Abstract
Scalable expansion of cells for regenerative cell therapy or to produce large quantities for high-throughput screening remains a challenge for bioprocess engineers. Laboratory scale cell expansion using t-flasks requires frequent passaging that exposes cells to many poorly defined bioprocess forces that can cause damage or alter their phenotype. Microcarriers offer a potential solution to scalable production, lending themselves to cell culture processes more akin to fermentation, removing the need for frequent passaging throughout the expansion period. One main problem with microcarrier expansion, however, is the difficulty in harvesting cells at the end of the process. Therefore, therapies that rely on cell delivery using biomaterial scaffolds could benefit from a microcarrier expansion system whereby the cells and microcarriers are transplanted together. In the current study, we used bioactive glass microcarriers doped with 5% TiO2 that display a controlled rate of degradation and conducted experiments to assess biocompatibility and growth of primary fibroblast cells as a model for cell therapy products. We found that the microcarriers are highly biocompatible and facilitate cell growth in a gradual controlled manner. Therefore, even without additional biofunctionalization methods, Ti-doped bioactive glass microcarriers offer potential as a cell expansion platform.
Keywords: Microcarriers, bioactive phosphate-based glass, cell expansion, titanium
Introduction
The emerging field of regenerative medicine incorporates strategies including cell therapy, biomaterial scaffolds and small molecules to stimulate regeneration in the body. In particular, direct cell delivery or tissue engineering approaches have the aim of restoring function of injured or diseased body parts by delivery of cells or engineered tissues to the defect site.1 This promising strategy depends on the assumption that cells can be expanded to produce therapeutic quantities and then be differentiated into the appropriate cell type required in the defect site.
Current limitations to cell therapy include expanding cells to the desired number for clinical use and the fact that their extended expansion in culture leads to loss of multipotency and senescence.2 For example, bioprocess forces experienced during serial passaging of cells (including centrifugal, hydrodynamic and capillary flow shear stress) can have poorly defined and, indeed, undesired effects on the cells. Moreover, interactions of adhesion molecules with extracellular matrix (ECM) ligands required to support cell survival are cyclically interrupted by trypsinization and replating during every passage in 2-dimensional (2D) monolayer expansion.3 Therefore, reliable and efficient ex vivo cell expansion methods need to be explored as alternatives to conventional 2D culture systems. Microcarriers that offer large surface areas for growth of anchorage-dependent cells have a potential advantage in that they can be used for the expansion of adherent cells in a 3D bioreactor using processes that are more akin to fermentation processes than traditional 2D monolayer cell culture. In recent years, several methods of expanding adherent cells have been reported using different types of microcarriers as substrates for cell attachment.4,5 Bead size, surface area and functional ability to promote cell attachment are all critical factors that can affect cell adhesion and ultimately their expansion potential.6
Recent advances in the biomaterials field have led to the development of composite materials, biofunctionalized to complement and enhance biological responses in living tissues without compromising the natural biological process. These materials include glasses and glass-ceramics and can be considered as bioactive materials.7 One of the measures of bioactivity is the utilization of simulated body fluid (SBF) in which apatite formed on the surface of a material is taken as an indicator that the material is bioactive.8 Phosphate-based glasses are particularly attractive as they are easy to produce, biodegradable and of known biocompatibility with multiple cell types.9,10 They can also be doped with a variety of metal oxides to modify their physical properties or induce a specific function such as antimicrobial capacity.11 However, they have been shown to not form an apatite layer in vitro,12 and yet do upregulate genes associated with bone formation in cell culture.13
The potential of microcarriers made of such materials for tissue engineering is significant because they present a potential way in which large quantities of material could be produced in many small individual cell-microcarrier units that can then be pieced together after expansion is accomplished.
In the current study, we selected microcarriers made from a stable bioactive phosphate glass doped with 5 mol% TiO2 to assess the attachment and expansion of primary fibroblasts as a model system for somatic cells used in cell therapy and tissue engineering procedures.
