Abstract
Individuals with mutation in the lysosomal enzyme glucocerebrosidase (GBA) gene are at significantly high risk of developing Parkinson’s disease with cognitive deficit. We examined whether visual short-term memory impairments, long associated with patients with Parkinson’s disease, are also present in GBA-positive individuals—both with and without Parkinson’s disease. Precision of visual working memory was measured using a serial order task in which participants observed four bars, each of a different colour and orientation, presented sequentially at screen centre. Afterwards, they were asked to adjust a coloured probe bar’s orientation to match the orientation of the bar of the same colour in the sequence. An additional attentional ‘filtering’ condition tested patients’ ability to selectively encode one of the four bars while ignoring the others. A sensorimotor task using the same stimuli controlled for perceptual and motor factors. There was a significant deficit in memory precision in GBA-positive individuals—with or without Parkinson’s disease—as well as GBA-negative patients with Parkinson’s disease, compared to healthy controls. Worst recall was observed in GBA-positive cases with Parkinson’s disease. Although all groups were impaired in visual short-term memory, there was a double dissociation between sources of error associated with GBA mutation and Parkinson’s disease. The deficit observed in GBA-positive individuals, regardless of whether they had Parkinson’s disease, was explained by a systematic increase in interference from features of other items in memory: misbinding errors. In contrast, impairments in patients with Parkinson’s disease, regardless of GBA status, was explained by increased random responses. Individuals who were GBA-positive and also had Parkinson’s disease suffered from both types of error, demonstrating the worst performance. These findings provide evidence for dissociable signature deficits within the domain of visual short-term memory associated with GBA mutation and with Parkinson’s disease. Identification of the specific pattern of cognitive impairment in GBA mutation versus Parkinson’s disease is potentially important as it might help to identify individuals at risk of developing Parkinson’s disease.
Keywords: visual short-term memory, working memory, Gaucher’s disease, glucocerebrosidase
Introduction
One of the key priorities in Parkinson’s disease research is to detect the disease at its earliest stage. Cognitive deficits, including those in visual working memory or the storage component of working memory, referred to as visual short-term memory (VSTM), are an important feature of Parkinson’s disease, often apparent at very early stages of the disease (Owen et al., 1992, 1993, 1997; Dujardin et al., 1999; Muslimovic et al., 2005; Verbaan et al., 2007; Savica et al., 2010). Such impairments potentially provide a means for early detection. However, screening for deficits on a population-wide basis would be an extremely challenging undertaking (Berg et al., 2012).
An alternative strategy is to target individuals who are at particularly high risk of developing Parkinson’s disease due, for example, to genetic factors. Recently mutation in the gene encoding the lysosomal enzyme glucocerebrosidase (GBA) has been identified as the highest genetic risk factor for developing Parkinson’s disease (Clark et al., 2009; Neumann et al., 2009; Sidransky et al., 2009). Mutations in this gene have classically been associated with Gaucher’s disease, a condition that results from homozygosity or compound heterozygosity for GBA mutations (Pastores and Hughes, 1993). The odds ratio of having a GBA mutation in Parkinson’s disease is >5 (Sidransky et al., 2009), whereas the lifetime risk of developing Parkinson’s disease has been found to be 21-fold greater in patients with Gaucher’s disease compared to control subjects (Bultron et al., 2010).
Individuals with GBA mutations are therefore a potentially important group of people to screen for impairments that might be associated with Parkinson’s disease. Here we focused on cognitive deficits, which have long been associated with established Parkinson’s disease (Bradley et al., 1989; Owen et al., 1992, 1993, 1997; Owen, 2004), most prominently impairments in working memory and attention (Cooper et al., 1991; Owen et al., 1992; Dujardin et al., 1999; Muslimovic et al., 2005).
It has now been established that cognitive impairments in patients with Parkinson’s disease who are heterozygous carriers of GBA mutations are more frequent and severe than in patients with GBA-negative Parkinson’s disease, as indexed by the Montreal Cognitive Assessment (Brockmann et al., 2011). Furthermore, visual working memory performance is severely impairment in cases with GBA-positive early-onset Parkinson’s disease compared to patients with GBA-negative Parkinson’s disease (Alcalay et al., 2012). Finally, GBA-positive individuals with Parkinson’s disease are more likely to develop dementia than those without (Winder-Rhodes et al., 2013). Together, these findings suggest that GBA mutation is an important risk factor for cognitive impairments in Parkinson’s disease.
But based on these results alone, one cannot conclude whether such deficits are due to GBA pathology, manifestation of Parkinson’s disease or a combination of these factors. Indeed GBA-positive individuals without Parkinson’s disease have also been shown to be impaired on the Montreal Cognitive Assessment as well as a test of olfaction, a potential marker for early neurodegeneration (McNeill et al., 2012). Clarification of this issue and identification of the signature of cognitive impairment in people with GBA mutation versus those with Parkinson’s disease is potentially important as it might help to identify individuals at risk of developing Parkinson’s disease.
