Croft et al. present fMRI and functional connectivity analyses of the language network in children with epilepsy and healthy controls. In both groups, the network is organised into dorsal and ventral systems. Activation of the ventral network is reduced in children with epilepsy, in association with poorer language function.
Keywords: epilepsy, seizure, language processing, functional brain imaging, cognition
Abstract
Children with focal epilepsy are at increased risk of language impairment, yet the neural substrate of this dysfunction is not yet known. Using functional magnetic resonance imaging we investigated the impact of focal epilepsy on the developing language system using measures of network topology (spatial organization of activation) and synchrony (functional connectivity). We studied healthy children (n = 48, 4–12 years, 24 females) and children with focal epilepsy (n = 21, 5–12 years, nine females) with left hemisphere language dominance. Participants performed an age-adjusted auditory description decision task during functional magnetic resonance imaging, to identify perisylvian language regions. Mean signal change was extracted from eight left perisylvian regions of interest and compared between groups. Paired region of interest functional connectivity analysis was performed on time course data from the same regions, to investigate left network synchrony. Two principal component analyses were performed to extract (i) patterns of activation (using mean signal change data); and (ii) patterns of synchronized regions (using functional connectivity data). For both principal component analyses two components (networks) were extracted, which mapped onto the functional anatomy of dorsal and ventral language systems. Associations among network variables, age, epilepsy-related factors and verbal ability were assessed. Activated networks were affected by age and epilepsy [F(2,60) = 3.74, P = 0.03]: post hoc analyses showed, for healthy children, activation in both ventral and dorsal networks decreased with age (P = 0.02). Regardless of age and task performance, children with epilepsy showed reduced activation of the ventral network (P < 0.001). They also showed a trend for increased activation of the dorsal network (P = 0.08) associated with improved task performance (r = 0.62, P = 0.008). Crucially, decreased activation of the ventral network in patients predicted poorer language outcome ( = 0.47, P = 0.002). This suggests childhood onset epilepsy preferentially alters maturation of the ventral language system, and this is related to poorer language ability.
= 0.47, P = 0.002). This suggests childhood onset epilepsy preferentially alters maturation of the ventral language system, and this is related to poorer language ability.
Introduction
Children with focal epilepsy have an increased risk of language impairment (Parkinson, 2002). However, it is not known how childhood-onset epilepsy affects functional language networks. Studies of adults with epilepsy have shown network-level changes associated with language compromise, using functional connectivity analysis. Functional connectivity is a measure of how tightly distinct regions of the brain are synchronized (Friston et al., 1993), providing a window into network dynamics. It has been shown that poor language function in adults with epilepsy correlates with reduced functional connectivity (synchrony) between left hemisphere language regions (Vlooswijk et al., 2010; Pravata et al., 2011). We used functional MRI to investigate if changes in the dynamics (synchronization) and spatial organization (topology) of language networks contribute to poorer language ability in children with focal epilepsy.
Theoretical models of language propose that dorsal and ventral processing streams connect Broca’s region with temporal and parietal language cortex (Scott et al., 2000; Wise, 2003; Hickok and Poeppel, 2004). These processing streams are supported by distinct white matter tracts, and may be optimized for particular language functions (Vigneau et al., 2006; Makris et al., 2007, 2009; Schmahmann and Pandya, 2007; Catani and Mesulam, 2008; Frey et al., 2008; Saur et al., 2008, 2010; Makris and Pandya, 2009; Friederici et al., 2011; Friederici, 2012). The ventral stream is connected via the extreme capsule, uncinate fasciculus, middle longitudinal fasciculus and inferior longitudinal fasciculus, and has been implicated in semantic processing. The dorsal system is supported by the superior longitudinal and arcuate fasciculi, and has been implicated in phonological processing, syntactic processing and working memory. Recent evidence suggests that white matter tracts of the two language streams mature at different stages during development: the tracts of the ventral stream appear to mature early in infancy, whereas the dorsal stream tract (arcuate fasciculus) continues to develop into adolescence (Brauer et al., 2011, 2013). These findings suggest the ventral stream may be important for early language development, and may also be relatively vulnerable to early insult (such as childhood onset epilepsy).
We applied principal component analysis (PCA)—a data-driven method—to functional MRI signal change and functional connectivity measures, to extract patterns (networks) of commonly activated or connected nodes across individuals. In contrast to previous studies, we divided classic Broca’s and Wernicke’s areas—as well as Geschwind’s territory (Catani et al., 2005)—into functional subregions guided by previous cytoarchitectonic, anatomical connectivity and functional imaging studies (Brodmann, 1909; Geschwind, 1965; Bookheimer, 2002; Catani et al., 2005; Vigneau et al., 2006; Anwander et al., 2007; Frey et al., 2008; Saur et al., 2008; Dick and Tremblay, 2012). These small regions of interest are hypothesized to reflect the functional subcomponents of the language network, thus providing a more detailed estimation of functional networks supporting task performance.
We aimed to investigate language networks in terms of: (i) their functional maturation with age; (ii) their vulnerability to childhood-onset epilepsy; and (iii) their relation to language ability. We expected PCA to extract networks consistent with the dorsal and ventral systems proposed in theoretical models and found in healthy adults. Based on findings from adults with epilepsy, we hypothesized that children with focal epilepsy would show reduced synchrony within the language system—specifically the ventral system—in association with poorer language function.
Materials and methods
Participants
Data for 48 healthy children (4–12 years) and 21 children with left focal epilepsy (5–12 years) were collected prospectively as part of a larger study investigating the effects of epilepsy on language organization (Table 1). All participants were native English speakers with unremarkable epilepsy MRI protocol (at 1.5 T), did not have comorbid mood disorders or any systemic disorder that might affect CNS function. All participants with epilepsy were deemed to have focal epilepsy with impaired consciousness (Berg et al., 2010) based on medical history, clinical examination and (video) EEG, with normal MRI and unknown cause. Routine genetic testing was not performed (unavailable at time of testing). Thus, subtle focal cortical dysplasia and genetic causes of focal epilepsy cannot be entirely excluded. Lateralization and localization of epilepsy were based on clinical description of seizures, post-ictal physical examination when available, and (video) EEG. The majority of patients (76%) experienced left hemisphere seizures. Seizure onset was most often localized to the frontal or temporal lobes (81% of all patients; 75% of patients with left hemisphere onset). Patients were taking one to three antiepileptic medications at the time of scanning (mode = 1): one patient was on topiramate. All patients were seizure-free in the 24 hours before functional MRI scanning.
Table 1.
