Abstract
Bipolar disorder is characterized by impaired decision-making captured in impulsivity and risk-taking. We sought to determine whether this is driven by a failure to effectively weight the lower-order goal of obtaining a strongly desired reward in relation to higher-order goals, and how this relates to trait impulsivity and risk-taking. We hypothesized that in bipolar disorder the weighting of valuation signals converging on ventromedial prefrontal cortex are more heavily weighted towards ventral striatum inputs (lower-order), with less weighting of dorsolateral prefrontal cortex inputs (higher-order). Twenty euthymic patients with bipolar disorder not in receipt of antipsychotic medication and 20 case-matched controls performed a roulette task during functional magnetic resonance imaging. Activity in response to high-probability (‘safe’) and low-probability (‘risky’) prospects was measured during both anticipation, and outcome. In control subjects, anticipatory and outcome-locked activity in dorsolateral prefrontal cortex was greater for safe than risky reward prospects. The bipolar disorder group showed the opposite pattern with preferential response to risky rewards. This group also showed increased anticipatory and outcome-locked activity in ventral striatum in response to rewards. In control subjects, however, ventromedial prefrontal activation was positively associated with both ventral striatum and dorsolateral prefrontal activity; patients evidenced a strong positive association with ventral striatum, but a negative association with dorsolateral prefrontal cortex. Response to high-probability rewards in dorsolateral prefrontal cortex was inversely associated with trait impulsivity and risk-taking in the bipolar disorder group. Our findings suggest that clinically impulsive and risky decision-making are related to subjective valuation that is biased towards lower-order preference, with diminished integration of higher-order goals. The findings extend a functional neuroanatomical account of disorders characterized by clinically impulsive decision-making, and provide targets for evaluating interventions that foster self-control.
Keywords: bipolar disorder, mania, reward, striatum, accumbens, risk
Introduction
Impulsive and risky decision-making is characteristic of several psychiatric disorders including substance dependence (Rogers et al., 2010), bipolar disorder (Swann et al., 2009), attention deficit hyperactivity disorder (Scheres et al., 2010), pathological gambling (Rogers et al., 2010) and psychopathy (Vitacco and Rogers, 2001). Impulsivity can be conceptualized as a diminished self-control to suppress behaviours that afford preferred (highly desirable) outcomes, but which contravene higher-order or longer-term goals and so are ultimately suboptimal in the long run (Hare et al., 2009; Diekhof et al., 2012). Common frontostriatal regions are implicated in optimal versus impulsive decision-making, whether the decision involves selecting a tastier over a healthier food option (Hare et al., 2011), or a safe but low-return prospect over a risky but potentially more lucrative investment (Peters and Büchel, 2009). Here, we present recent developments on the functional basis of self-control in decision-making to examine suboptimal decision-making in bipolar disorder during a probabilistic task in which safe and risky reward prospects are evaluated.
The functional basis of optimal decision-making
Ventral frontostriatal regions have largely been implicated both in motivating behaviour towards obtaining desired outcomes—or rewards—and, subsequently, in the hedonic impact of rewards once obtained. The nucleus accumbens, part of the ventral striatum, has been shown to code both probability and delay features of rewards indiscriminately, pointing towards a common ‘neural currency’ (Peters and Büchel, 2009). This region responds preferentially to (i) low-probability rewards that are better-than-expected and so more subjectively gratifying (Yacubian et al., 2006; Smith et al., 2009); and (ii) immediate rewards over those that are superior in the longer-term (McClure et al., 2004). In contrast, activity in dorsofrontal structures, most notably the dorsolateral prefrontal cortex (PFC), is associated with mediating behaviour in the direction of safer over risky prospects (Campbell-Meiklejohn et al., 2008) and with delaying gratification in favour of superior but delayed rewards (McClure et al., 2004). Underlying the role of the dorsolateral PFC in decision-making is its central ability to detect and direct attention towards goal- or task-relevant stimuli (Banich et al., 2000; MacDonald et al., 2000).
Signals from ventral striatum and dorsolateral PFC converge in the ventral PFC, an area understood to encode an integrated valuation of prospects taking account of the trade-off between lower-order preference and longer-term profitability (Hare et al., 2009) and which cuts across both delay and probability domains (Peters and Büchel, 2009). Activation of the ventromedial PFC reflects subjective preference curves for different prospects (Kable and Glimcher, 2007) and predicts behavioural choice (Plassmann et al., 2006).
Regulation of activity in the ventromedial PFC via the dorsolateral PFC has been demonstrated in tasks requiring self-control over behaviours that would secure strongly desired outcomes, but which are suboptimal in the long run. This includes, for example, selecting a healthier but non-preferred food option (Hare et al., 2009), or deciding not to chase increasingly risky rewards after experiencing losses (Campbell-Meiklejohn et al., 2008). The dorsolateral PFC is also recruited in non-choice contexts when subjects are instructed to cognitively reappraise a rewarding prospect or outcome, accomplishing this through the modulation of the ventral striatal signal in the ventral frontostriatal pathway (Staudinger et al., 2009, 2011). The functional outcome of this modulation is a differential weighting (in the ventromedial PFC) of the lower-order preference, mediated by ventral striatum, against higher-order goals represented in dorsolateral PFC activity.