Given that visual working memory/short-term memory deficits have been reported in early Parkinson’s disease (Owen et al., 1992, 1993, 1997; Muslimovic et al., 2005) and in patients with GBA-positive Parkinson’s disease (Alcalay et al., 2012), we focused on VSTM in GBA-positive individuals with and without Parkinson’s disease, as well as sporadic (GBA-negative) cases of Parkinson’s disease to investigate whether the pattern of VSTM deficit associated with GBA and Parkinson’s disease pathology is dissociable. We used a relatively new experimental method of measuring VSTM that, unlike conventional clinical measures of memory ‘span’, examines the resolution with which items are maintained and later recalled. In tasks similar to the one we used, participants are asked to remember visual features and reproduce the exact qualities of those features when, after a retention period, they are probed to recall an item (Bays et al., 2009; Gorgoraptis et al., 2011; Zokaei et al., 2011; Pertzov et al., 2013).
Importantly, such VSTM precision tasks also provide a means to dissect out sources of error contributing to the pattern of performance. In paradigms similar to the one we used, error can potentially arise from three sources. First, it can be due to variability in memory for the probed item; how well it is stored. Second, participants may make random errors because, on some trials, they are simply guessing, e.g. they failed to encode the probed item or to retrieve it. Finally, and importantly, error can arise from misreporting features of non-probed items that were presented in the memory array, instead of reporting the features that belonged to the probed item. In other words, an observer’s response might be systematically biased by other objects that were encoded into VSTM. By applying a recent analytical technique (Bays et al., 2009) we were able to estimate the proportion of responses arising from each of these sources of error and to find signature VSTM deficits associated with GBA mutation and Parkinson’s disease.
Materials and methods
Patients and age-matched control subjects
Twenty GBA-positive individuals without Parkinson’s disease were tested: nine with type 1 Gaucher’s disease and 11 heterozygote GBA mutation carriers (without Gaucher’s disease). The patients with type 1 Gaucher’s disease were not receiving substrate reduction therapy. There was no difference in all our measures (i.e. overall VSTM performance, proportion of target, non-target and random responses) between patients with Gaucher’s disease and individuals with heterozygote GBA mutation and hence these two groups, for the rest of the analysis, have been collapsed (n = 20). GBA-positive individuals were recruited from the Lysosomal Storage Disorder Unit at the Royal Free Hospital, London and UK Gaucher Disease Association. All GBA-positive individuals scored zero for all subscales of the Unified Parkinson’s Disease Rating Scale (UPDRS). All GBA-positive individuals were aware of their GBA status.
Eighteen GBA-positive patients with Parkinson’s disease (daily levodopa equivalent dose: 628 mg) (Tomlinson et al., 2010) and 15 GBA-negative patients with Parkinson’s disease (daily levodopa equivalent dose: 647 mg) were also tested. GBA-positive and GBA-negative cases with Parkinson’s disease were recruited from Oxford Parkinson’s Disease Centre (OPDC) cohort study (PD discovery) and were aware of their GBA status or underwent genetic testing by the Lysosomal Storage Disorder Unit at the Royal Free Hospital London after being diagnosed with Parkinson’s disease in the Department of Neurology, UCL and were later informed of their GBA status. None of the patients with GBA-positive Parkinson’s disease were related to one another or related to GBA-positive cases. Patient demographics are presented in Table 1.
Table 1.
Demographic information on all patient groups and healthy controls
| Age (years) | Gender | Years of education | |
|---|---|---|---|
| Mean (SD) | Male/female | Mean (SD) | |
| GBA-positive non-Parkinson’s disease cases (n = 20) | 61 (9) | 11/9 | 15 (2) |
| GBA-negative Parkinson’s disease patients (n = 15) | 63 (6) | 9/6 | 13.5 (5) |
| GBA-positive Parkinson’s disease patients (n = 15) | 60 (8) | 7/8 | 14 (3) |
| Healthy controls (n = 17) | 61.5 (10) | 9/8 | 15 (3) |
SD = standard deviation.
All patients scored 30/30 on the Mini-Mental State Examination (Folstein et al., 1975) except three GBA-positive patients with Parkinson’s disease who were excluded from the rest of the analysis (information on UPDRS scores of patients with Parkinson’s disease, both GBA-negative and positive are presented in Supplementary Tables 1 and 2). For comparison 17 healthy control participants, matched to patients for age and years of education, also participated in this study (Table 1). Control participants had no neurological disease or history of Gaucher’s disease in their family.