Sample characteristics and group comparisons
| Patients (n = 21) | Controls (n = 48) | P | |
|---|---|---|---|
| Descriptive statistics | |||
| Age (years) | 8.67 (±2.2) | 9.06 (±2.48) | 0.57 |
| Sex (females, males) | 9,12 | 24,24 | 0.72 |
| Language lateralization (laterality indices) | |||
| Broca’s area | 0.68 (±0.21) | 0.71 (±0.16) | 0.37 |
| Wernicke’s area | 0.54 (±0.42) | 0.63 (±0.32) | 0.42 |
| Neuropsychological performance | |||
| General verbal ability composite (Z-score) | −0.73 (±0.8) | 0.32 (±0.9) | <0.001 |
| Verbal IQ (SS) | 100 (±13) | 117 (±16) | <0.001 |
| CELF-IV Core language (SS) | 100 (±12) | 113 (±11) | <0.001 |
| EOWPVT (SS) | 98 (±14) | 114 (±16) | <0.001 |
| Performance (non-verbal) IQ (SS) | 99 (±11) | 110 (±14) | 0.002 |
| In-scanner measures | |||
| Movement (mm) | 1.87 (±2.88) | 1.97 (±3.2) | 0.9 |
| Task performance (% correct) | 65.56% (±19) | 78.88% (±17) | 0.008 |
| Epilepsy characteristics | |||
| Age of seizure onset (years) | 5.01 (±2.85) | ||
| Duration of seizures (years) | 4.23 (±3.54) | ||
| Seizure frequency | |||
| Daily | 5% | ||
| Weekly | 14% | ||
| Monthly | 33% | ||
| ≤1 per six months | 43% | ||
| Seizure lateralization | |||
| Left | 76% | ||
| Right | 24% | ||
| Seizure localization | |||
| Frontal | 14.30% | ||
| Temporal | 57.10% | ||
| Parietal | 4.80% | ||
| Fronto-temporal | 9.50% | ||
| Temporo-parietal | 4.80% | ||
| Central | 4.80% | ||
| Undetermined | 4.80% | ||
Demographic and epilepsy variables, as well as standard scores (SS) on neuropsychological assessments for children with focal epilepsy (patients) and healthy children (controls), including significant group differences (independent samples t-tests). Healthy children had no history of developmental, learning, neurological or psychiatric disorder. In-scanner task performance data were available for 47 healthy controls and 18 patients. Age of onset and seizure frequency data were not available for one participant.
EOWPVT = Expressive One Word Picture Vocabulary Test.
Language dominance
We included only children with left hemisphere language dominance, to control for the influence of language reorganization when investigating intrahemispheric connectivity. Language lateralization was determined using a threshold-independent laterality index calculated using the LI toolbox (Wilke and Schmithorst, 2006), where LI = (L − R) / (L + R). Language dominance was categorized according to the laterality index calculated in the inferior frontal gyrus (Broca’s area), such that participants with indices ≥0.2 were considered left-dominant, and participants with indices <0.2 were considered to show atypical (right or bilateral) language dominance, consistent with previous studies (Berl et al., 2014).
Neuropsychological testing
Intelligence and language were assessed according to standardized administration of age-appropriate measures: IQ was assessed with either the Differential Scales of Ability (DAS, ages 4–5 years) or the Wechsler Abbreviated Scale of Intelligence (WASI, ages 6–12 years) (Elliott, 1990; Wechsler, 1999). Fundamental language skills were assessed with the Clinical Evaluation of Language Fundamentals, preschool version (CELF-P, age 4 years) or fourth edition (CELF-IV, ages 5–12 years) (Semel et al., 2003; Wiig et al., 2004). Single word retrieval (naming) was assessed using the Expressive One Word Picture Vocabulary Test (EOWPVT, ages 2–80 years) (Gardner, 1990). Verbal measures (verbal IQ, core language ability and word retrieval) were combined using PCA to produce a general verbal ability composite score, which accounted for 74.36% of the variance. This composite score was used to reduce the number of comparisons made when investigating the relationship of network measures to cognition.
Functional magnetic resonance imaging
Functional data were acquired using a 3.0 T Siemens Magnetom Trio equipped with a standard CP head coil. Anatomical images of participants were collected using a sagittal T1 MPRAGE sequence, slice thickness of 1.0 mm, repetition time of 1600 ms and echo time of 3.37 ms, which served to screen for anatomical abnormalities. Blood oxygen level-dependent changes were measured using a whole brain echo planar imaging sequence with parameters: repetition time = 3000 ms, echo time = 30 ms, field of view = 192 mm, and effective voxel size = 3.0 × 3.0 × 3.0 mm. Axial images were collected parallel to the anterior commissure–posterior commissure plane, which served as an origin of reference. Whole-brain volumes consisted of 50 axial slices of 2.8-mm thickness with a 0.2-mm gap between slices.
Language task
All children had a mock scanning session to enhance compliance and task performance. During the Auditory Description Decision Task (ADDT) participants heard pre-recorded sentences of true or false definitions of common objects (e.g. ‘A long yellow fruit is a banana’). The child pressed the button on the MRI compatible response box when definitions were true. This task engages semantic processing regions along the temporal and inferior parietal cortices associated with sentence comprehension, and inferior frontal and inferior parietal regions associated with semantic predictions and word retrieval (Gaillard et al., 2007; Berl et al., 2014). This task also offers the benefit of performance monitoring and developmentally matched levels; easy (4–6 years), medium (7–9 years) and hard (10–12 years). Seventy per cent of items were correct targets and 30% were foils. During the baseline condition participants listened to reversed speech and pressed the button upon hearing a tone. This allowed for the subtraction of bilateral activation due to primary audition and was matched to the experimental condition for motor response, length of utterance and volume of presentation. The task was presented in a blocked design with 10 alternating 30-s blocks, five active and five baseline, resulting in a total scan time of 5 min. Individual stimuli were presented every 3 s with a total of 10 stimuli per block. This task was performed as part of a larger imaging protocol performed during the same scanning session.
Data preprocessing
Image data preprocessing and group analyses were performed using Statistical Parametric Mapping software (SPM8; University College London) and Matlab (The MathWorks Inc.). Images were spatially normalized to the MNI standard anatomical space, spatially smoothed using an 8 mm full-width at half-maximum Gaussian kernel and temporally filtered (high-pass filter: 128 s). Individual t-maps were generated with movement parameters as covariates of no interest, to control for in-scanner movement. In-scanner movement did not differ between controls and patients (Table 1). Group maps were generated using a random-effects model.
