic acid decarboxylase autoantibodies in nonparaneoplastic stiff person syndrome. JAMA Neurol. 2013;70:1140–9. doi: 10.1001/jamaneurol.2013.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clardy SL, Lennon VA, Dalmau J, Pittock SJ, Jones HR, Jr, Renaud DL, et al. Childhood onset of stiff-man syndrome. JAMA Neurol. 2013;70:1531–6. doi: 10.1001/jamaneurol.2013.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerinx K, Breban T, Schrooten M, Leite MI, Vincent A, Verschakelen J, et al. Progressive encephalomyelitis with rigidity and myoclonus: resolution after thymectomy. Neurology. 2011;76:303–4. doi: 10.1212/WNL.0b013e318207b008. [DOI] [PubMed] [Google Scholar]
- Costa M, Saiz A, Casamitjana R, Castaner MF, Sanmarti A, Graus F, et al. T-cell reactivity to glutamic acid decarboxylase in stiff-man syndrome and cerebellar ataxia associated with polyendocrine autoimmunity. Clin Exp Immunol. 2002;129:471–8. doi: 10.1046/j.1365-2249.2002.01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas MC, Fujii M, Li M, Lutfi B, Kyhos J, McElroy B. High-dose intravenous immune globulin for stiff-person syndrome. New Engl J Med. 2001;345:1870–6. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio J, Leite MI, Coutinho E, Waters P, Woodhall M, Santos MA, et al. Progressive encephalomyelitis with rigidity and myoclonus: the first pediatric case with glycine receptor antibodies. JAMA Neurol. 2013;70:498–501. doi: 10.1001/jamaneurol.2013.1872. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Glycinergic synaptic currents in Golgi cells of the rat cerebellum. Proc Natl Acad Sci USA. 1995;92:1441–5. doi: 10.1073/pnas.92.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298:1116–22. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J Biol Chem. 2012;287:40216–23. doi: 10.1074/jbc.R112.408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Chen R. Rigidity and spasms from autoimmune encephalomyelopathies: stiff-person syndrome. Muscle Nerve. 2006;34:677–90. doi: 10.1002/mus.20653. [DOI] [PubMed] [Google Scholar]
- Geis C, Grunewald B, Weishaupt A, Wultsch T, Toyka KV, Reif A, et al. Human IgG directed against amphiphysin induces anxiety behavior in a rat model after intrathecal passive transfer. J Neural Transm. 2012;119:981–5. doi: 10.1007/s00702-012-0773-3. [DOI] [PubMed] [Google Scholar]
- Geis C, Weishaupt A, Hallermann S, Grunewald B, Wessig C, Wultsch T, et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133:3166–80. doi: 10.1093/brain/awq253. [DOI] [PubMed] [Google Scholar]
- Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, Cross H, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84:748–55. doi: 10.1136/jnnp-2012-303807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N, Grunewald B, Weishaupt A, Colaco MN, Toyka KV, Sommer C, et al. Human Stiff person syndrome IgG-containing high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp Neurol. 2013;239:202–9. doi: 10.1016/j.expneurol.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–7. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Holmøy T, Skorstad G, Roste LS, Scheie D, Alvik K. Stiff person syndrome associated with lower motor neuron disease and infiltration of cytotoxic T cells in the spinal cord. Clin Neurol Neurosurg. 2009;111:708–12. doi: 10.1016/j.clineuro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–2. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Leite MI, Lang B, Waters P, Urano Y, Miyakawa S, et al. Glycine receptor antibodies are detected in progressive encephalomyelitis with rigidity and myoclonus (PERM) but not in saccadic oscillations. J Neurol. 2012;259:1566–73. doi: 10.1007/s00415-011-6377-2. [DOI] [PubMed] [Google Scholar]
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–67. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6:383–92. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8:e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain. 2008;131:1940–52. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–95. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–9. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Mas N, Saiz A, Leite MI, Waters P, Baron M, Castano D, et al. Antiglycine-receptor encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. 2011;82:1399–401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–8. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto JY, Harvey RJ, McEvoy KM, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol. 2013;70:44–50. doi: 10.1001/jamaneurol.2013.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A, Robinson MT, McEvoy KM, Matsumoto JY, Lennon VA, Ahlskog JE, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol. 2012;69:230–8. doi: 10.1001/archneurol.2011.991. [DOI] [PubMed] [Google Scholar]
- Meinck HM. Startle and its disorders. Clin Neurophysiol. 2006;36:357–64. doi: 10.1016/j.neucli.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Meinck HM, Thompson PD. Stiff man syndrome and related conditions. Mov Disord. 2002;17:853–66. doi: 10.1002/mds.10279. [DOI] [PubMed] [Google Scholar]
- Paton JF, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J Physiol. 1995;484:505–21. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters E, Vanacker P, Woodhall M, Vincent A, Schrooten M, Vandenberghe W. Supranuclear gaze palsy in glycine receptor antibody-positive progressive encephalomyelitis with rigidity and myoclonus. Mov Disord. 2013;27:1830–2. doi: 10.1002/mds.25239. [DOI] [PubMed] [Google Scholar]
- Piotrowicz A, Thumen A, Leite MI, Vincent A, Moser A. A case of glycine-receptor antibody-associated encephalomyelitis with rigidity and myoclonus (PERM): clinical course, treatment and CSF findings. J Neurol. 2011;258:2268–70. doi: 10.1007/s00415-011-6078-x. [DOI] [PubMed] [Google Scholar]
- Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–55. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Saiz A, Blanco Y, Sabater L, Gonzales F, Bataller L, Casamijana R, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–63. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- Song W, Chattipakorn SC, McMahon LL. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J Neurophysiol. 2006;95:2366–79. doi: 10.1152/jn.00386.2005. [DOI] [PubMed] [Google Scholar]
- Stern WM, Howard R, Chalmers RM, Woodhall MR, Waters P, Vincent A, et al. Glycine receptor antibody mediated Progressive Encephalomyelitis with Rigidity and Myoclonus (PERM): a rare but treatable neurological syndrome. Pract Neurol. 2014;14:123–7. doi: 10.1136/practneurol-2013-000511. [DOI] [PubMed] [Google Scholar]
- Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology. 2011;77:439–43. doi: 10.1212/WNL.0b013e318227b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–43. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–72. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Baer K, Eady E, Allen KL, Gilbert RT, Mohler H, et al. Differential localization of gamma-aminobutyric acid type A and glycine receptor subunits and gephyrin in the human pons, medulla oblongata and uppermost cervical segment of the spinal cord: an immunohistochemical study. J Comp Neurol. 2010;518:305–28. doi: 10.1002/cne.22212. [DOI] [PubMed] [Google Scholar]
- Warren JD, Scott G, Blumbergs PC, Thompson PD. Pathological evidence of encephalomyelitis in the stiff man syndrome with anti-GAD antibodies. J Clin Neurosci. 2002;9:328–9. doi: 10.1054/jocn.2001.1014. [DOI] [PubMed] [Google Scholar]
- Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, et al. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci. 2009;2:6. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley AM, Swash M, Urich H. Progressive encephalomyelitis with rigidity. Brain. 1976;99:27–42. doi: 10.1093/brain/99.1.27. [DOI] [PubMed] [Google Scholar]
- Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–9. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- Xu TL, Gong N. Glycine and glycine receptor signaling in hippocampal neurons: diversity, function and regulation. Prog Neurobiol. 2010;91:349–61. doi: 10.1016/j.pneurobio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. 2012;83:638–45. doi: 10.1136/jnnp-2011-301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.