Decision-making in bipolar disorder
Bipolar disorder is characterized by marked difficulty in regulating the pursuit of goals (Johnson, 2005), with the onset of manic and depressive episodes linked to the attainment and failure to obtain goals, respectively (Johnson et al., 2008). Although particularly elevated during mania, impulsivity represents a trait feature of the disorder (Strakowski et al., 2010) and there is evidence of altered frontostriatal processing of reward prospects and outcomes across mood episodes. A recent study of euthymic patients demonstrated hyperactivation of ventral striatum and ventromedial PFC when anticipating rewarding outcomes (Nusslock et al., 2012), but no difference at outcome. Similarly in a non-clinical sample exhibiting subsyndromal hypomania, striatal activity during anticipation was more strongly modulated by the reward value of prospects, compared with control subjects (O'Sullivan et al., 2011). Other studies of this at-risk population have shown both a greater hedonic impact of reward outcomes generally (Mason et al., 2012a) as well as a preference for immediate over delayed-but-superior rewards (Mason et al., 2012b), as indexed by an event-related potential originating from the ventral frontostriatal pathway (Carlson et al., 2011).
The findings are not clear-cut for how these frontostriatal systems are modulated by affective state. One study of patients experiencing mania reported an elevated ventral striatal response to omission of reward outcomes, but no difference in response to expected reward outcomes (Abler et al., 2007a), although unexpected rewards, which may be more valued, were not available in their design. The failure to deactivate ventral striatum for reward omission may point towards a reduced appreciation of the cost of these null outcomes on the higher-order goal of maximizing profit. In another study, patients experiencing mania showed hyperactivation of ventral prefrontal cortex given the prospect of increasing reward, but relatively reduced activation when faced with increasing loss (Bermpohl et al., 2010). Healthy controls showed the inverse pattern, indicative of an appreciation that larger losses contravene the higher-order goal of maximizing task earnings, whereas manic individuals were less concerned about this and instead were motivated by larger gains.
Taken together, these studies indicate that goal dysregulation in bipolar disorder manifests neurally as a strong lower-order preference for rewarding prospects (Bermpohl et al., 2010; O'Sullivan et al., 2011; Nusslock et al., 2012) and reduced appreciation for risky prospects that are suboptimal with regards to a higher-order goal (Bermpohl et al., 2010). The present study aimed to test whether impulsive and risky decision-making results from a valuation in ventromedial PFC that is biased towards strongly desired but risky prospects (mediated by ventral striatum) over those that are better in the long-run (dorsolateral PFC-mediated). To reduce medication effects, we recruited euthymic patients that were not receiving antipsychotics, which are likely to be the most problematic class for studying reward processing (Pessiglione et al., 2006; Abler et al., 2007b). A second aim, in light of the equivocal findings in the above studies, was to examine how trait differences in frontostriatal regions are modulated by affective symptoms.
To this end we assessed how activity in the dorsolateral PFC, ventromedial PFC and ventral striatum was influenced by safe and risky gambles, determined by probability of reward in a roulette task. Consistent with a role in coordinating the higher-order goal of maximizing overall winnings, we expected that the dorsolateral PFC would activate preferentially for safe gambles and that this would be negatively associated with real-life impulsivity and risk-taking traits. In contrast, we predicted that activity in ventral striatum would preferentially respond to risky gambles and that this would be positively correlated with trait impulsivity and risk-taking, in keeping with a stronger lower-order preference for unexpected (low probability) rewards. We assumed that optimal integration of these signals in ventromedial PFC in healthy controls would manifest as a stronger correlation with dorsolateral PFC activity than with ventral striatal activity (i.e. final valuation being more contingent on the higher-order goal of being successful in the long run with safe gambles). We predicted that patients with bipolar disorder would show the opposite pattern, with ventromedial PFC activity correlating more strongly with ventral striatum, consistent with final valuation being more contingent on lower-order preference. A separate set of analyses looked at the impact of state fluctuation in symptoms.
Materials and methods
Participants
Twenty patients with bipolar disorder in remission were case-matched with 20 healthy control subjects by age, gender and level of education (Table 1). Key inclusion criteria were age 18–45 years, no current alcohol problem (weekly intake ≤25 units) or substance use in the past 4 months. In addition we excluded participants that had received antipsychotic medication in the past 6 months to reduce the effect of medication on reward-related activations (Pessiglione et al., 2006; Abler et al., 2007b).
Table 1.
Demographics and behavioural data
| Remitted bipolar disorder |
Healthy controls |
|||||
|---|---|---|---|---|---|---|
| Mean or proportion | SD | Mean or proportion | SD | Statistic | P-value | |
| Age | 35.95 | 8.34 | 33.25 | 9.32 | t(38) = −0.965 | 0.34 |
| Female | 10/20 | 11/20 | χ2(1) = 0.1 | 0.75 | ||
| Education (years) | 14.08 | 2.47 | 14.70 | 2.29 | t(38) = 0.829 | 0.41 |
| Episodes mania | 4.38 | 4.88 | ||||
| Episodes hypomania | 6.13 | 6.27 | ||||
| Episodes depression | 7.04 | 4.79 | ||||
| Primary diagnosis | ||||||
| BD-I | 18/20 | |||||
| BD-II | 2/20 | |||||
| Current comorbidity | ||||||
| GAD | 2/20 | |||||
| Lifetime diagnoses | ||||||
| Alcohol/SUD | 10/20 | |||||
| Panic disorder | 4/20 | 1/20 | ||||
| GAD | 2/20 | |||||
| OCD | 1/20 | |||||
| Medications | ||||||
| Lithium | 8/20 | |||||
| Valproate | 5/20 | |||||
| Lamotrigine | 2/20 | |||||
| SSRI | 3/20 | |||||
| SNRI | 3/20 | |||||
| Benzodiazepine | 1/20 | |||||
| z hypnotic | 3/20 | |||||
| None | 4/20 | |||||
| HRSD-17 | 3.82 | 3.04 | 0.63 | 1.06 | t(38) = −4.45 | ≤0.001 |
| MAS-12 | 3.23 | 2.78 | 0.40 | 1.13 | t(38) = −4.21 | ≤0.001 |
| VAS-Anxiety | 3.00 | 6.55 | 1.42 | 4.23 | t(37) = −0.889 | 0.38 |
| VAS-Sadness | −2.61 | 13.60 | −1.58 | 5.01 | t(37) = 0.232 | 0.82 |
| Response time (ms) | 724.5 | 188.0 | 741.6 | 191.8 | t(38) = 0.285 | 0.78 |
| Response (%) | ||||||
| Choice 1 | 25.1 | 4.51 | 25.0 | 5.48 | t(38) = −0.07 | 0.95 |
| Choice 2 | 27.9 | 6.40 | 25.6 | 5.04 | t(38) = 1.23 | 0.23 |
| Choice 3 | 24.3 | 4.13 | 24.9 | 4.45 | t(38) = −0.49 | 0.63 |
| Choice 4 | 17.8 | 5.02 | 19.2 | 4.34 | t(38) = −0.89 | 0.38 |
Patients with bipolar disorder did not differ from the controls in their response time or proportions of each option chosen.