Left hemisphere regions of interest
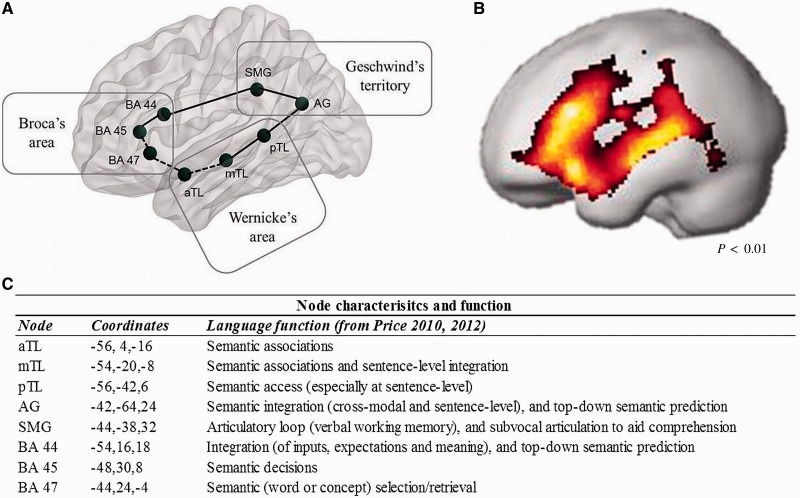
Using the automatic anatomic labels (Tzourio-Mazoyer et al., 2002) in the Wake Forest Pick Atlas (Maldjian et al., 2003, 2004), we created eight anatomic regions of interest in the left hemisphere (Fig. 1A) according to known anatomical boundaries within Broca’s area (inferior frontal gyrus), Wernicke’s area (middle and superior temporal gyri) and Geschwind’s territory (inferior parietal lobe) (Brodmann, 1909). Anatomic regions of interest were overlaid on the control group activation map (Fig. 1B) and a 4-mm radius sphere was drawn around the global point maxima within each region. These regions of interest were used as network nodes for the analysis of mean functional MRI signal change and functional connectivity in the dominant (left hemisphere) language network. We classified nodes as dorsal or ventral (Fig. 1A) to interpret our results in relation to current functional neuroanatomical models of language (Hickok and Poeppel, 2004). To ensure placement of regions of interest was not unduly influenced by our sample, we confirmed these are common areas for language activation by entering peak coordinates into a functional MRI database (http://beta.neurosynth.org), which verified that >70 studies of language also found activation within six voxels of our peak activation.
Figure 1.
Network nodes (regions of interest) placed within classic language regions (A) according to peak maxima in the control group activation map (B), and their functional characteristics (C). Regions of interest were placed along the dorsal (solid line) and ventral (dashed line) language streams (A). Coordinates in MNI space and the associated roles of each node in the language network are listed (C), according to consistent structure–function relationships which have emerged from 20 years of language functional MRI and PET studies (taken from Price, 2010, 2012). Network nodes include BA 47 (pars orbitalis), BA 45 (pars triangularis), BA 44 (pars opercularis), anterior temporal lobe (aTL), middle temporal lobe (mTL), posterior temporal lobe (pTL), supramarginal gyrus (SMG) and angular gyrus (AG).
Region of interest analyses
Nodal activation
Percent mean functional MRI signal change was extracted from each region of interest using MarsBaR software (Brett et al., 2002). We used repeated measures analysis of variance (ANOVA) to investigate the effect of group (between-subject factor) and node (within-subject factor) on activation strength.
Paired region of interest functional connectivity analysis
Mean time courses were extracted from each of the eight network nodes. Pearson’s r correlation coefficients were calculated between mean time courses for all node pair combinations (25 in total) as a measure of functional connectivity strength, and were converted to Fisher’s z-scores for statistical analyses. Mixed ANOVA were performed to investigate the effect of group (between-subject factor), connection (within-subject factor) and connection type (within-subject factor; frontotemporal, frontoparietal and temporo-parietal) on functional connectivity strength.
Identification of language networks using principal component analysis
Two PCAs were performed across all participants (n = 69), using a direct oblimin rotation with 25 iterations and corrected with the Kaiser criterion (including variables with an eigenvector >1). Only variables with factor loadings >0.4 (which explain at least 16% of the variance in a given factor) were reported (Field, 2005). Activated networks were investigated (PCA-A) by entering mean functional MRI signal change values for each node. Factors from this analysis represent a cluster of brain regions that behave similarly across individuals (they share between-subject variability in task-related functional MRI signal change). As it is their collective response that defines them as a single factor, we interpret these regions as representing a functional network. Synchronized networks were investigated (PCA-S) by entering all 25 z-scores from the paired region of interest functional connectivity analysis. Here factors represent networks of connections with shared between-subject variance in functional connectivity strength. Regression factor scores for individual participants were generated for each PCA (activated and synchronized), to quantify how strongly the extracted networks were activated (PCA-A) or synchronized (PCA-S) for each individual. A factor score of 0 represents the average for the sample. Positive factor scores indicate higher-than-average activation or synchrony, and negative factor scores indicate lower-than-average activation or synchrony.
The relation of language networks to age, epilepsy, performance and cognition
Two mixed ANOVA were performed to investigate the effects of component (network), diagnostic group and age group (4–6, 7–9 and 10–12 years) on regression factor scores from PCA-A and PCA-S. The association of age of onset, duration of epilepsy, in-scanner task performance and general verbal ability to factor scores was investigated using Pearson’s r correlation coefficient. Networks showing a significant relationship with the general verbal ability composite were investigated further in relation to individual neuropsychological measures. This stepwise approach served to reduce the number of comparisons made. Finally, all networks were investigated as predictors of language function (Core Language composite scores on CELF-IV) with multiple linear regression, to identify which network and which parameter (functional MRI signal change or functional connectivity) were most informative for predicting language outcome in children with epilepsy. All P-values were adjusted for multiple comparisons using the Bonferroni procedure.
Results
Neuropsychological performance
Patients had lower verbal and non-verbal abilities than controls (Table 1). Verbal ability (as measured by the composite score) did not differ between age groups and did not correlate with age in controls (P = 0.76 and 0.17, respectively) or patients (P = 0.75 and 0.81, respectively). Verbal ability was not correlated with age of onset or duration of epilepsy in patients (P-values > 0.42). Task performance did not differ between age groups in controls (accuracy P = 0.08, reaction time P = 0.48) or patients (accuracy P = 0.32, reaction time P = 0.44). In the control group, there was a trend for a correlation of performance accuracy with age (P = 0.06) which was driven by one outlier. There was no correlation once this outlier was removed (P = 0.21).
Group activation
Both groups activated the language network during Auditory Description Decision Task performance, including left superior temporal regions, temporal pole, inferior parietal lobe, Broca’s area [left Brodmann area (BA) 44 and 45] and middle frontal gyrus, bilateral BA 47, basal ganglia, anterior cingulate cortex and right cerebellum (Fig. 1B).