Materials and methods
Glass preparation and microsphere formation
Glasses were synthesized from the precursors: sodium dihydrogen orthophosphate (NaH2PO4), calcium carbonate (CaCO3), phosphorous pentoxide (P2O5) and titanium dioxide (TiO2) as previously described.14 Three different compositions, Ti3, Ti5 and Ti7 were made, according to the TiO2 content used (Table 1). The precursors were weighed and mixed and then preheated to 700℃ for 30 min to remove CO2 and H2O. The mixture was then melted at 1300℃ for 3 h and then rapidly quenched onto a steel plate. The phosphate glasses were ball-milled and sieved down to 75–106 µm. The glass particles were then passed through a flame spheroidization device to produce microspheres as previously described.12 The size distribution of the resulting microspheres was 10–210 µm and a size range of 63–106 µm was selected for use in subsequent experiments.
Table 1.
Glass structural composition.
| Glass compositions (mol%) | ||||
|---|---|---|---|---|
| Glass codes | P2O5 | CaO | Na2O | TiO2 |
| P50C40N7Ti3 (Ti3) | 50 | 40 | 7 | 3 |
| P50C40N5Ti5 (Ti5) | 50 | 40 | 5 | 5 |
| P50C40N3Ti7 (Ti7) | 50 | 40 | 3 | 7 |
Characterization of dissolution and ion release
To determine the change in weight with time, 250 mg of microspheres were placed in 25 ml of deionised water at pH 7. At 0, 1, 3, 7, 14 and 21 days, the solution was removed and the samples were oven dried and then reweighed and the weight loss was calculated as a percentage of the original weight. A unique set of samples was prepared for each time point and each time point was measured in triplicate.
ICS-1000 ion chromatography system (Dionex, UK) was used to measure Na+ and Ca2+ release. The separation was performed using methanesulfonic acid (Fluka, UK) eluent and a 4 × 250 mm Ion Pac® CS12A separator column. Prior to running a sample, ion chromatograph was calibrated against a four-point calibration curve using a predefined calibration routine. The Chromeleon® software package was used for data analysis.
The phosphate anion measurements were carried out using a Dionex ICS-2500 ion chromatography system. Polyphosphates were eluted using a 4 × 250 mm Ion Pac® AS16 anion-exchange column packed with anion exchange resin. A Dionex anion self-regenerating suppressor (ASRS®) was used at 242 mA. The Dionex EC40 eluent generator equipped with a potassium hydroxide (KOH) cartridge was used in conjunction with the ASRS®. The sample run time was set for 20 min where the gradient program started from 30 mM KOH for 10 min, then increased from 30 to 60 mM KOH over 5 min, followed by remaining at 60 mM KOH for 3 min and finally the KOH returned to 30 mM for 2 min.
Average microcarrier diameter measurements
Average microcarrier diameter was determined from light micrograph images of microcarrier monolayers. The diameter of 200 microcarriers was measured using the Image J software. Frequency histograms of size distribution were created in order to determine the size range of microcarriers employed.
Isolation and culture of primary fibroblasts
Primary fibroblasts were derived from E12.5-13.5 CD-1 mouse embryos in accordance with local animal welfare guidelines and regulations. Fibroblasts were liberated from embryonic tissue using the collagenase digestion method described previously.15 Cultures were established by plating digested tissue in T-25 flasks and cultured with Dulbecco's Modified Eagle Medium (DMEM) (4.5 g/L glucose) supplemented with FBS (10%) and non-essential amino acids (1%) (all from Invitrogen, Paisley, UK). Cell cultures were maintained at 37℃/5% CO2 and passaged when 80-90% confluent using trypsin-EDTA solution for 3 min at 37℃. Cell number was quantified using a Neubauer haemocytometer and viability was determined using the Trypan blue dye exclusion method.
Relative attachment of primary fibroblasts to microcarriers versus cell culture-grade plastic
Bioactive glass microcarriers (15 mg/well) were placed into wells of a 96-well microwell plate (Greiner Bio-One, Stonehouse, UK) in a monolayer and UV sterilized for 1 h and 40 min. Microcarriers were then equilibrated in 50 µL of pre-warmed medium to ‘wet’ them and avoid putting the cell suspension directly onto a dry monolayer of microcarriers. Fibroblasts were seeded on microcarriers (1.5 × 104 cells in 50 µL of medium) and the plates were then incubated at 37℃ and 5% CO2 for 24 h. Observations of cell attachment to microcarriers compared to the surrounding cell culture plastic were made using light microscopy and subsequently using fluorescence microscopy after staining with DAPI.