*SUD = substance use disorder; GAD = generalized anxiety disorder; OCD = obsessive-compulsive disorder; HRSD = Hamilton Depression Score; MAS = Bech-Rafaelsen Mania Score; VAS = visual analogue scale score (−50 to +50); BD = bipolar disorder; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor.
Diagnosis was established using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 2002), with remission defined as not meeting the criteria for manic or depressive episodes in the past 2 months. Residual symptoms of depression and mania were assessed using the 17-item Hamilton Depression Rating Scale and 12-item Bech-Rafaelsen Mania Scale, respectively. Informed written consent was obtained from all participants, and the study was conducted in accordance with the Declaration of Helsinki. Additional details of the recruitment procedure are reported in the online Supplementary material.
To generate behavioural measures of real-life impulsivity and risk-taking that could be used in further validating the association of dorsolateral PFC and ventral striatum activity with impulsivity and risk-taking, participants completed the 11-item version of the Barratt Impulsivity Scale (Patton et al., 1995) and the Domain-Specific Risk-Taking Scale (Blais and Weber, 2006).
Task
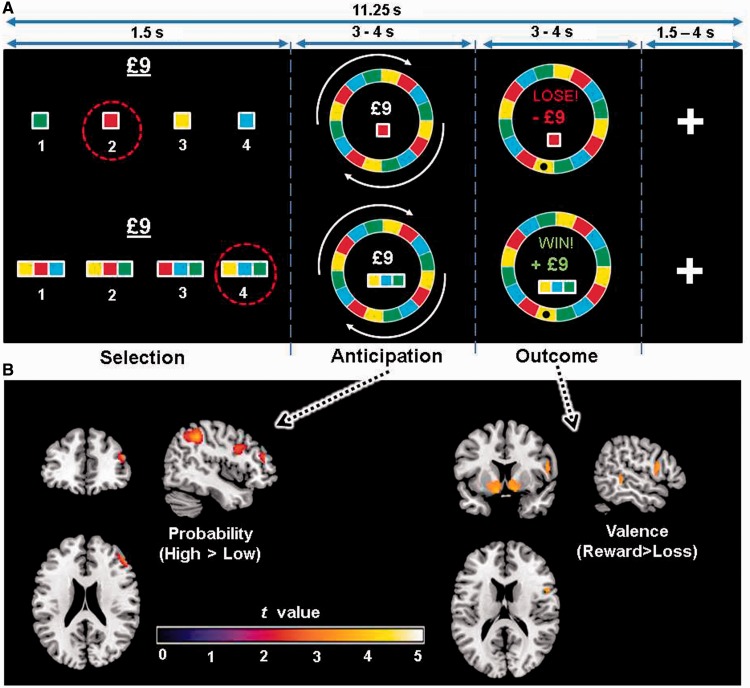
Participants played a modified version of a previously validated Roulette task (van Eimeren et al., 2009) comprising three time stages (Fig. 1A): selection, anticipation, and outcome (see Supplementary material for timing information). In this task, participants select between four choices that are equivalent in terms of available knowledge of reward probability, with the only difference being colour. Two variables were manipulated: probability of reward and magnitude, and each condition was presented as a separate trial, rather than as opposing choices within a given trial. Given that patients with bipolar disorder behave differently in decision-making tasks (Swann et al., 2003; Adida et al., 2011), this design precluded the eventuality of a mismatch in the numbers of responses for each condition. In the low probability (25% reward; ‘risky’) conditions, the four options were any one of the four colours that made up the roulette wheel. In the high probability conditions (75% reward; ‘safe’), participants chose between four sets of three colours each, and won if the Roulette wheel stopped on any of the three colours in the set that they chose. The stake was also fixed, and varied equally between trials of low (£3) and high (£9) magnitude. The magnitude at stake was presented during the selection phase. During the anticipation phase, the wheel spun. It stopped spinning at the outcome phase to reveal whether the participant had won or lost. Losses were penalized by the magnitude that had been at stake on the particular trial.
Figure 1.
Trial schematic and associated neural activity. (A) Participants made bets on which colour would win in a Roulette gamble. The trial sequence comprised three phases: selection; anticipation while the wheel spun; and outcome evaluation when the ball stopped on one of the colours, signifying the delivery of the reward or loss. (B) Whole-brain analysis of probability and valence during anticipation and outcome, respectively. Dorsolateral prefrontal cortex activated more during anticipation of high compared to low reward probability (cluster threshold P = 0.011 uncorrected, see Supplementary Table 1). At outcome, the nucleus accumbens was more active for rewards than losses (left and right cluster threshold P < 0.05 familywise error corrected).