Region of interest analyses
Nodal activation
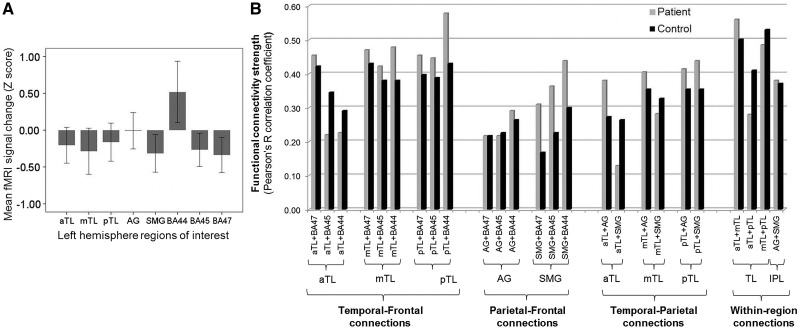
Patients showed lower mean functional MRI signal change than controls [main effect of group: F(1,64) = 10.74, P = 0.002]. Post hoc independent samples t-tests confirmed signal change values were lower in patients for all nodes (P-values < 0.02) except BA 44 (which was higher in patients; P = 0.047) and the angular gyrus (which did not differ between groups; P = 0.95) (Fig. 2A).
Figure 2.
(A) Mean functional MRI signal change (Z-scores) in network nodes in the patient group. Z-scores show the mean functional MRI signal strength in network nodes for patients, expressed in relation to the control group mean and standard deviation (with 95% confidence intervals). (B) Strength of individual functional connections by region and by diagnostic group. Pearson’s R correlation coefficient values for patients (grey) and controls (black); no significant group differences. fMRI = functional MRI; aTL = anterior temporal lobe; mTL = middle temporal lobe; pTL = posterior temporal lobe; AG = angular gyrus; SMG = supramarginal gyrus.
Paired region of interest functional connectivity analysis
Mean functional MRI signal time courses were positively correlated for all 25 network node pairings (P-values <0.001). There was no main effect or interaction effect of diagnostic group on overall functional connectivity [F(1,67) = 0.34, P = 0.56], nor on frontotemporal, frontoparietal or temporo-parietal connections (all P-values > 0.31) (Fig. 2B).
Identification of language networks with principal component analysis
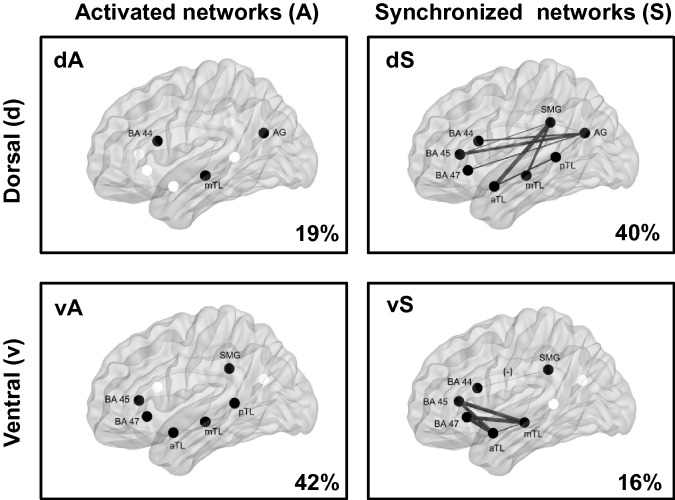
Two factors were extracted from both PCA-A and PCA-S (Fig. 3): dorsal and ventral activated networks (dA and vA, respectively) and dorsal and ventral synchronized networks (dS and vS, respectively). To confirm this factor solution provided a valid representation of the typical network structure, we recalculated PCA-A in healthy controls alone. Encouragingly, this analysis also extracted two factors (explaining 55% of the variance) that distinguished between dorsal and ventral regions: the dorsal factor showed activation in the inferior frontal gyrus (BA 44 and 47) and inferior parietal lobe (angular gyrus and supramarginal gyrus) and explained 39% of between-subject variability. The ventral factor showed combined activation of all temporal nodes and BA 45, and explained 16% of between-subject variability.
Figure 3.
Language networks extracted by PCAs. Dorsal (d; top row) and ventral (v; bottom row) principal components extracted from PCA-A (left) and PCA-S (right). Components from PCA-A (activated networks; A) represent networks of nodes (black) with shared between-subject variability in task-related functional MRI signal fluctuations across individuals. Components from PCA-S (synchronized networks; S) represent networks of node-pairings with shared between-subject variability in functional connectivity. The percentage of between-subject variability explained is depicted for each component (bottom right corner). For synchronized networks, line thickness is weighted by mean factor loading, where thicker lines indicate higher factor loadings. Negative factor scores are highlighted (–) for ventral synchronized networks (vS). dA = dorsal activated; vA = ventral activated; dS = dorsal synchronized; aTL = anterior temporal lobe; mTL = middle temporal lobe; pTL = posterior temporal lobe; AG = angular gyrus; SMG = supramarginal gyrus.
Activated language networks
Effect of age
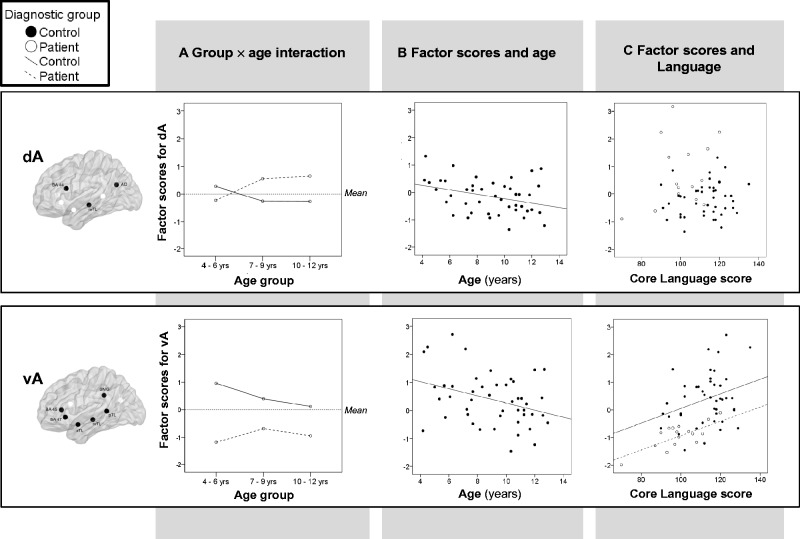
An interaction of age and diagnostic group [F(2,60) = 3.74, P = 0.03] showed that, in the control group, younger children (4–6 year olds) had higher factor scores than older children (10–12 year olds) (P = 0.02; Fig. 4A). Correlation analyses also showed a decrease in factor loadings with age in the control group, for both components (r = −0.38 and −0.34, P-values < 0.03; Fig. 4B). This correlation remained significant when controlling for task performance (accuracy and reaction time) and verbal ability (as measured by the composite score) for ventral activated networks (r = −0.32, P = 0.045) and at trend level for dorsal activated networks (r = −0.27, P = 0.09). There were no age effects in the patient group.