Primary fibroblast expansion on microcarriers in low-adhesion microwell plates
Low attachment 96-well plates (Costar®) were used for cell proliferation studies to prevent undesired cell attachment to the microwell surface. After sterilizing as above, microcarriers were equilibrated in 50 µL of pre-warmed medium. Fibroblasts in 50 µL of medium were then seeded onto microcarriers at a concentration of 1.5 × 104 cells/well and the plates were incubated at 37℃/5% CO2 for different time periods of up to 14 days. Medium was replaced every 48 h.
Cell staining procedures
Live cell staining was performed by incubating a confluent T-25 flask of fibroblasts with staining solution consisting of 10 µL Vybrant DiO labeling dye (Invitrogen) in 2 mL of normal growth medium at 37℃ for 20 min. Staining medium was removed and cells washed three times with PBS.
4′, 6-diamidino-2-phenylindole (DAPI) staining was performed after fixing cells with 4% paraformaldehyde (PFA) for 10 min at room temperature (RT) and washing with PBS. Cultures were stained with DAPI (working solution 1:1000) for 5 min at 37℃ and then rinsed with PBS.
For actin cytoskeleton labeling, cells were fixed with 4% PFA for 10 min at RT and then permeabilized with 0.5% Triton X-100 in PBS for 5 min at RT. Cultures were stained with Acti-stain™ 488 phalloidin (1:140 dilution in PBS) (Cytoskeleton, Inc.) for 30 min at RT in the dark. Counter-staining with propidium iodide (PI) was performed by fixing cells as above, then permeabilizing with 0.1% Triton X-100 in PBS for 5 min at RT. Cells were stained with PI (10 µg/mL; Sigma) for 5 min at RT in the dark.
PI and phalloidin labeling were visualized by confocal microsopy using a Radiance 2100 confocal microscope (BioRad, Loughborough, UK).
Cell proliferation assays
Cell proliferation was measured the Cell Counting Kit-8 (CCK-8; Sigma). Fibroblasts were seeded onto monolayers of bioactive glass microcarriers (15 mg/well) in 96-well low adhesion microwell plates at a concentration of 1.5 × 104 cells/well and incubated at 37℃. At different time points up to 14 days, optical density was measured at 450 nm in a multi-well Safire2 plate reader (Tecan). Cells cultured in 96-well tissue culture grade plates were used as a positive control due to their capacity to enhance cell proliferation. All samples were assayed in triplicate and for each experiment the mean ± standard deviation was calculated.
Scanning electron microscopy
For scanning electron microscopy (SEM), fibroblasts (3 × 104 cells/well) were seeded onto bioactive glass microcarriers (40 mg/well) in 24-well plate tissue culture inserts (Greiner Bio-One, Stonehouse, UK). After culture for 1, 3 or 7 days, cells on microcarriers were prepared SEM using established protocols.16 Briefly, cells were fixed (3% glutaraldehyde in 0.1 M cacodylate buffer for 24 h at 4℃), dehydrated, critical-point dried using hexamethyldisilazane, air-dried and then mounted onto aluminium pin stubs and coated with gold/palladium. Samples were examined with a JEOL 5410LV SEM (JEOL UK) operating at 10 kV.