Participants were instructed to respond within the fixed selection time and informed that a random choice would be automatically made if a timely response was not issued. Participants completed a total of 272 trials over eight runs (∼6 min each), with probability and stake being equally distributed across each run. Participants were informed that they would be paid the actual winnings from the task at the end of the experiment.
Functional magnetic resonance imaging data analysis
Standard functional MRI data acquisition and preprocessing are described in detail in the Supplementary material. We modelled the influence of motivational factors at anticipation and outcome. At anticipation, reward probability (25%, 75%) and magnitude (£3, £9) were modelled. At outcome, the factors were valence (gain, loss) probability (low, high) and magnitude (£3, £9). The selection phase was modelled as a regressor of no interest. We did not model motivational factors for this phase because of confounds from motor response and the short duration of this phase (1.5 s), which would reduce the power to reliably disambiguate neural activities related to this stimulus. Group (bipolar disorder or healthy control) was entered as a between-groups factor in each analysis of variance. Current depressive and manic symptoms were included as covariates in a separate analysis of covariance. A separate analysis of variance examined the trial-wise modulation of brain activity in relation to expected value and prediction error. Due to space constraints, both this analysis and those exploring interaction with mood symptoms are reported as Supplementary material.
The Harvard-Oxford probabilistic atlas (http://www.fmrib.ox.ac.uk/fsl/) was used to generate region of interest masks for bilateral nucleus accumbens and the medial frontal gyrus portion of dorsolateral PFC. The region of interest mask for the ventromedial PFC, unavailable in the Harvard-Oxford atlas, was functionally defined from prior publication (Hare et al., 2009), using 10 mm spheres around peak coordinates reported. The mean signal (beta values) across voxels in these regions of interest for individual trials were extracted for each contrast using the MarsBaR SPM toolbox (http://marsbar.sourceforge.net/) and exported to Statistical Package for the Social Sciences (SPSS) for all further analyses. This enabled a range of additional analyses (including post hoc tests) to be performed within a single and widely used package, as well as enabling versatile graphs and plots to be generated.
Modulation of ventromedial prefrontal cortex valuation by dorsolateral prefrontal cortex and ventral striatum
We evaluated the degree to which activity in ventral striatum (lower-order preference) and dorsolateral PFC (long-term goal) predicted activity in ventromedial PFC (weighted by both lower-order preference and the long-term goal), and how this differed between groups. To address this question we used a simplified regression model restricted to just these three key brain regions, which were identified a priori by previous theoretical and empirical work (Hare et al., 2009, 2011). In our analysis, outcome-locked activity in ventromedial PFC in response to gains only was the dependent variable, with (gain) outcome-locked activities in ventral striatum and dorsolateral PFC as covariates and group was a fixed factor. Although directionality cannot be inferred from our analysis, previous work using causal modelling (Hare et al., 2011) highlights ventromedial PFC as being confluence of valuation signals that receives ventral striatum and dorsolateral PFC inputs. A second analysis step assessed state-related modulation of the main effects by adding residual affective symptoms (scores on the Hamilton Depression Rating and Bech-Rafaelsen Mania Scales) to the above model as covariates. Correlations explored significant effects identified by this ANCOVA. Region of interest masks were an average across hemispheres, unless there was a main effect of hemisphere. In this latter eventuality, mean activity in the maximal hemisphere was taken, to increase statistical power.
Effects of trait impulsivity and risk-taking on the processing of safe and risky gains
Repeated measures ANOVA were conducted on neural activity in response to gain outcomes in ventral striatum, dorsolateral PFC and ventromedial PFC separately. Probability and group were fixed factors, and total impulsivity (Barratt Impulsivity Scale) and risk-taking (Domain-Specific Risk-Taking Scale) scores were entered as covariates. Significant interactions with covariates were followed up with partial correlation, controlling for group.
Results
Clinical and behavioural data
Demographics, clinical variables, medications, and behavioural results are reported in Table 1. Patients with bipolar disorder showed higher levels of state-related symptomology in depression (Hamilton Depression Rating Scale) and mania (Bech-Rafaelsen Mania Scale) scores relative to healthy control subjects (although all in the low range of ≤8). Patients also scored higher on trait impulsivity [total score: t(1,38) = 5.86, P < 0.001; motoric subscale: P = 0.06; non-planning: P = 0.089] and risk-taking behaviours [total score: t(1,38) = 2.13, P = 0.04], reflecting the higher levels of behavioural impulsivity and risk-taking in bipolar disorder.
Functional magnetic resonance imaging findings
This section reports the a priori region of interest analyses (see Supplementary Tables 1–3 for activated foci from a whole-brain analysis). The analyses are reported in the following order: (i) group comparisons; (ii) modulation of valuation by ventral and dorsal systems; and (iii) trait effects of impulsivity and risk-taking. The main analysis of state effects of residual affective symptoms on activity is reported as Supplementary material.
Group comparisons
Anticpation: The dorsolateral PFC region of interest showed main effects of reward probability [F(1,38) = 12.64, P < 0.001] and magnitude [F(1,38) = 6.91, P = 0.012] as well as a probability by group interaction [F(1,38) = 4.1, P = 0.05]. Across all participants, the dorsolateral PFC was more active for prospects that afforded a high probability of reward, and for prospects of larger magnitude. This is consistent with activity in dorsolateral PFC aligning with the higher-order goal of maximizing rewards. In the bipolar disorder group, however, the effect of probability was reduced, such that these patients showed a smaller increase in dorsolateral PFC activation for high-probability (relative to low-probability) prospects [t(19) = −4.61, P < 0.001], compared to controls [t(19) = −0.96, P = 0.35].