Figure 4.
(A) Significant interaction of epilepsy and age group on factor scores from dorsal (top) and ventral (bottom) networks extracted from PCA-A. Mean factor scores for controls (solid line) and patients (dashed line) on dorsal activated (dA) and ventral activated (vA) networks are depicted across three age categories. Factor scores for ventral activated networks were significantly reduced in the patient group (bottom), while patients showed a trend for higher factor scores on dorsal activated networks (top). In the control group, factor scores were significantly higher for 4–6 year olds than 10–12 year olds (P = 0.02) for both ventral and dorsal activated networks. (B) Significant correlation between factors scores from PCA-A and age in healthy children. (C) Relationship of factor scores with language ability as measured by core language composite scores on the CELF-IV for dorsal activated (insignificant; P > 0.05) and ventral activated networks (controls: r = 0.31, P = 0.04, patients: r = 0.69, P = 0.002). The corresponding network is depicted next to the y-axis for each graph, to aid interpretation.
Effect of epilepsy
There was an interaction of diagnostic group and component [F(1,60) = 47.1, P < 0.001; Fig. 4A], suggesting between-group differences in factor scores for dorsal and ventral networks. Post hoc ANOVAs investigating the effects of age and epilepsy on factor scores for each component separately revealed children with epilepsy (regardless of age) had greatly reduced factor scores for ventral activated networks compared to healthy children [F(1,60) = 44.3, P < 0.001; Fig. 4A, bottom row], even when controlling for group differences in task performance (P < 0.001). In contrast, for the dorsal activated network (dA) there was a trend for an interaction of age and group, suggesting older children with epilepsy (>7 years of age) showed increased activation in the dorsal network relative to healthy children of the same age [F(2,60) = 2.69, P = 0.08, Fig. 4A, top row]. However, this was no longer a trend when controlling for group differences in task performance accuracy (P = 0.14). Further analyses showed factor scores for dA were strongly correlated with task performance accuracy for children with epilepsy (r = 0.62, P = 0.008), but not for controls (r = −0.08, P = 0.63), such that children with epilepsy who performed better inside the scanner showed increased activation in this dorsal network. Neither ventral nor dorsal activated networks were related to age of onset or duration of epilepsy.
Synchronized language networks
There was no main effect or interaction of component, age or diagnostic group on factor scores from PCA-S. Factor loadings from PCA-A and PCA-S were not correlated (r < 0.08, P > 0.49) suggesting they measure distinct attributes of the language network.
The relation of network measures to task performance and language ability
Due to the main effect of diagnostic group on factor scores from PCA-A, correlation analyses were performed in control and patient groups separately. In-scanner task performance was not associated with network measures when controlling for age. Higher factor scores for vA associated with better general verbal ability for controls (r = 0.30, P = 0.04) and patients (r = 0.47, P = 0.04), specifically: higher verbal IQ (r = 0.29, P < 0.05) and CELF-IV core language composite scores in controls (r = 0.31, P = 0.04; Fig. 4C), and higher CELF-IV core language composite scores in patients (r = 0.69, P = 0.002; Fig. 4C). The correlation of vA factor scores and core language composite scores remained significant when controlling for in-scanner task performance (controls: r = 0.35, P = 0.02, patients: r = 0.75, P = 0.002) and when controlling for age for patients (controls: r = 0.28, P = 0.06, patients: r = 0.68, P = 0.001). There were no significant correlations between factor scores from PCA-S and the general verbal ability composite.
Predictors of language ability
To investigate the independent contribution of different network measures (vA, dA, vS, dS) to language ability in children with and without epilepsy, stepwise linear regression was performed in both groups separately. In both groups, only activation strength in the ventral network (vA) predicted language ability (CELF-IV core language composite scores); explaining 47% of language performance in children with epilepsy ( = 0.47, P = 0.002) and 8% in healthy children (
= 0.47, P = 0.002) and 8% in healthy children ( = 0.077, P < 0.04).
= 0.077, P < 0.04).
Discussion
Using novel methods we provide new evidence for activated and synchronized networks, which suggest the developing language system—like the mature language system—shows a division of labour between dorsal and ventral systems (Scott et al., 2000; Wise, 2003; Hickok and Poeppel, 2004). Although both networks were recruited to support language performance in typically developing children, particularly early in life (4–6 years), children with focal epilepsy failed to recruit the ventral system, and this was a marker of poorer language ability. These findings highlight the importance of the ventral language system for typical language development, and provide a potential explanation for the increased incidence of language impairment in children with epilepsy.
Dorsal and ventral systems in the typically developing language system
Through the novel application of PCA to both functional MRI signal change and functional connectivity data, we were able to extend findings from single-region and single-connection comparisons, to investigate developmental changes in language network topology and synchrony. Both PCAs extracted two main components (networks), which we interpreted as having a dorsal or ventral stream topology, based on known white matter connections. These provide novel evidence for a degree of functional separation within the language system, previously only seen in structural connectivity studies. As we did not overtly manipulate aspects of task demands, the function of each network cannot be inferred directly. However, structure-to-function mappings from the conventional functional MRI literature (Price, 2012) provide a strong basis for interpreting the functional roles of these sub-networks.
Ventral network
We identified active and tightly synchronized networks predominantly involving BA 45 and 47, and the anterior and middle temporal nodes. These regions are implicated in semantic processing of sentences and semantic decision-making, which are necessary skills for auditory comprehension. The activated ventral component (vA) also included activation in the posterior temporal and supramarginal nodes, implicated in sentence-level semantic access and sub-vocal articulation to support comprehension, respectively. We found a strong correlation between activation strength in vA and the core language composite on the CELF-IV, which is comprised of several tasks requiring receptive language (concepts & following directions, recalling sentences etc.) and further supports the role of the ventral system in auditory comprehension. The supramarginal gyrus and BA 44 are together implicated in a dorsal phonological working memory system (Vigneau et al., 2006; Price, 2012). Synchrony between these regions was attenuated in vS, and may reflect reduced demands on dorsal working memory or attentional systems when comprehension is achieved mainly via semantic processing in the ventral system.