Results
Microcarrier dissolution and ion release
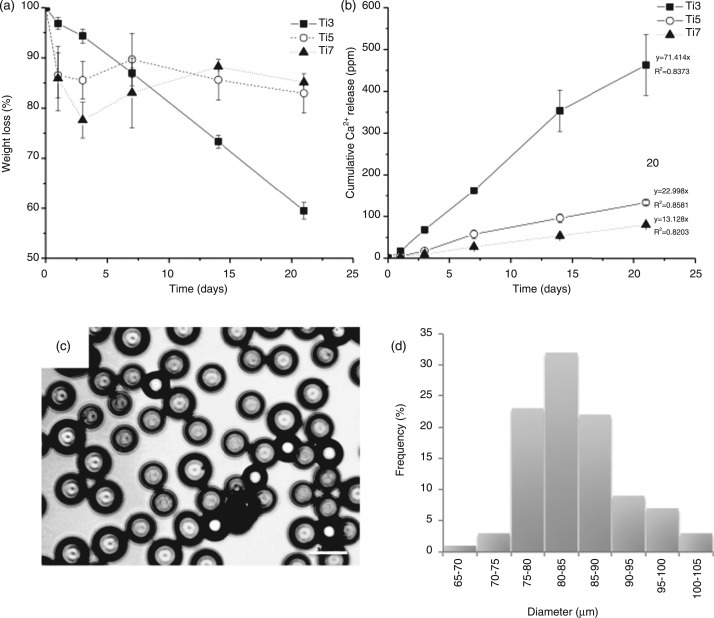
Microcarriers doped with three different concentrations of TiO2 were subjected to dissolution studies to determine their stability. Figure 1(A) shows the weight change over time for beads containing 3, 5 and 7 mol% TiO2. As expected, the glass with the lowest TiO2 content showed the highest degradation rate and is relatively linear with time. The glasses containing 5 and 7 mol% TiO2 however appeared to be more stable and showed very similar patterns of degradation behavior, with an initial drop in weight up to day 3 and overall losing around 15% of their total weight at 21 days. Ca2+ release data also confirmed this (Figure 1(B)), with the lowest TiO2 content glass releasing Ca2+ at a greater rate due to rapid degradation. The glasses with 5 and 7 mol% TiO2 showed relatively similar Ca2+ release profiles, with the glass containing 5 mol% TiO2 having a slightly higher Ca2+ release profile. All three glasses showed relatively linear Ca2+ release with time. The ionic release of other ionic species is shown in Table 2. The release rates were calculated from the respective graphs of ion release with time, whereby a straight line was fitted through the data, passing through the origin. All the other ions showed a similar release pattern to the Ca2+ (data not shown), differing only in the rates at which the ions were released. The Ti4+ was not measured due to the very low levels released, making the measurements unreliable. Due to the relatively similar stability of Ti5 and Ti7, we used Ti5 microcarriers for subsequent experiments using fibroblasts. The mean microcarrier diameter of the Ti5 batch was 84.2 + 19.4 µm and approximately 1450 microcarriers were required to cover the surface of one well of a 96-well microplate. Microscopic appearance and size distribution of a sample of 200 microcarriers are shown in Figure 1(C) and (D).
Figure 1.