In ventral striatum, a three-way group by probability by hemisphere interaction approached significance [F(1,38) = 3.81, P = 0.058], which indicated that in left ventral striatum, the increase in activity for high-probability rewards (relative to low) was more pronounced in the bipolar disorder compared to healthy control group.
The ventromedial PFC region of interest showed no main effects of interactions (P-values ≥ 0.16).
Outcome: Task-related effects in the dorsolateral PFC region of interest included effects of valence [F(1,38) = 19.2, P < 0.001], hemisphere [F(1,38) = 6.16, P = 0.018], and a trend for magnitude [F(1,38) = 3.55, P = 0.067]. These effects indicated that dorsolateral PFC activation was (i) greater for gains than losses; (ii) greater for large than small outcomes; and (iii) greater in right dorsolateral PFC, across conditions, relative to left.
A three-way valence × probability × group interaction [F(1,38) = 4.96, P = 0.03] also emerged (Fig. 2). Follow-up ANOVAs on gain and loss outcomes separately showed that the interaction between probability and group was specific to gain outcomes [F(1,38) = 5.62, P = 0.023; losses, P = 0.58). T-tests showed that the groups differed in high probability gains [t(38) = 2.46, P = 0.019; low probability, P = 0.84], with greater dorsolateral PFC activity observed in controls.
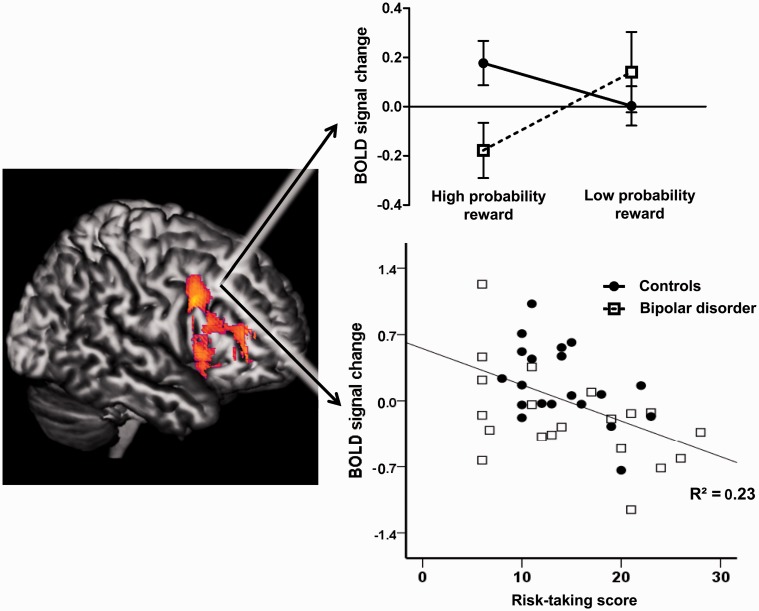
Figure 2.
Abnormal dorsolateral prefrontal cortex (PFC) activity in bipolar disorder correlates with risk-taking. Left: Effect of probability (High > Low) in whole-brain analysis of gamble outcomes. Right: Blood oxygen level-dependent signal from a priori region of interest analysis. Top right: Whereas controls preferentially activate dorsolateral PFC for high-probability (safe) reward outcomes, the bipolar disorder group show the opposite pattern, activating this region more for low-probability (risky) rewards. Bottom right: Greater real-life risk-taking in bipolar disorder group is associated with a reduced response to safe reward outcomes in right dorsolateral prefrontal cortex [r = −0.629, P = 0.005].
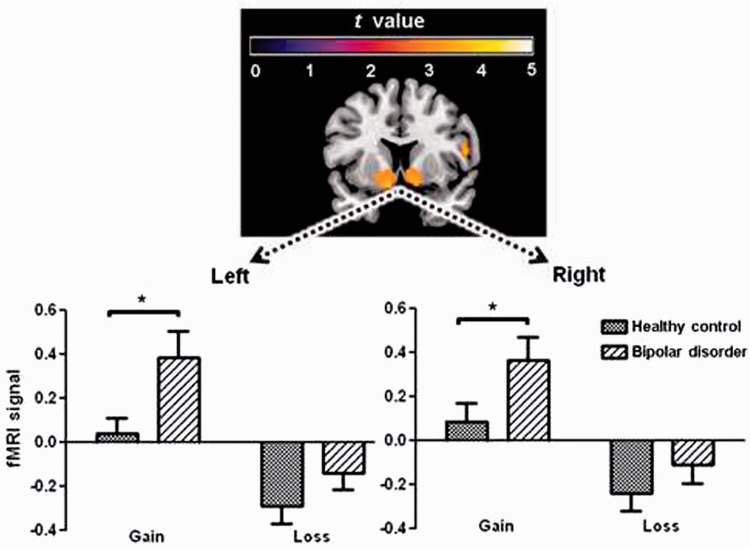
In the ventral striatum region of interest, task effects of valence [F(1,38) = 53.7, P < 0.001], magnitude [F(1,38) = 5.51, P = 0.024] were significant, in addition to valence × probability [F(1,38) = 7.71, P = 0.008] and valence × magnitude [F(1,38) = 4.05, P = 0.051] interactions. These effects indicated greater activity for gains than losses (Figs 1B and 3), and for large compared to small outcomes. As expected, the interactions signified that low probability and large magnitude outcomes increased ventral striatum activity for gains, but reduced activity for losses. These findings are consistent with the ventral striatum playing a role in the savouring of rewards, particularly those that are unexpected and large. An overall effect of group was significant [F(1,38) = 4.51, P = 0.04], and group interacted with valence [F(1,37) = 4.06, P = 0.05]. Separate ANOVAs for gain and loss outcomes showed that the groups only differed in ventral striatal activity for gain outcomes [F(1,38) = 5.16, P = 0.029; losses, P = 0.17], with the stronger response in the bipolar disorder patients signalling greater response to rewards (Fig. 3).