Dorsal network
We identified a more dorsal network with activation increases in BA44, the angular gyrus and middle temporal node (dA). We also identified a widely connected network (dS) showing tight synchrony of inferior parietal nodes with inferior frontal and temporal nodes. This pattern of functional connectivity mirrors the underlying white matter tracts of the dorsal system (arcuate fasciculus), which connects the inferior parietal lobe to both the frontal and temporal lobes (Catani et al., 2005). As such, we consider this a dorsal network, which may be involved in working memory and phonological processing (Vigneau et al., 2006; Saur et al., 2008). Crucially, this network shows functional connectivity among both dorsal and ventral nodes, emphasizing the overlap of these systems during naturalistic use of language (Weiller et al., 2009).
A middle temporal hub
Over the course of development the internal sensorimotor, memory and affective representations of words become strongly associated over time and due to extensive experience with language. Although highly controlled language tasks can distinguish between dorsal and ventral systems in healthy adults (Saur et al., 2010), these associations firmly integrate the two pathways into a synergistic system during naturalistic use of language (Pulvermüller and Fadiga, 2010; Rolheiser et al., 2011). The middle temporal gyrus is a potential substrate for integration of dorsal and ventral systems due to its extensive anatomical connectivity, and may be a critical language hub (Turken and Dronkers, 2011). Our findings support this hypothesis; the middle temporal node was involved in both dorsal and ventral networks identified with PCA.
Maturation of dorsal and ventral networks
Longitudinal and cross-sectional studies in children using conventional functional MRI have shown both increases and decreases in language network activation with age (Ahmad et al., 2003; Brown et al., 2005; Plante et al., 2006; Schmithorst et al., 2006; Szaflarski et al., 2006, 2012; Brauer and Friederici, 2007; Berl et al., 2010, 2014; Yeatman et al., 2010; Lidzba et al., 2011). Findings are inconsistent and conflicting however, most likely due to use of different language tasks and inconsistent control over in-scanner task performance and language proficiency. For example, up to 50% of apparent age-related changes in network activation can be due to task performance differences (Brown et al., 2005). We therefore provide novel evidence that whole-network activation in dorsal and ventral language systems reduces with age during typical development, when task performance and language proficiency are well-controlled. Increased network activation in younger children may suggest the task was more difficult for these children (Yeatman et al., 2010), despite an age-adjusted task design and equivalent performance accuracy across age groups. Alternatively, reduced whole-network activation in older children may reflect refining of systems with age, with only a subset of nodes supporting proficient task performance later in life. For example, young children (<10 years) show activation along the extent of the inferior frontal gyrus during a syntactic processing task, whereas older children (>10 years) show localized activation around BA 44 (Nunez et al., 2011). As such, our findings could be considered as evidence supporting the interactive specialization framework of cognitive development, which predicts refining of cognitive networks with age (Johnson, 2011).
We also found evidence to suggest healthy children with more proficient language—regardless of age and in-scanner task performance—show increased activation throughout the ventral network. Conventional functional MRI studies have also shown increased language proficiency is associated with increased and more extensive activity in left hemisphere language regions (Yeatman et al., 2010; Lidzba et al., 2011; Nunez et al., 2011).
In contrast to findings of network activation, we did not find evidence for age-related changes in functional connectivity. Although left hemisphere functional connectivity differs when comparing very young children to adults (Friederici et al., 2011), another study which investigated changes across childhood also failed to find evidence for major age-related connectivity changes (Wilke et al., 2009). This may reflect the early establishment of fundamental white matter structures in the brain, despite their ongoing refinement after 5 years of age (Lebel et al., 2008). Alternatively, the poor temporal resolution of functional MRI or the influence of hidden dynamics may limit our ability to detect changes in network synchrony. Dynamic causal modelling (Friston et al., 2003) may be better placed to investigate subtle, or more complex, changes in network dynamics across childhood and adolescence, for example by accounting for possible age-related alterations in the haemodynamic response function (Friston et al., 2013).
The effects of childhood-onset focal epilepsy on language network topology
PCA suggested ventral language regions work together in a coordinated network, which is compromised in children with epilepsy. The typical developmental trajectory of activation in this ventral network for healthy children was not seen in children with epilepsy, nor was there evidence for developmental delay. Instead, activation of this network was consistently decreased for children with epilepsy across childhood, suggesting a pattern of atypical development. In support of this interpretation, reduced activation in this network was associated with poorer language function, predicting almost half the variance in language ability in children growing up with epilepsy. Previous studies also suggest the ventral system may be particularly crucial for typical language development, due to its relatively early maturation compared to the dorsal system (Brauer et al., 2013). Further, the relevance of the ventral system to (receptive) language outcome has been documented in several patient populations (Crinion and Price, 2005; Warren et al., 2009; Agosta et al., 2013). For example, in a large sample of adults with acute stroke, auditory comprehension deficits were associated with lesions affecting the extreme capsule (a ventral tract) (Kummerer et al., 2013). There is also accumulating evidence to suggest early structural damage to the ventral system has important consequences for language development. For example, damage to the extreme capsule and uncinate fasciculus—and not the arcuate fasciculus—is associated with language impairment in children born preterm (Northam et al., 2012). In addition, lesions encroaching on the ventral system are associated with functional reorganization of language in children with drug-resistant epilepsy (Pahs et al., 2013), suggesting damage to this system impacts the wider language network. Unlike these previous studies, our sample had no overt structural lesion. We therefore hypothesize changes in the ventral system in this study are related to epilepsy variables or its remote cause. Given the relatively short duration of epilepsy, however, we could not fully investigate the contribution of longer duration of epilepsy, which is best addressed by longitudinal studies. Our initial findings however, do suggest the topology of ventral network activation may provide insights into the neural mechanisms of language impairment in this population.
In contrast with ventral network nodes, our analysis of mean functional MRI signal change showed activation in BA 44 was increased in patients. This region has been associated with the dorsal stream, and is involved in processing complex syntax, syntactic working memory, phonological processing and lexical retrieval (Fiebach et al., 2005; Friederici, 2006; Friederici et al., 2006; Heim et al., 2009a, b). Principal Component Analysis revealed BA 44 shared between-subject variance in task-related activation with the middle temporal node and supramarginal gyrus (dA), and was functionally connected with regions anatomically connected via the arcuate fasciculus (dS). Activation of the dorsal network (dA) was increased in patients (especially those >7 years of age), providing novel evidence that reduced activation in one stream may be accompanied by increased activation in the other. Increased activation of the dorsal network was associated with improved task performance in the patient group. This finding suggests that flexibility in recruitment of the two streams enables functional compensation.