(A) Microcarrier degradation and (B) associated Ca2+ release from Ti3, Ti5 and Ti7 microcarriers over time in deionized water. (C) Light micrograph of microscopic appearance and (D) size distribution histogram of Ti5 microcarriers used in subsequent experiments. Bar = 100 µm.
Table 2.
Release rate of ionic constituents from microcarriers.
| Glass code | Ti3 | Ti5 | Ti7 |
|---|---|---|---|
| Anion (ppm h−1) | |||
| 37.921 | 5.2563 | 3.6023 | |
| 46.864 | 13.304 | 7.4739 | |
| 15.084 | 3.2237 | 2.2045 | |
| 18.761 | 8.1531 | 5.5788 | |
| Cation (ppm h−1) | |||
| Ca2+ | 45.778 | 15.202 | 7.462 |
| Na+ | 15.571 | 1.9666 | 0.772 |
Microscopic characterization and fibroblastic cell affinity of TiO2-doped microcarriers
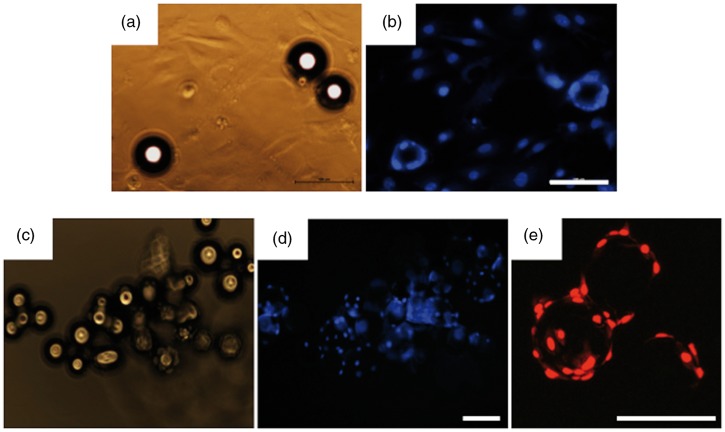
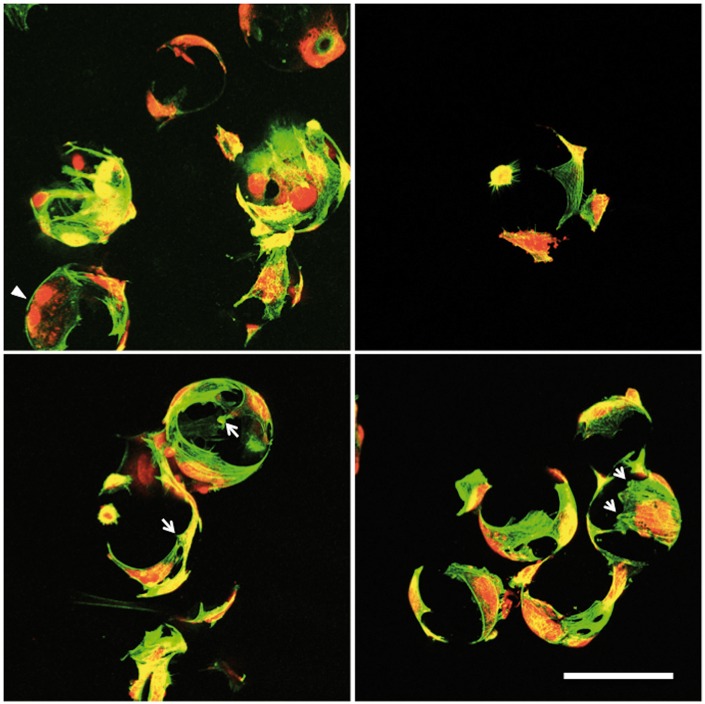
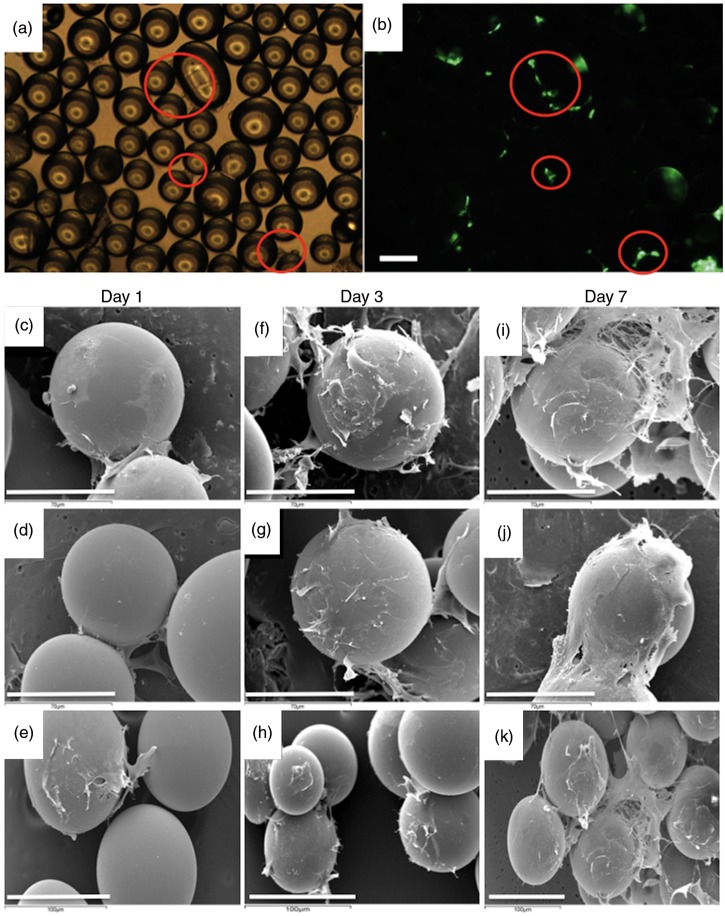
Examinations of cell attachment to 5 mol% TiO2 glass microcarriers was conducted initially in standard cell culture plates to determine whether cells can attach to the microcarrier surface or instead show preference for the highly permissive cell culture grade plastic surface. We observed that even though many fibroblasts were established on the tissue culture plastic by 24 h, fibroblasts could readily adhere to the microcarrier surfaces where they came into contact with them (Figure 2(A) and (B)). Having confirmed that the microcarriers are not inhibitory to cell growth when placed in competition with tissue culture plastic, fibroblasts were then seeded onto microcarriers in low-adhesion microwell plates and after 72 h all cells attached to the microcarriers and not the bottom of the microwell (Figure 2(C)–(E)). Morphology was assessed in greater detail on 5 mol% TiO2-doped microcarriers after 3 days of culture (Figure 3). Labeling of the actin cytoskeleton (green) revealed that fibroblasts wrap themselves around the microcarrier and prominent focal adhesion sites are evident (arrows). Cell nuclei staining also revealed cells actively dividing on the microcarrier surface (arrowheads).