Figure 3.
Hyper-hedonic response to reward in bipolar disorder. Top: Activity in ventral striatum and the ventromedial prefrontal cortex in response to gains [Gain > Loss contrast across all subjects (whole-brain analysis; P < 0.05, familywise error corrected)]. Bottom: blood oxygen level-dependent signal from a priori region of interest analysis of left and right ventral striatum for gain and loss outcomes. After a Roulette gamble, patients with euthymic bipolar disorder show a hyper-hedonic response to winning, and a smaller response to losing. This may result in greater swaying by the ‘feel-good’ highs and less influence of the potential hazards of risky choices. Asterisk indicates significant group difference (P ≤ 0.05).
Task-related effects in the ventromedial PFC region of interest included effects of valence [F(1,38) = 28.6, P < 0.001], probability [F(1,38) = 3.91, P = 0.05], in addition to an interaction between valence and probability [F(1,38) = 15.0 P < 0.001]. As per the ventral striatum findings, follow-up ANOVAs on gain and loss outcomes separately showed that an effect of probability in the ventromedial PFC was specific to gain [F(1,38) = 13.6, P < 0.001; losses, P = 0.15]. This finding shows that the ventromedial PFC was most responsive to low-probability rewards. Also resembling the pattern within ventral striatum, valence interacted with group [F(1,38) = 4.62, P = 0.038]. Follow-up t-tests on gain and loss outcomes showed that, relative to controls, patients with bipolar disorder responded more strongly to gains [t(1,38) = 1.71, P = 0.08] but not losses [t(1,38) = 0.14, P = 0.89], consistent with a stronger lower-order preference for rewards.
Modulation of valuation by dorsolateral prefrontal cortex and ventral striatum
The aim of this analysis was to see how activity in ventral striatum and dorsolateral PFC related to activity in ventromedial PFC during processing of gain outcomes, and whether this differed across groups. Outcome-locked activity in ventromedial PFC in response to gains was entered as a dependent variable in an ANCOVA, with outcome-locked activity in the ventral striatum and dorsolateral PFC in response to gains as covariates, and group as a fixed factor.
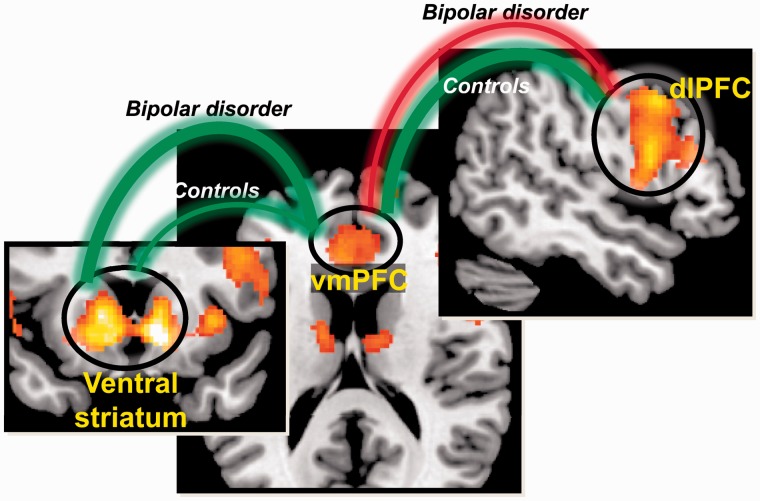
Activity in dorsolateral PFC interacted with ventral striatum [F(1,39) = 3.45, P = 0.01]: stronger combined activity between dorsolateral PFC and ventral striatum predicted greater activity in ventromedial PFC. A combination of outcomes that were subjectively preferred in addition to being perceived to promote the higher-order goal of maximizing outcomes promoted strongest activity in ventromedial PFC. Group separately interacted with ventral striatum [F(1,39) = 7.32, P = 0.01] and with dorsolateral PFC [F(1,39) = 12.1, P < 0.001], indicating that the two groups differed in strength or sign of the association between activity in these two regions and ventromedial PFC. Follow-up correlations were used to assess these possibilities. With regard to the relationship between ventromedial PFC and ventral striatum activations, the correlation was stronger for patients [r(20) = 0.662, P < 0.001] than controls [r(20) = 0.464, P = 0.04], although this difference did not reach significance with follow-up testing (Fisher’s Z = 0.86, P = 0.19). With regard to the relationship between dorsolateral PFC and ventromedial PFC, the correlation was positive in controls [r(20) = 0.704, P < 0.001]; however, it was negative in the bipolar disorder group [r(20) = −0.478, P = 0.03]. These correlations significantly differed between groups (Fisher’s Z = 2.78; P < 0.001; Fig. 4).
Figure 4.
Outcome valuation in bipolar disorder is driven more by ventral striatal than dorsal prefrontal cortical signals. In controls, ventromedial prefrontal cortical (vmPFC) activity showed moderate positive associations with both ventral striatum and with dorsolateral prefrontal cortex (dlPFC), consistent with comparable influences of both lower-order (ventral pathway) and higher-order (dorsal pathway) goals. In contrast, the bipolar disorder group showed a strong positive association in the ventral pathway (between ventral striatum and ventromedial PFC), and an inverse association in the dorsal pathway (between dorsolateral PFC and ventromedial PFC), suggesting that decision-making is influenced more by lower-order rather than higher-order aspects. Green and red lines denote positive and negative correlation, respectively. Thickness of connecting line denotes the strength of correlation coefficients.