The effect of childhood-onset focal epilepsy on language network synchrony
Previous studies suggest that functional connectivity in the left hemisphere language network is reduced in adults with epilepsy regardless of side of seizure onset, and that this is associated with poorer language function (Waites et al., 2006; Vlooswijk et al., 2010; Pravata et al., 2011). Contrary to these findings—and our own hypotheses—we found no group differences in functional connectivity at either level of analysis; single-connection (paired region of interest functional connectivity analysis) or network (PCA). Our findings may differ from previous studies because of three main methodological differences: first, previous studies performed functional connectivity analyses using large anatomical regions of interest, comprised of functionally distinct brain regions. Large regions of interest may conceal changes in connectivity associated with specific functional networks. For example, a region of interest based on the entire inferior frontal gyrus will average time courses from BA 44, 45 and 47, which show a posterior–anterior gradient for phonological, syntactic and semantic processing, respectively (Bookheimer, 2002). For this reason, we placed small regions of interest within functional subdivisions of classic language regions. Large regions of interest may also result in overlap with other (non-linguistic) networks supporting task performance, such as attention or executive functioning. Indeed, previous studies using large regions of interest report changes in functional connectivity most often involving the anterior and posterior cingulate cortices (Waites et al., 2006; Vlooswijk et al., 2010). These regions may be involved in attentional processes supporting task performance, rather than linguistic processing per se (Fu et al., 2002; Leech et al., 2011). We aimed to retain the focus of our analysis to core language processing regions, by placing small regions of interest within specific language regions.
Second, in contrast with previous studies, we investigated functional connectivity in children who retained left hemisphere language dominance. Because atypical (right or bilateral) language lateralization is more common in people with epilepsy compared to healthy controls (Rosenberger et al., 2009), it is difficult to differentiate the effects of language reorganization from the effects of epilepsy on functional connectivity in previous studies. The one study that did control for language dominance (i.e. only participants with left hemisphere language dominance were included) found no evidence for reduced left hemisphere functional connectivity in adults with epilepsy (Protzner and McAndrews, 2011). Previous findings of reduced functional connectivity between language regions in adults with epilepsy may therefore reflect differences in language dominance, rather than the effect of epilepsy per se. To further emphasize this point, we failed to find any association between epilepsy variables and network measures in this study. Even in the aforementioned studies of adults with epilepsy, neither age of onset, frequency of seizures, drug load, nor seizure focus accounted for variation in language network functional connectivity (Vlooswijk et al., 2010). However, initial research does suggest seizure duration (chronicity) may be an important variable for network connectivity; a prolonged duration of epilepsy has been shown to reduce functional connectivity (Luo et al., 2011) and promote a more random network configuration (van Dellen, 2009). In addition, a prolonged duration of epilepsy may also be accumulatively detrimental to language function (Caplan, 2009). As such, it is possible that children in this study may not have experienced a sufficient duration of epilepsy to demonstrate changes in functional connectivity compared to adults with epilepsy. However, previous research in adults with epilepsy also provides little support for a direct influence of epilepsy on functional connectivity in the language system.
Third, we adjusted task difficulty according to developmental ability, to reduce variability of in-scanner task performance and effort. In this context, we were unable to identify group differences in functional connectivity, despite differences in network topology which remained highly significant when controlling for in-scanner performance. Studies that have reported reduced functional connectivity between core language regions in adults with epilepsy (Vlooswijk et al., 2010; Pravata et al., 2011) used a one-size-fits-all approach to task design, and did not control for in-scanner task performance. As such, differences in functional connectivity reported in these studies may reflect differences in performance accuracy or effort between healthy participants and participants with epilepsy. Indeed, changes in functional connectivity are related to poorer performance on similar language tasks outside the scanner (Vlooswijk et al., 2010).
Finally, in adults it has been shown that changes in language network connectivity are more pronounced in patients with a greater degree of cognitive impairment (Vlooswijk et al., 2011). It is therefore possible that functional connectivity changes may be apparent in children with more severe epilepsy and cognitive impairment; for example, those with drug-resistant epilepsy.
Aside from these methodological differences, our results may suggest functional connectivity is less vulnerable to the effects of childhood-onset epilepsy than adult-onset epilepsy. This requires empirical support, however, particularly with respect to the effect of epilepsy duration on language networks. Future studies investigating the relationship of functional connectivity to disease status, task performance and language ability in adults compared to children would be highly valuable, to assess the sensitivity of connectivity measures to variation in disease state and ability across the lifespan.
The effects of epilepsy on reorganization of language networks has been well documented in both adults and children with epilepsy (Janszky et al., 2003; Liegeois et al., 2004; Gaillard et al., 2007; Mbwana et al., 2009; Rosenberger et al., 2009; You et al., 2011; Pahs et al., 2013). However little is known about the neural correlates of language in the significant portion of children with epilepsy (∼60%) who do not reorganize function to the unaffected hemisphere. We highlight the importance of the ventral language system to language outcome in these children. This ventral network substantially overlaps with brain systems critical for speech intelligibility (Scott et al., 2000, 2006; Vagharchakian et al., 2012), making it a good candidate region for developmental language dysfunction.
Conclusion
The typically developing language system is composed of dorsal and ventral networks. The ventral system is particularly important for receptive language and appears to develop in early childhood, while the dorsal system shows a more protracted developmental course. The current study demonstrates that childhood-onset epilepsy preferentially affects functional development of the ventral language network, and that failure to activate this system predicts poorer language ability in children with epilepsy. These findings, along with accumulating evidence from developmental populations with early-onset structural brain lesions, suggest the ventral system may be the ‘Achilles heel’ of the developing language system. It therefore represents an important target for future research which aims to understand the neuroanatomical basis of the increased risk for language disorder, and the propensity for compensation and plasticity, in children with epilepsy.
Acknowledgements
The authors thank the families who participated in this study and Ben Yerys for technical help.