Some patients and healthy controls were also tested on verbal working memory task (forwards and backwards digit span) with no significant difference in performance between groups (Supplementary Table 3). All participants had normal or corrected to normal vision and colour vision and provided written informed consent to the procedure of the experiment, approved by the local ethics committee.
Genetic testing
Patients with GBA-positive Parkinson’s disease were screened for N370S and L444P mutations in the GBA gene by extracting genomic DNA from blood samples using the AutoPure LS® (QIAGEN). PCR reactions were carried out with AmpliTaq Gold® DNA polymerase (Applied Biosystems). Primer sequences used for N370S were: 5’- GCCTTTGTCCTTACCCTC*G -3’ and 5’- GACAAAGTTACGCACCCAA-3’. A mismatch was engineered into the forward primer in order to create a XhoI restriction site in the PCR product from participants carrying the N370S mutation. For the L444P mutation primers used were: 5’-GGAGGACCCAATTGGGTGCGT-3’ and 5’-ACGCTGTCTTCAGCCCACTTC-3’. The resulting PCR products were digested with XhoI (NEB) for N370S and NciI (NEB) for L444P and resolved by agarose gel electrophoresis. Mutations were then confirmed by sequencing. Briefly, DNA was treated with an ExoSAP reaction as follows: 1 × SAP buffer, shrimp alkaline phosphatase (500 U; SAP, Promega), Exonuclease I (2 U; NEB). Samples were incubated at 37°C for 1 h and then 80°C for 20 min. The sequencing reaction was performed according to BigDye® Terminator v3.1 Cycle Sequencing protocol (Applied Biosystems). Following a clean-up step, the sequencing read was performed on a 3700 DNA Analyser (Applied Biosystems) sequencing platform.
GBA mutation in non-Parkinson’s disease cases was confirmed by sequencing exons 1 to 11 of the GBA gene using published protocol (Neumann et al., 2009) with PCR designed for regions of the GBA gene not found in pseudogene. After amplification by PCR the product was run on 1% agarose gel with ethidium bromide and size-checked to ensure intronic sequences using the Dye Terminator Sequencing Kit (Applied Biosystems) on an ABI 3700xl genetic analyser. Of the 20 tested GBA-positive individuals, three had mutations other than the two most common mutations N370S and L444P (RecNcil, V3494L and W184R).
Experimental visual short-term memory task
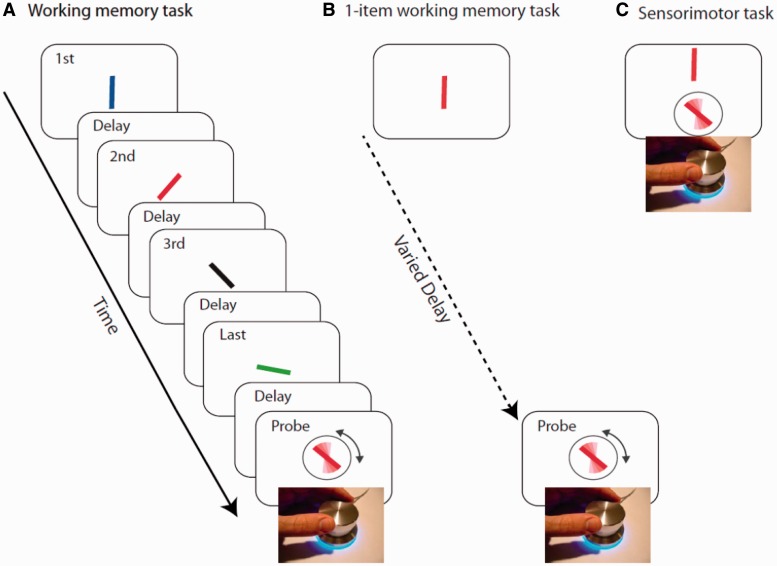
The task was adapted from that previously used by Gorgoraptis et al. (2011). Stimuli were presented on a laptop monitor (32° × 19°) at a viewing distance of ∼52 cm. A schematic representation of the task is shown in Fig. 1A. In each trial, a sequence of four coloured bars (2° × 0.2°) was presented at screen centre on a grey background. Each bar was presented for 500 ms followed by a 500 ms blank interval before the presentation of the next bar. The colours in each trial were selected randomly—with no repetition within a trial—from five easily distinguished colours (red, yellow, green, blue and purple). Minimum angular separation between the orientation of bars within the same sequence was 10°; the orientation was chosen randomly otherwise. Participants were asked to remember the orientation of each bar.
Figure 1.