The above analysis was repeated, but only in the bipolar disorder group, with depression and mania symptom scores included in the model. Mania interacted with ventral striatal activity [F(1,13) = 9.39, P = 0.009]. No other effects or interactions reaching significance. Subsequent partial correlations (controlling for depression scores) were performed using the predicted scores of the interaction (mania with ventral striatal activity) on ventromedial PFC. These showed that mania score augmented the positive relationship between ventral striatal and ventromedial PFC activities [r(17) = 0.636, P = 0.003 for correlation between ventral striatum and ventromedial PFC; r(17) = 0.842, P < 0.001 for the ventral striatal interaction with mania and ventromedial PFC]. This is consistent with state mania augmenting the role that lower-order preference plays in the evaluation of gain outcomes in ventromedial PFC.
Effects of trait impulsivity and risk-taking on the processing of safe and risky gains
As hypothesized, interactions emerged between personality traits (impulsivity, risk-taking) and neural activity related to reward probability. The probability by risk-taking interaction was significant in dorsolateral PFC [F(1,37) = 3.89, P = 0.05] and marginally significant in ventral striatum [F(1,37) = 3.86, P = 0.057]. Although in the direction hypothesized, the probability by impulsivity interactions failed to reach significance in dorsolateral PFC (P = 0.07) and ventral striatum (P = 0.13). There were no further effects or interactions in these structures, or in ventromedial PFC (P ≥ 0.31).
To explore the above interactions, correlations were performed between trait measures and the neural activity in response to low-probability and high-probability gain outcomes, separately. As predicted, the neural response to high-probability gains was negatively correlated with impulsivity in dorsolateral PFC [r(40) = −0.36, P = 0.023)] and with risk-taking score in both dorsolateral PFC [r(40) = −0.479, P = 0.002; Fig. 2] and ventral striatum [r(40) = −0.50, P < 0.001]. These correlations remained significant when controlling for group (partial correlation P ≤ 0.02). Neither trait impulsivity nor risk-taking was related to activations for low-probability gains (P ≥ 0.24).
Discussion
This study sought to specify the neural basis of suboptimal decision-making in bipolar disorder through examining activity during the anticipation and experience of safe and risky prospects. Recent research into the functional anatomical basis of optimal decision-making demonstrates interplay between frontostriatal systems. Whereas longer-term goals are represented in dorsolateral PFC and lower-order preference in ventral striatum, ventromedial PFC has been proposed to integrate these signals into a weighted valuation that ultimately drives subsequent behaviour (Plassmann et al., 2006). Our findings suggest that in bipolar disorder, and potentially other disorders characterized by impulsivity, the weighting of these signals (in ventromedial PFC) may be biased towards the ventral striatal contribution, and away from the dorsolateral PFC signal. In this way lower-order, strongly desired outcomes are favoured above and beyond those that fit with the long-term goal (Fig. 4).
Patients with bipolar disorder evidenced hyperactivation of ventral striatum both during anticipation and experience of rewards. When anticipating outcomes, these patients showed a greater increase in left ventral striatal activation for high reward probability gambles, compared with control subjects. This finding fits with recent research both in euthymic patients (Nusslock et al., 2012) and in people vulnerable to bipolar disorder (O'Sullivan et al., 2011), and may indicate that when rewards are likely to be available, this group have a greater drive to obtain them compared with control subjects. This may explain their elevated levels of goal-striving and willingness to expend effort to obtain reward (Johnson et al., 2012). Subsequently, when processing reward outcomes, patients with bipolar disorder showed hyperactivation of both ventral striatum and ventromedial PFC, compared with control subjects. This fits with our hypothesis that a trait feature of bipolar disorder is a greater hedonic impact of rewards, thereby making reward-seeking behaviours more enticing. Ventral striatal activation is associated with selecting immediate over delayed rewards (McClure et al., 2004), and so the dominance of this signal on integrated valuation (ventromedial PFC) reported here may be causally related to the impulsive delay discounting trajectory previously reported in bipolar disorder (Strakowski et al., 2010; Mason et al., 2012b). Although further work will be needed to examine this possibility, manic symptoms in the present study were associated with a strengthening of the association between ventral striatum and ventromedial PFC. This provides a link to the increases in impulsive and unrestrained reward-seeking behaviour associated with mania.
The divergent profile of activity in dorsolateral PFC between groups further points towards an overvaluation of lower-order goals in bipolar disorder. Consistent with our hypothesis, controls preferentially activated dorsolateral PFC for rewards of high (relative to low) probability—both during anticipation and delivery stages. These trials likely held the greatest attentional relevance in terms of the overarching goal of maximising earnings across the task, adding to past research that links dorsolateral PFC to the pursuit of longer-term or superordinate goals (Hare et al., 2009; Staudinger et al., 2011; Diekhof et al., 2012). In contrast, patients with bipolar disorder showed the opposite pattern of results to controls, with dorsolateral PFC preferentially responding to low-probability (i.e. more risky) rewards. This provides initial evidence that in bipolar disorder, this dorsal control system fails to suppress or devalue behaviours associated with immediate payoff in favour of those that fit with a longer-term, superordinate goal (Hare et al., 2009; Staudinger et al., 2011; Diekhof et al., 2012). This interpretation is further validated by the inverse association between dorsolateral PFC activation in response to safe rewards and both impulsivity and risk-taking traits (Fig. 2). From this finding it is reasonable to infer that other disorders characterized by impulsivity, both psychiatric and neurological, may be similarly characterized; by absent or reduced dorsolateral PFC-mediated upregulation of behaviours affording to longer-term or higher-order goals. Further research will be needed to examine this possibility.