Figure 2.
(A) Phase-contrast and (B) DAPI staining of fibroblasts seeded onto standard tissue culture plastic dispersed with microcarriers after 24 h. (C–D) Adhesion of fibroblasts to microcarriers in ultra-low attachment microwell plates 72 h after seeding. (E) Clear cell adhesion and growth on microcarriers is evident with propidium iodide (PI) staining at high power after 72 h. Bar = 100 µm.
Figure 3.
Morphology of fibroblasts cultured on 5 mol% TiO2-doped microcarriers. Green = phalloidin f-actin; Red = propidium iodide; arrows = focal adhesions; arrowhead = dividing cell. Bar = 100 µm.
Fibroblast proliferation capacity on microcarrier surfaces
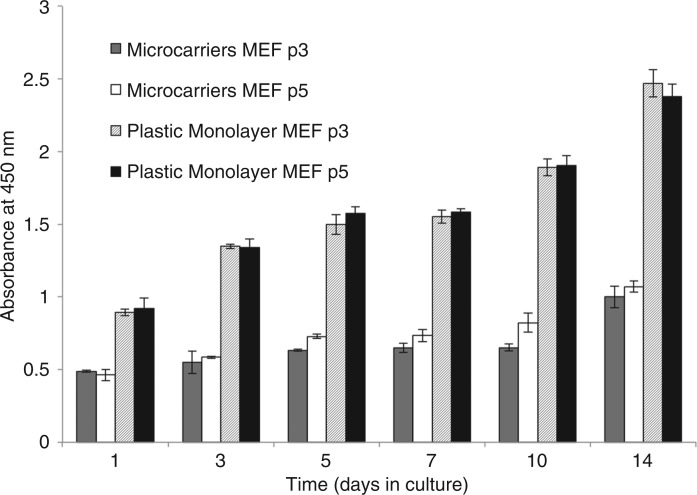
Proliferation of primary fibroblasts on TiO2-doped microcarriers in low-adhesion microwell plates was assayed under static conditions and compared to routine cell culture-grade microwell plates that provide optimal substrate properties to enhance cell expansion. The CCK proliferation assay revealed that a steady, sustained expansion of the population was achievable over 14 days of culture for 2 different passages of cells tested, culminating in a 2-fold increase in the population size after 14 days (p < 0.01) (Figure 4). However, proliferation was only 50% of that in routine cell culture grade microwell plates.
Figure 4.
Proliferation of fibroblasts cultured on 5 mol% TiO2-doped microcarriers and on standard tissue culture plastic. Values = mean ± standard deviation.
Interconnectivity and network forming capacity of fibroblast-microcarrier cultures
Fibroblasts are able not only to adhere to microcarriers but also readily form 3D networks in static culture as soon as 24 h after seeding by bridging across to adjacent microcarriers and to cells located on adjacent microcarriers (Figure 5(A) and (B)). SEM reveals this to an even greater extent over a period of 7 days culture and the extensive network of cell processes between microcarriers reveals that a high degree of biocompatibility is evident (Figure 5(C)–(K)) and that the combined fibroblast-microcarrier system was capable of forming a large interconnected 3D network.
Figure 5.
(A) Phase contrast and (B) fluorescent images of fibroblasts labelled with Vibrant DiO after 24 h. (C–K) Scanning electron microscopy (SEM) images showing morphology of fibroblasts over time. Bar A, B, E, H, K = 100 µm; C, D, F, G, I, J = 70 µm.