(A) A sequence of four coloured oriented bars were presented sequentially. Any of the bars could be probed by colour of the response stimuli and participants were asked to adjust the orientation of the response stimuli to the orientation of the bar with same colour. (B) An example of the one-item working memory task. A rotating dial is used to orient the probe bar (surrounded by circle) to match the orientation of the target bar presented following a delay. (C) An example sensorimotor task. A rotating dial is used to orient the probe bar (surrounded by circle) to match the orientation of the target bar presented above the probe.
At the end of each trial, a probe bar oriented randomly, in the same colour as one of the bars in the sequence was presented. A circle surrounding this probe made it easier to distinguish from bars in the sequence. Participants were instructed to use a rotating dial (which controlled the orientation of the probe bar) to match the orientation of the probe with the same coloured bar they had seen in the sequence. The black circle surrounding the probe item disappeared upon rotating the dial. Participants clicked on the dial once they had rotated the dial to their selected orientation. Stimuli presented in any of the serial positions within the sequence were probed with equal probability and participants did not know beforehand which item would be tested.
Control tasks
It is possible that poor performance in our experimental VSTM task might be due to factors other than the ability to maintain four items. The following tasks, administered in random order across participants, were used as controls to ensure that various issues were not a concern for subsequent interpretation.
Pre-cueing condition
Stimuli were identical to those in the experimental VSTM task except for the following. At the beginning of each block, a 100% valid cue would inform participants to maintain the orientation of a bar presented in a specific colour in the subsequent sequence of four oriented bars. Participants were always probed on the cued item at the end of the sequence. This task was employed to control for any deficits in attentional filtering across groups.
One item working memory task
In each trial, participants were presented with a single oriented bar at screen centre for 500 ms. Following a blank variable delay, a probe bar of the same colour appeared and participants were asked to adjust the probe bar’s orientation to match the orientation of the target bar (Fig. 1B). The maintenance period preceding the probe was randomly chosen from one of the following delays: 500, 1500, 2500 and 3500 ms. The duration of the retention periods matched the durations from presentation of probed bars at different serial positions within four-item sequences used in the experimental VSTM task. This task was used to control for temporal decay of information in working memory.
Sensorimotor control task
In each trial a coloured oriented bar was presented at screen centre. Five hundred milliseconds after the presentation of the bar, a probe bar of the same colour surrounded by a black circle appeared below the target bar. Participants were asked to adjust the probe bar’s orientation on screen to match the orientation of the target which remained on screen until response (Fig. 1C). The orientation of the target and the probe were independently randomized on each trial.
For each of the experimental VSTM, pre-cueing and one-item VSTM tasks GBA-positive individuals completed 100 trials, patients with GBA-negative and positive Parkinson’s disease completed 50–200 trials (depending on their availability) and control participants performed 200 trials. All GBA-positive individuals, patients with GBA-negative and positive Parkinson’s disease and 10 aged-matched controls completed 20 trials of the sensorimotor control task.
Analysis
We calculated recall precision as the reciprocal of the circular standard deviation of response error (the difference in response and target angle) (Philipp, 2009). Precision is a measure of response variability, less variability corresponding to more precise memory.
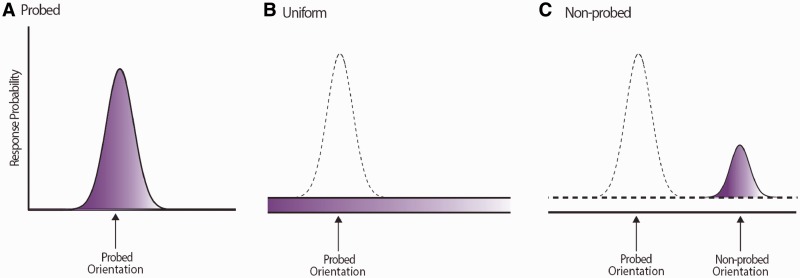
To identify mechanisms underlying VSTM impairment associated with Parkinson’s disease and GBA mutation, we also fit a mixture model that dissociates different sources of error in memory previously introduced by Bays et al. (2009) (see also http://www.sobell.ion.ucl.ac.uk/pbays/code/JV10/). The mixture model is described by the following equation:
| (1) |
Several sources of error can contribute to impaired performance. Error can arise due to increased variability in memory for orientation of the probed item, captured by model parameter κ, where higher κ corresponds to lower variability in memory for the orientation (Fig. 2A). Increase in random responses, i.e. guesses (Fig. 2B), captured by γ in the model. Successful performance also requires memory for correct combination of orientation and colour. Therefore errors can also arise as a result of incorrect conjunction of colour and orientation (misbinding errors). In such trials participants make an error centred on the orientation of other (non-probed) items in memory (Fig. 2C). In more concrete terms: if the probed item is red (as in Fig. 1A) but the participants responds with the orientation of one of the other coloured bars in the sequence, this would be classified as a misbinding error.