Finally, although dorsolateral PFC activity was positively correlated with that of ventromedial PFC in controls, these two regions were negatively correlated in bipolar disorder group. This fits with an existing finding in euthymic patients of both reduced frontopolar (dorsal) cortical activation and increased ventromedial PFC activation during a gambling task (Jogia et al., 2012). Collectively, these findings suggest that valuation of outcomes in bipolar disorder is biased in favour of lower-order preference, even when this conflicts with the long-term goal of maximizing winnings (Fig. 4).
Although we attempted to reduce the impact of medication on reward-related neural activity by excluding antipsychotic medication, future work should examine reward processing in medication-naïve participants. Further, rates of (historic) alcohol and substance use were higher in the bipolar disorder group. Because addiction has been associated with differences in reward processing in its own right (Hommer et al., 2011), it cannot be ruled out that this also contributed to the group differences. However, supplementary analyses failed to show any effect of substance use history. It has also been suggested that bipolar disorder and addiction have shared vulnerability factors (Alloy et al., 2009). As with many reward tasks in the literature, the task employed in the present study could not disentangle reward probability from surprise. Recent work has shown that these processes may be coded by distinct regions of the human ventral midbrain (Boll et al., 2013) and future work will be needed to delineate these processes in disorders characterized by reward dysregulation.
In summary, we report evidence that in bipolar disorder both ventral (striatum) and dorsal (dorsolateral PFC) frontostriatal reward systems attend to lower-order goals, overvaluing outcomes that are strongly desired, but suboptimal in the long run. These findings strongly suggest that bipolar disorder cannot be reduced to affective instability alone, highlighting the centrality of goal regulation in understanding the impulsive and risky decision-making that spans the course of the disorder. Our findings may also have theoretical implications for understanding other impulsivity disorders, as well as several implications for clinical intervention. First, they suggest that psychotherapeutic interventions might be enhanced by specifically attending to issues of goal regulation. Second, the neurophysiological markers of poor goal regulation that we have identified in this study, if confirmed by later investigations, suggest some targets for novel psychological and pharmacological treatments. Moreover, third, these markers may be useful in evaluating both kinds of interventions. In particular, interventions that bolster dorsolateral PFC-mediated cognitive control may be an important direction for future research.
Funding
This work was supported by an interdisciplinary doctoral studentship awarded to Liam Mason by the Medical Research Council.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- PFC
prefrontal cortex
References
- Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2007a;33:2217–27. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007b;191:823–33. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, et al. Bipolar spectrum-substance use co-occurrence: behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J Pers Soc Psychol. 2009;97:549–65. doi: 10.1037/a0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Brain Res Cogn Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31:958–69. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais AR, Weber E. A domain-specific risk-taking (DOSPERT) scale for adult populations. Judgm Decis Mak. 2006;1:373–98. [Google Scholar]
- Boll S, Gamer M, Gluth S, Finsterbusch J, Büchel C. Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur J Neurosci. 2013;37:758–67. doi: 10.1111/ejn.12094. [DOI] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD. Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry. 2008;63:293–300. doi: 10.1016/j.biopsych.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal blood oxygen level-dependent activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57:1608–16. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O. Impulsive personality and the ability to resist immediate reward: an fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Hum Brain Mapp. 2012;33:2768–84. doi: 10.1002/hbm.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–87. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, Gilman JM. Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- Jogia J, Dima D, Kumari V, Frangou S. Frontopolar cortical inefficiency may underpin reward and working memory dysfunction in bipolar disorder. World J Biol Psychiatry. 2012;13:605–15. doi: 10.3109/15622975.2011.585662. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: a review. Clin Psychol Rev. 2005;25:241–62. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Cueller AK, Ruggero C, Winett-Perlman C, Goodnick P, White R, et al. Life events as predictors of mania and depression in bipolar I disorder. J Abnorm Psychol. 2008;117:268–77. doi: 10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Ann Rev Clin Psychol. 2012;8:243–67. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mason L, O'Sullivan N, Bentall RP, El-Deredy W. Better than I thought: positive evaluation bias in hypomania. PLoS One. 2012a;7:e47754. doi: 10.1371/journal.pone.0047754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, O'Sullivan N, Blackburn M, Bentall R, El-Deredy W. I want it now! neural correlates of hypersensitivity to immediate reward in hypomania. Biol Psychiatry. 2012b;71:530–7. doi: 10.1016/j.biopsych.2011.10.008. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, LaBarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–60. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan N, Szczepanowski R, El-Deredy W, Mason L, Bentall RP. fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia. 2011;49:2825–35. doi: 10.1016/j.neuropsychologia.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–5. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–34. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, Kenning P, Deppe M, Kugel H, Schwindt W, Ahlert D. Muenster, Germany: University of Muenster; 2006. How brands twist heart and mind: neural correlates of the affect heuristic during brand choice. [Google Scholar]
- Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol Clin Exp Res. 2010;34:1319–33. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. 2010;67:641–8. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DGV, Hardin MG, Jazbec S, Fridberg D, Blair RJR, et al. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–9. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–21. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–88. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, et al. Impulsivity across the course of bipolar disorder. Bipolar Disord. 2010;12:285–97. doi: 10.1111/j.1399-5618.2010.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord. 2009;11:280–8. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–11. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in parkinson's disease. Neuropsychopharmacology. 2009;34:2758–66. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitacco MJ, Rogers R. Predictors of adolescent psychopathy: the role of impulsivity, hyperactivity, and sensation seeking. J Am Acad Psychiatry Law. 2001;29:374–82. [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain-and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.