Discussion
The degradation rate of phosphate glasses, and consequently their biocompatibility, is mainly dependent on the glass composition; therefore, suitable modifications to the glass chemistry are required in order to obtain stable substrates that degrade slowly and exhibit high biocompatibility. In the current study, microcarriers were doped with several different concentrations of TiO2 and while 3 mol% microcarriers were found to degrade too rapidly to be useful for either scalable cell expansion or clinical delivery, the 5 and 7 mol% compositions were structurally stable over the time period examined. There was evidence of more rapid initial degradation in the 5 and 7 mol% glass and this may be due to the rate at which Na+ is initially released, which may come out of these glasses more quickly. However, the rest of the structure is relatively more stable in solution, hence the reduction in weight loss. The 5 mol% TiO2-doped microcarriers were used for subsequent cytocompatibility work using primary fibroblasts and it was found that cell adhesion and proliferation was supported on the microcarriers. This is attributable to the known biocompatibility of TiO2. 17 Furthermore, after a static culture period of 7 days, cells formed a large amount of bridging between adjacent microcarriers. However, for scalable expansion processes, dynamic bioreactor culture would be employed to ensure homogeneous dispersion of microcarriers and efficient mixing of nutrients. Significant cell bridging between microcarriers after 7 days of culture raises the possibility that they would be suitable for forming dynamic tissue-engineered structures after an initial period of dynamic culture.
A dynamic approach to scalable cell expansion amenable to tissue engineering by producing tissue-engineered microunits would be highly beneficial for tissue regeneration purposes. However, our aim in the current study was to demonstrate the biocompatibility of TiO2-doped glass microspheres and feasibility of the surface to serve as a suitable surface for cell adhesion and growth. While cell growth was not as rapid as on commercially available tissue culture plastic designed and optimised to enhance cell growth, surface modifications to the TiO2-doped microcarriers should overcome this concern. Cell culture grade plastic is surface-modified to enhance cell growth and subsequent improvements to the microcarriers such as biofunctionalization of the surface may be a suitable strategy to enhance cell growth. Biofunctionalization methods, such as surface coating with ECM molecules or short peptides that can mimic extracellular or growth factor motifs,18 have been successful for polymer-based scaffolds of low intrinsic biocompatibility.
Traditional tissue engineering strategies employ a “top-down” approach, in which cells are seeded on a biodegradable scaffold. Using this approach, the cells are expected to populate the scaffold and create the appropriate ECM and microarchitecture. However, it is very difficult to replicate tissue microarchitecture using a top-down approach even in spite of the wealth of advanced surface biofunctionalization methods and native decellularized scaffolds available. In a “bottom-up” approach, modular tissue engineering aims to address the challenge of recreating biomimetic structures by designing structural features on the micron scale to build modular tissues that can be used as building blocks to create larger tissues.19 The use of TiO2-doped microcarriers for modular tissue engineering is particularly promising for bone regeneration strategies as titanium is widely used for orthopaedic and dental procedures, supporting osteogenesis even when a disease such as diabetes dampens the intrinsic regeneration response.20–22
Compared to pre-fabricated bulk scaffolds, microcarriers offer a range of unique features: (a) expansion capacity that can be scaled according to the individual patient requirements such as size of defect; (b) the possibility of easy and quick injection without larger surgical procedures23; (c) enhanced versatility in fitting the unique, site-specific shape of a defect as compared to a pre-fabricated bulk scaffold and (d) homogeneous distribution of cells throughout an agglomerated construct of cell-loaded microcarriers. These features make microcarriers a promising alternative to bulk scaffolds.
Conclusions
TiO2-doped bioactive glass microcarriers offer great potential as a scalable expansion tool for the cell therapy industry and may also serve a dual purpose by doubling as a dynamic method for tissue engineering. Surface modifications for further biofunctionalization could yield microcarriers that are valuable tools for cell expansion and tissue engineering.
Funding
This work was part supported by WCU Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. R31-10069).