Figure 2.
Three sources of error in memory used for mixture modelling. This figure is a schematic presentation of the three possible sources of error that can occur in working memory. (A) A Von Mises (circular Gaussian) distribution with concentration parameter κ, centred on the probed value, capturing variability in memory for target, with the area under the distribution (shaded) being proportional to the probability of responding to the probe. (B) A uniform distribution of error corresponding to random error, with the area under this distribution corresponding to the proportion of random responses. (C) Von Mises distribution with concentration parameter κ, centred on one of the non-probe value, resulting from errors in identifying which orientation belonged with the probed colour (binding failures). The area under the distribution corresponds to the proportion of non-probed responses.
The probability of responding to non-probed items is captured by parameter β in the model, the probability of responding with the probed orientation is given by α. Maximum likelihood parameters of κ, α, β and γ were obtained using the well-established expectation maximization (Myung, 2003) procedure for each participant (full details are given in Bays et al., 2009).
Results
Visual short-term memory impairments in individuals with GBA mutation and Parkinson’s disease
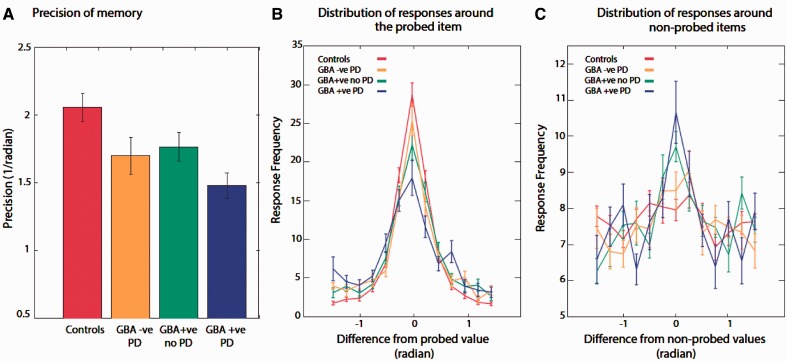
There was a main effect of both Parkinson’s disease and GBA mutation on precision of recall [F(1,63) = 12.5, P = 0.001 and F(1,63) = 7.6, P < 0.01, respectively; Fig. 3A]. Compared to healthy participants, performance was significantly worse—less precise—in patients with Parkinson’s disease, both GBA-negative and GBA-positive carriers [t(30) = 2.9, P < 0.01 and t(30) = 4.99, P < 0.001, respectively]. In addition, GBA-positive individuals without Parkinson’s disease were significantly impaired compared to controls [t(35) = 2.3, P < 0.05]. Importantly, patients with GBA-positive Parkinson’s disease had significantly less precise memory compared to cases with GBA-negative Parkinson’s disease [t(28) = 2.1, P < 0.05] as well as GBA-positive individuals without Parkinson’s disease [t(33) = 2.04, P < 0.05].
Figure 3.
Performance of all participants in experimental working memory task. (A) Precision of memory. Memory precision in all patients groups was significantly worse compared to healthy controls, patients with GBA-positive Parkinson’s disease (PD) performed significantly worse compared to all tested participants (**P < 0.025, *P < 0.05). (B) Distribution of responses around the probed orientation for patient groups and healthy participants. GBA carrier Parkinson’s disease patients made significantly less responses around the probed orientation, followed by individuals with GBA mutation and non-carrier patients with Parkinson’s disease compared to healthy controls. (C) Distribution of responses around non-probed orientations. Individuals with GBA mutation, with or without Parkinson’s disease made significantly more responses centred on non-probed orientations.
Sources of error in visual short-term memory impairments associated with GBA mutation and Parkinson’s disease
Although all three groups of interest were significantly impaired in the VSTM task compared to healthy participants, the overall performance does not inform us about the sources of error associated with Parkinson’s disease and GBA mutation. Are these the same or can they be distinguished?
Examination of the distribution of error in relation to the orientation of the probed item revealed a main effect of group at the peak of the distribution [F(3,63) = 4.95, P < 0.005]. At the peak of the distribution, there was a significant decrease in proportion of responses in GBA-positive individuals, either with [t(30) = 4.06, P < 0.001] or without Parkinson’s disease [t(35) = 2.99, P = 0.005], compared to healthy controls. The proportion of responses falling close to the orientation of the probed item was lowest in patients with GBA-positive Parkinson’s disease (Fig. 3B).