Glossary
Abbreviation
- PCA
principal component analysis
Funding
This publication was made possible by the Charlotte and Yule Bogue Research Fellowship in honour of Sir Charles Lovatt Evans and A.J. Clark, from University College London, as well as; The Child Health Research Appeal Trust (to L.J.C), Great Ormond Street Hospital Children’s Charity (to L.J.C and T.B.), Epilepsy Research UK (to T.B.), The National Institutes of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH) [R01 NS44280 to W.D.G., K23NS065121-01A2 to M.M.B]; Grant HD040677-07 from the National Institutes of Childhood Diseases (NICHD), NIH, and Grant M01RR020359 from the National Centre for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Agosta F, Galantucci S, Canu E, Cappa SF, Magnani G, Franceschi M, et al. Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang. 2013;127:157–66. doi: 10.1016/j.bandl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, Von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca's area. Cerebal Cortex. 2007;17:816–25. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Van Emde Boas, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, Vanmeter J, et al. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114:115–25. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Mayo J, Parks EN, Rosenberger LR, Vanmeter J, Ratner NB, et al. Regional differences in the developmental trajectory of lateralization of the language network. Hum Brain Mapp. 2014;35:270–84. doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex. 2011;21:459–66. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Perani D, Friederici AD. Dorsal and ventral pathways in language development. Brain Lang. 2013;127:289–95. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brauer J, Friederici AD. Functional neural networks of semantic and syntactic processes in the developing brain. J Cogn Neurosci. 2007;19:1609–23. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. In: Eighth International Conference on Functional Mapping of the Human Brain, Sendai, Japan. 2002. Region of interest analysis using the MarsBar toolbox for SPM 99. [Google Scholar]
- Brodmann K. Brodmann's localisation in the human cortex. London, UK: Smith-Gordon; 1909. [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Vona P, Stahl L, Bailey C, Gurbani S, et al. Language in pediatric epilepsy. Epilepsia. 2009;50:2397–407. doi: 10.1111/j.1528-1167.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–71. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–50. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales: introductory and technical handbook. San Antonio: The Psychological Corp; 1990. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, Von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. London, UK: SAGE Publications; 2005. [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Broca's area and the ventral premotor cortex in language: functional differentiation and specificity. Cortex. 2006;42:472–5. doi: 10.1016/s0010-9452(08)70380-0. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Language development and the ontogeny of the dorsal pathway. Front Evol Neurosci. 2012;4:3. doi: 10.3389/fnevo.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc Natl Acad Sci USA. 2006;103:2458–63. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS One. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston K, Moran R, Seth AK. Analysing connectivity with Granger causality and dynamic causal modelling. Curr Opin Neurobiol. 2013;23:172–8. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Morgan K, Suckling J, Williams SCR, Andrew C, Vythelingum GN, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demands. NeuroImage. 2002;17:871–9. [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–71. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gardner MF. Novato, California: Academic Therapy Publications; 1990. EO-WPVT-R.: expressive one-word picture vocabulary test, revised. [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–94. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Amunts K. Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. Neuroimage. 2009a;48:616–24. doi: 10.1016/j.neuroimage.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Friederici AD, Amunts K. Left cytoarchitectonic area 44 supports selection in the mental lexicon during language production. Brain Struct Funct. 2009b;213:441–56. doi: 10.1007/s00429-009-0213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Kloppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136:619–29. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidzba K, Schwilling E, Grodd W, Krageloh-Mann I, Wilke M. Language comprehension vs. language production: age effects on fMRI activation. Brain Lang. 2011;119:6–15. doi: 10.1016/j.bandl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–36. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32:438–49. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–58. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2009;19:777–85. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN. The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. Neuroimage. 2007;37:1100–11. doi: 10.1016/j.neuroimage.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132:347–56. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Tournier JD, Croft LJ, Johns PN, Chong WK, et al. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain. 2012;135(Pt 12):3781–98. doi: 10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez SC, Dapretto M, Katzir T, Starr A, Bramen J, Kan E, et al. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Dev Cogn Neurosci. 2011;1:313–23. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahs G, Rankin P, Helen Cross J, Croft L, Northam GB, Liegeois F, et al. Asymmetry of planum temporale constrains interhemispheric language plasticity in children with focal epilepsy. Brain. 2013;136:3163–75. doi: 10.1093/brain/awt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson GM. High incidence of language disorder in children with focal epilepsies. Dev Med Child Neurol. 2002;44:50–6. doi: 10.1017/s0012162201002511. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–21. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Pravata E, Sestieri C, Mantini D, Briganti C, Colicchio G, Marra C, et al. Functional connectivity MR imaging of the language network in patients with drug-resistant epilepsy. AJNR Am J Neuroradiol. 2011;32:532–40. doi: 10.3174/ajnr.A2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann NY Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–47. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner AB, McAndrews MP. Network alterations supporting word retrieval in patients with medial temporal lobe epilepsy. J Cogn Neurosci. 2011;23:2605–19. doi: 10.1162/jocn.2010.21599. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Fadiga L. Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010;11:351–60. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci. 2011;31:16949–57. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72:1830–6. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Kupper H, Kellmeyer P, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49:3187–97. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. J Hist Neurosci. 2007;16:362–77. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29:254–66. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Rosen S, Lang H, Wise RJ. Neural correlates of intelligibility in speech investigated with noise vocoded speech—a positron emission tomography study. J Acoust Soc Am. 2006;120:1075–83. doi: 10.1121/1.2216725. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord W. Clinical evaluation of language fundamentals-IV. Marickville: Harcourt Assessment; 2003. [Google Scholar]
- Szaflarski JP, Altaye M, Rajagopal A, Eaton K, Meng X, Plante E, et al. A 10-year longitudinal fMRI study of narrative comprehension in children and adolescents. Neuroimage. 2012;63:1188–95. doi: 10.1016/j.neuroimage.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Sys Neurosci. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vagharchakian L, Dehaene Lambertz C, Pallier C, Dehaene S. A temporal bottleneck in the language comprehension network. J Neurosci. 2012;32:9089–102. doi: 10.1523/JNEUROSCI.5685-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen E, Douw L, Baayen JC, Heimans JJ, Ponten SC, Vandertop WP, et al. Long-term effects of temporal lobe epilepsy on local neural networks: a graph theoretical analysis of corticography recordings. PloS ONE. 2009;4:1–9. doi: 10.1371/journal.pone.0008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vlooswijk MC, Jansen JF, Majoie HJ, Hofman PA, De Krom MC, Aldenkamp AP, et al. Functional connectivity and language impairment in cryptogenic localization-related epilepsy. Neurology. 2010;75:395–402. doi: 10.1212/WNL.0b013e3181ebdd3e. [DOI] [PubMed] [Google Scholar]
- Vlooswijk MC, Vaessen MJ, Jansen JF, De Krom MC, Majoie HJ, Hofman PA, et al. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011;77:938–44. doi: 10.1212/WNL.0b013e31822cfc2f. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weiller C, Musso M, Rijntjes M, Saur D. Please don't underestimate the ventral pathway in language. Trends Cogn Sci. 2009;13:369–70; 370–1. doi: 10.1016/j.tics.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Wiig EH, Secord W, Messing Semel E. CELF preschool 2: clinical evaluation of language fundamentals preschool. San Antonio: Pearson/PsychologicalCorp; 2004. [Google Scholar]
- Wilke M, Lidzba K, Krageloh-Mann I. Combined functional and causal connectivity analyses of language networks in children: a feasibility study. Brain Lang. 2009;108:22–9. doi: 10.1016/j.bandl.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–30. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Wise RJ. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. Br Med Bull. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM. Individual differences in auditory sentence comprehension in children: an exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114:72–9. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Adjouadi M, Guillen MR, Ayala M, Barreto A, Rishe N, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: a multisite study. Hum Brain Mapp. 2011;32:784–99. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]