References
- 1. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926 [DOI] [PubMed] [Google Scholar]
- 2. Banfi A, Bianchi G, Notaro R, et al. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng 2002; 8: 901–910 [DOI] [PubMed] [Google Scholar]
- 3. Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev 2005; 24: 425–439 [DOI] [PubMed] [Google Scholar]
- 4. Eibes G, dos Santos F, Andrade PZ, et al. Maximizing the ex vivo expansion of human mesenchymal stem cells using a microcarrier-based stirred culture system. J Biotechnol 2010; 146: 194–197 [DOI] [PubMed] [Google Scholar]
- 5. Santos F, Andrade PZ, Abecasis MM, et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods 2011; 17: 1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol 2001; 281: F12–F25 [DOI] [PubMed] [Google Scholar]
- 7. Kokubo T, Kim HM, Kawashita M, et al. Bioactive metals: preparation and properties. J Mater Sci Mater Med 2004; 15: 99–107 [DOI] [PubMed] [Google Scholar]
- 8. Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006; 27: 2907–2915 [DOI] [PubMed] [Google Scholar]
- 9. Ahmed I, Collins CA, Lewis MP, et al. Processing, characterisation and biocompatibility of iron-phosphate glass fibres for tissue engineering. Biomaterials 2004; 25: 3223–3232 [DOI] [PubMed] [Google Scholar]
- 10. Bitar M, Salih V, Mudera V, et al. Soluble phosphate glasses: in vitro studies using human cells of hard and soft tissue origin. Biomaterials 2004; 25: 2283–2292 [DOI] [PubMed] [Google Scholar]
- 11. Neel EA, Ahmed I, Pratten J, et al. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005; 26: 2247–2254 [DOI] [PubMed] [Google Scholar]
- 12. Lakhkar NJ, Park JH, Mordan NJ, et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater 2012, http://dx.doi.org/10.1016/j.actbio.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 13. Abou Neel EA, Mizoguchi T, Ito M, et al. In vitro bioactivity and gene expression by cells cultured on titanium dioxide doped phosphate-based glasses. Biomaterials 2007; 28: 2967–2977 [DOI] [PubMed] [Google Scholar]
- 14. Abou Neel EA, Chrzanowski W, Knowles JC. Effect of increasing titanium dioxide content on bulk and surface properties of phosphate-based glasses. Acta Biomater 2008; 4: 523–534 [DOI] [PubMed] [Google Scholar]
- 15. Wall IB, Moseley R, Baird DM, et al. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J Invest Dermatol 2008; 128: 2526–2540 [DOI] [PubMed] [Google Scholar]
- 16. Ahmadi R, Mordan N, Forbes A, et al. Enhanced attachment, growth and migration of smooth muscle cells on microcarriers produced using thermally induced phase separation. Acta Biomater 2011; 7: 1542–1549 [DOI] [PubMed] [Google Scholar]
- 17. Lakhkar NJ, Lee IH, Kim HW, et al. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv Drug Deliv Rev 2012, http://dx.doi.org/10.1016/j.addr.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 18. Yun YR, Jang JH, Jeon E, et al. Administration of growth factors for bone regeneration. Regen Med 2012; 7: 369–385 [DOI] [PubMed] [Google Scholar]
- 19. Nichol JW, Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 2009; 5: 1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donos N, Retzepi M, Wall I, et al. In vivo gene expression profile of guided bone regeneration associated with a microrough titanium surface. Clin Oral Implants Res 2011; 22: 390–398 [DOI] [PubMed] [Google Scholar]
- 21. Ivanovski S, Hamlet S, Retzepi M, et al. Transcriptional profiling of "guided bone regeneration" in a critical-size calvarial defect. Clin Oral Implants Res 2011; 22: 382–389 [DOI] [PubMed] [Google Scholar]
- 22. Retzepi M, Lewis MP, Donos N. Effect of diabetes and metabolic control on de novo bone formation following guided bone regeneration. Clin Oral Implants Res 2010; 21: 71–79 [DOI] [PubMed] [Google Scholar]
- 23. Senuma Y, Franceschin S, Hilborn JG, et al. Bioresorbable microspheres by spinning disk atomization as injectable cell carrier: from preparation to in vitro evaluation. Biomaterials 2000; 21: 1135–1144 [DOI] [PubMed] [Google Scholar]