A plot of the distribution of error in relation to the non-probed orientations (i.e. the orientations of other items held in VSTM that were not probed) demonstrated a significant increase around non-probed orientations at the peak of the distribution in both GBA-positive Parkinson’s disease [t(30) = 3.12, P < 0.005] and GBA-positive cases without Parkinson’s disease [t(35) = 5.65, P < 0.001 compared to healthy controls (Fig. 3C). The peak of non-probed responses was significantly higher in patients with GBA-positive Parkinson’s disease compared to cases with GBA-negative (sporadic) Parkinson’s disease [t(28) = 2.15, P < 0.05]. Thus, regardless of whether they had Parkinson’s disease, individuals who were GBA-positive demonstrated increased error due to responding to non-probed items. In other words, these patients erroneously misbound the colour of the probed orientation with non-target orientations (i.e. orientations of other items in the sequence).
To quantify the possible sources of error, we next applied the mixture model of response error described above to our data (Bays et al., 2009) (see above). Maximum likelihood estimates of the probability of responding with the probed orientation (α), proportion of responding to non-probed orientations (β), and proportion of random responses or guessing (γ) were estimated together with the variability in recall of target orientation (κ). After model fitting, any individual with outlier parameter values (defined as values 2.5 standard deviations from the mean of each parameter) were excluded. One healthy participant, one individual with GBA mutation, one Parkinson’s disease GBA-negative and two Parkinson’s disease GBA-positive patients were excluded.
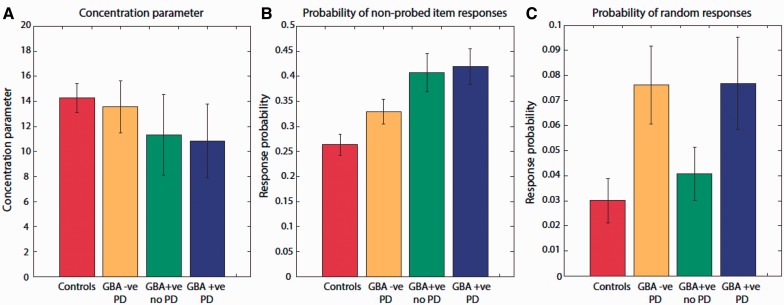
In line with the distribution of error around the probed item (Fig. 3B), there was a main effect of both Parkinson’s disease and GBA mutation on α, the probability of responses to this orientation [F(1,59) = 7.4, P < 0.01 and F(1,59) = 8.4, P = 0.005, respectively]. Thus, GBA-positive Parkinson’s disease, GBA-negative Parkinson’s disease and GBA-positive carriers without Parkinson’s disease were all impaired in terms of proportion of responses to the probed item (Fig. 4A). There was no significant difference in κ (model parameter measuring variability around probed item orientation) between all four groups demonstrating that change in the resolution of information in VSTM does not contribute to the impairments observed.
Figure 4.
Model estimates for different sources of error in experimental working memory task. (A) Concentration parameter did not differ significantly between all patient groups and healthy controls. (B) Probability of non-probed responses was significantly higher in individuals with GBA mutation, with or without Parkinson’s disease (PD) compared to healthy controls and non-carrier patients with Parkinson’s disease. (C) Probability of random responses was significantly higher in patients with Parkinson’s disease—with or without GBA mutation—compared to healthy controls and individuals with GBA mutation without Parkinson’s disease.
Importantly, there was only a main effect of GBA-positive status on β, the proportion of responses to non-probed items [F(1,59) = 8.6, P = 0.005], but no effect of Parkinson’s disease on these responses [F(1,59) = 3, P < 0.05; Fig. 4B]. This is the pattern observed previously on simply plotting the distribution of responses around non-probed orientations (Fig. 3C). Hence GBA-positive individuals, regardless of whether they had Parkinson’s disease, made significantly more misbinding errors, responding with the orientations of non-probed items compared to healthy controls and patients with GBA-negative Parkinson’s disease.
Conversely, there was only a main effect of Parkinson’s disease on γ, the proportion of random responses [F(1,59) = 6, P < 0.05], with no effect of GBA positivity on these responses [F(1,59) = 0.07, P = not significant; Fig. 4C]. Thus although the presence of both Parkinson’s disease and GBA mutation resulted in impaired VSTM performance, the sources of error associated with each was different.
Visual short-term memory deficits cannot be explained by impairments in control tasks
There was no significant difference between the three groups and healthy participants in the pre-cueing and one-item VSTM tasks. Thus any difference in performance between groups is unlikely to be due to being able to attend to different items presented sequentially or due to differences in how information decays over time for single items (although this does not mean that there is no influence of maintenance duration on memory precision). Finally, there was no significant difference in performance between the groups on the sensorimotor control task. This is particularly important in the context of testing patients with Parkinson’s disease who might have difficulties with dexterity in using the dial. Our findings show that this is not a confounding factor in interpreting the data.
Discussion
This study sought to examine whether visual VSTM impairments associated with GBA mutation and Parkinson’s disease were due to the same or different underlying mechanisms. The results demonstrate that there are different patterns of deficit contributing to VSTM deficits in individuals with Parkinson’s disease and those who carry a GBA mutation. The results of three control tasks show that in all groups, impaired performance is unlikely to be explained by gross difficulties in attending to different serial positions in a sequence, differences in temporal decay of information or sensorimotor deficits.
Although all tested groups (GBA-positive Parkinson’s disease, GBA-negative Parkinson’s disease and GBA-positive subjects without Parkinson’s disease) were significantly less precise in VSTM recall than healthy controls, those who were GBA-positive and also had Parkinson’s disease were significantly worse than those who had either of these alone. Thus patients with GBA-positive Parkinson’s disease appear to suffer a double ‘hit’ in VSTM performance. What then is the nature of the mechanisms underlying VSTM deficits in GBA-positive and cases with Parkinson’s disease?
The paradigm used here allowed us to analyse the sources of error associated with GBA positivity and Parkinson’s disease. Error in recall can potentially arise from several different sources. First, it can be due to variability in memory for a feature, i.e. how well the feature is stored. Second, error can arise due to systematic corruption of the memory for the target item by other items maintained in VSTM: interference or misbinding errors. Finally, participants might guess due to failure to encode the probed item or alternatively to retrieve it, e.g. due to fluctuations in attention.
Those with GBA mutation, regardless of whether they had Parkinson’s disease, made significantly greater responses to non-probed items, responding more frequently with other orientations retained in memory. Thus their memory was more prone to be corrupted by misbinding errors in VSTM: failures to correctly bind features (colour and orientation) of encoded items in memory (Figs 3C and 4B). In contrast, those with Parkinson’s disease, regardless of their GBA-positive status, were more prone to making random responses or guesses. Thus there was a double dissociation in the pattern of errors.
Human post-mortem specimens and GBA-positive mouse models provide some support for distinct memory impairments in Parkinson’s disease and GBA mutation. In our study GBA-positive individuals, regardless of whether they had Parkinson’s disease, showed increased misbinding errors. Both mouse models of GBA mutation (Sardi et al., 2011) and human Gaucher’s disease patients with dementia and Parkinson’s disease have pathological changes in hippocampal and medial temporal regions (Wong et al., 2004), which seem to play a critical role in binding of information in VSTM (Olson and Marshuetz, 2005; Parra et al., 2009, 2010; Della Sala et al., 2012; Pertzov et al., 2013). Interestingly, Della Sala and colleagues (2012) have also recently demonstrated that whereas patients with Alzheimer’s disease (with presumed medial temporal pathology) demonstrate misbinding errors, cases with Parkinson’s disease do not. These authors examined the binding problem by measuring measure VSTM performance for individual features alone as well as for binding of features.
Although, we did not examine recall precision for colour independently, we found no significant difference either between patients groups or compared to healthy controls in the concentration parameter model estimate (Fig. 4A). Thus the resolution of memory for orientation—independent of binding information—was not disrupted. Together these findings suggest that there might be specific VSTM binding deficits that occur in individuals with medial temporal pathology, including perhaps those with GBA mutation. These types of error might therefore be a marker for detecting medial temporal dysfunction in different patient populations.
By contrast, corruption of memory in patients with Parkinson’s disease—regardless of GBA status—was due to an increase in random responses, analogous to greater noise in a neural network, which might not necessarily be specific to any particular brain region but perhaps associated with fluctuations in performance, possibly related to cholinergic deficits in Parkinson’s disease (Kehagia et al., 2010). Alternatively, increased random responses can be due to decrease in signal-to-noise ratio due to deficits in the dopaminergic system in patients with Parkinson’s disease, leading responses to fall within the random range (Sawaguchi and Goldman-Rakic, 1991; Winterer and Weinberger, 2004; Kroener et al., 2009). Individuals who were GBA-positive and had Parkinson’s disease suffered from both types of error (increased misbinding and random responses) and as a consequence had far less precision in VSTM recall than those who were GBA-positive or had Parkinson’s disease alone.
Note that in our study, patients with Parkinson’s disease, regardless of GBA status, were all ON dopaminergic medication. Previously, we reported that VSTM precision, measured using similar techniques, improves in patients with Parkinson’s disease ON medication compared to OFF (Zokaei et al., 2012). Thus the impairments observed here in both Parkinson’s disease groups (GBA-positive and GBA-negative) cannot be attributed to dopaminergic medication, although this clearly can modulate performance, but in a beneficial way. One limitation of our study is that relatively small numbers meant that we might have missed differences in performance between cases with Gaucher’s disease and GBA carriers who did not have Parkinson’s disease (in our study the data from these groups was collapsed). Further investigation will be required to investigate this particular issue more definitively.
In summary, these findings demonstrate that GBA-positive individuals without Parkinson’s disease have deficits in VSTM, comparable to GBA-negative patients with Parkinson’s disease. However, the sources of error underlying impairments in Parkinson’s disease and GBA are dissociable. GBA-positive individuals who develop Parkinson’s disease suffer a ‘double’ hit and hence display worse performance overall. The results suggest that measurement of VSTM provides a potentially useful tool to probe cognitive deficits associated with GBA mutation and Parkinson’s disease. GBA mutation is an important risk factor for developing Parkinson’s disease but clearly most individuals who have this do not develop Parkinson’s disease. Future research might aim to examine whether it is possible to use the pattern of VSTM errors associated with Parkinson’s disease (increased random responses) to identify, at an early stage, individuals with GBA mutations or other risk factors who are beginning to develop Parkinson’s disease.
Supplementary Material
Acknowledgements
We are grateful to all the participants. We would also like to thank Hugo Fernandez for carrying out the GBA screening for GBA-positive patients with Parkinson’s disease at Oxford.
Glossary
Abbreviation
- VSTM
visual short-term memory
Funding
The study was supported by a grant to MH from the Wellcome Trust (WT098282) and Brain Research Trust (N.Z.), the Wellcome Trust/MRC Joint Call in 95 Neurodegeneration award (WT089698) to the UK Parkinson’s Disease Consortium (UKPDC), the Kattan Trust and Parkinson’s UK. A.H.V.S. is a NIHR Senior Investigator. This study was also funded by the Monument Trust Discovery Award, Parkinson’s UK and supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN).
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78:1434–40. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. J Vis. 2009;9:7.1–11. doi: 10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson’s disease: lessons from ongoing studies. Mov Disord. 2012;27:656–65. doi: 10.1002/mds.24985. [DOI] [PubMed] [Google Scholar]
- Bradley VA, Welch JL, Dick DJ. Visuospatial working memory in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1989;52:1228–35. doi: 10.1136/jnnp.52.11.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77:276–80. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- Bultron G, Kacena K, Pearson D, Boxer M, Yang R, Sathe S, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33:167–73. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–83. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain J Neurol. 1991;114(Pt 5):2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. 2012;50:833–40. doi: 10.1016/j.neuropsychologia.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Degreef JF, Rogelet P, Defebvre L, Destee A. Impairment of the supervisory attentional system in early untreated patients with Parkinson’s disease. J Neurol. 1999;246:783–8. doi: 10.1007/s004150050455. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gorgoraptis N, Catalao RFG, Bays PM, Husain M. Dynamic updating of working memory resources for visual objects. J Neurosci. 2011;31:8502–11. doi: 10.1523/JNEUROSCI.0208-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–13. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PEM, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PloS One. 2009;4:e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Duran R, Proukakis C, Bras J, Hughes D, Mehta A, et al. Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov Disord. 2012;27:526–32. doi: 10.1002/mds.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Myung IJ. Tutorial on maximum likelihood estimation. J Math Psychol. 2003;47:90–100. [Google Scholar]
- Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–94. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Marshuetz C. Remembering “what” brings along “where” in visual working memory. Percept Psychophys. 2005;67:185–94. doi: 10.3758/bf03206483. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, et al. Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia. 1993;31:627–44. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW. Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia. 1997;35:519–32. doi: 10.1016/s0028-3932(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala S. Short-term memory binding deficits in Alzheimer’s disease. Brain J Neurol. 2009;132:1057–66. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain J Neurol. 2010;133:2702–13. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Hughes DA. Gaucher disease. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviewsTM. Seattle, WA: University of Washington; 1993. [PubMed] [Google Scholar]
- Pertzov Y, Miller TD, Gorgoraptis N, Caine D, Schott JM, Butler C, et al. Binding deficits in memory following medial temporal lobe damage in patients with voltage-gated potassium channel complex antibody-associated limbic encephalitis. Brain. 2013;136(Pt 8):2474–85. doi: 10.1093/brain/awt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp B. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw. 2009;31:1–21. [Google Scholar]
- Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, et al. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108:12101–6. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Rocca WA, Ahlskog J. When does Parkinson disease start? Arch Neurol. 2010;67:798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic P. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HA, et al. Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1182–7. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain J Neurology. 2013;136:392–9. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–90. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Zokaei N, Gorgoraptis N, Bahrami B, Bays PM, Husain M. Precision of working memory for visual motion sequences and transparent motion surfaces. J Vis. 2011;11:1–18. doi: 10.1167/11.14.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N, Gorgoraptis N, Husain M. Dopamine modulates visual working memory precision. J Vis. 2012;12:350. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.