Abstract
The angiotensin-converting enzyme gene is a candidate gene of stroke. The present study involved 62 healthy volunteers and 148 patients with middle cerebral artery stenosis as confirmed by brain color ultrasound from a Han population in North China, and determined the peripheral blood angiotensin-converting enzyme genotype using PCR-restriction fragment length polymorphism analysis. The results showed that the frequencies of the DD genotype and D allele were increased in patients with middle cerebral artery stenosis, but the difference was not statistically significant compared with healthy controls. The findings of this study on the relationship between stroke genes and middle cerebral artery stenosis indicate no significant correlation between the frequencies of the DD genotype and D allele of angiotensin-converting enzyme and middle cerebral artery stenosis in this Han population from North China. In the future, studies will be carried out to investigate correlations between multiple stroke candidate gene synergy and middle cerebral artery stenosis to provide a foundation for the development of gene therapy.
Keywords: neural regeneration, brain injury, stroke, angiotensin-converting enzyme, gene, polymorphism, middle cerebral artery, angiostenosis, North China, Han population, neuroregeneration
Research Highlights
(1) PCR-restriction fragment length polymorphism analysis was used to determine angiotensin-converting enzyme genotypes in normal volunteers and patients with middle cerebral artery stenosis as confirmed by brain color ultrasound in a Han population from North China.
(2) The results indicate no significant correlation between the frequencies of the DD genotype and D allele of angiotensin-converting enzyme and middle cerebral artery stenosis in the Han population from North China.
INTRODUCTION
The renin-angiotensin system is associated with cardiovascular system regulation, water-salt balance, and maintenance of internal environmental homeostasis[1], and plays an important role in ischemic cerebrovascular disease[2]. The renin-angiotensin system mainly includes prehypertension and angiotensin-converting enzyme[3,4]. This system plays a critical role in the development of hypertension and arteriosclerosis, and angiotensin-converting enzyme participates in vascular remodeling and the development of atherosclerosis[5,6,7,8]. The angiotensin-converting enzyme gene comprises 26 exons and 25 introns[9] localized at the long arm of chromosome 17 (17q23), and intron 16 has or deletes a 287-bp Alu repetitive sequence, presenting insertion/deletion (I/D) polymorphism[10]. Deletion, but not insertion, is the cause of angiotensin-converting enzyme gene polymorphism[10]. Recent evidence indicates that the angiotensin-converting enzyme gene is a main candidate gene for hereditary susceptibility to various cardio-cerebrovascular diseases[11]. Intracranial atherosclerotic stenosis accounts for 8% to 10% of ischemic cerebrovascular disease and is higher in Asian, African, and Spanish individuals[12]. Approximately 10% of cases of ischemic stroke and 8% of cases of transient ischemic attack are caused by intracranial arteriostenosis[13], and the incidence of middle cerebral artery stenosis is the highest[14]. One study demonstrated that a high plasma concentration of angiotensin-converting enzyme can induce vessel wall thickening and vasculopathy and that the angiotensin-converting enzyme gene participates in the progression of vessel wall atherosclerosis and induces atherosclerotic cerebral infarction[15]. To date, there are few studies regarding the correlation between the angiotensin-converting enzyme gene and intracranial arteriostenosis, such as middle cerebral artery stenosis. No study has been conducted to investigate the correlation between the angiotensin-converting enzyme gene polymorphism and middle cerebral artery stenosis in the Han population of North China. Thus, the present study compared the angiotensin-converting enzyme gene in Han patients from North China with middle cerebral artery stenosis with that in normal controls to analyze whether there is a correlation between angiotensin-converting enzyme gene polymorphism and middle cerebral artery stenosis.
RESULTS
Quantitative analysis of participants
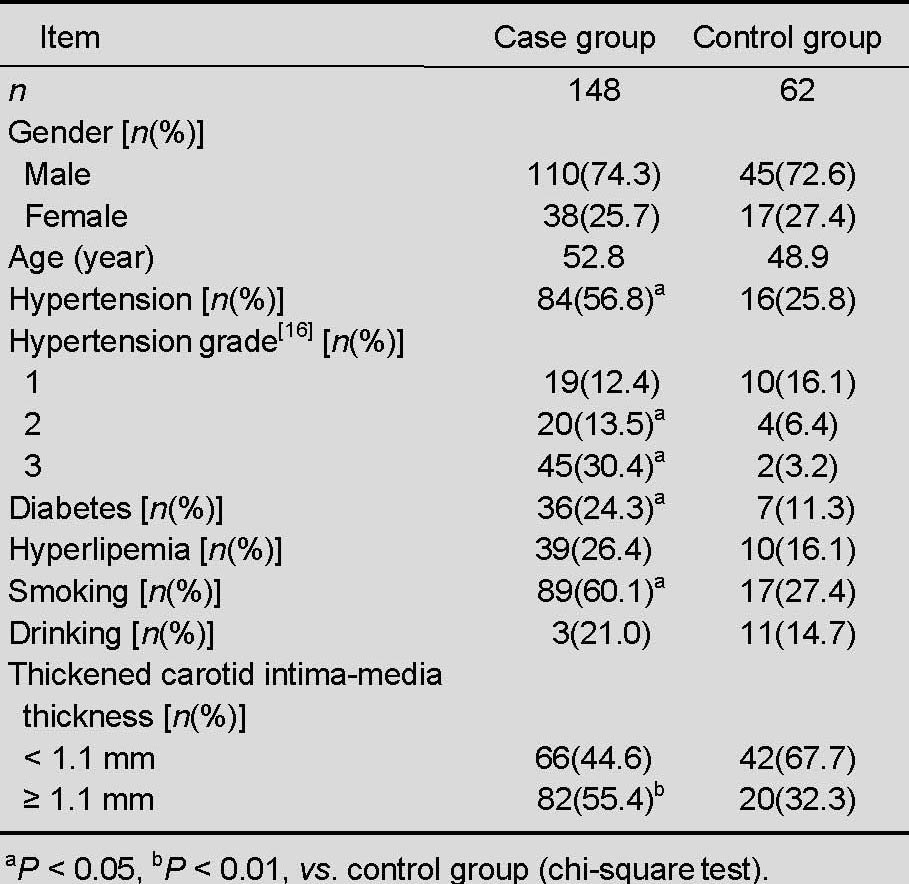
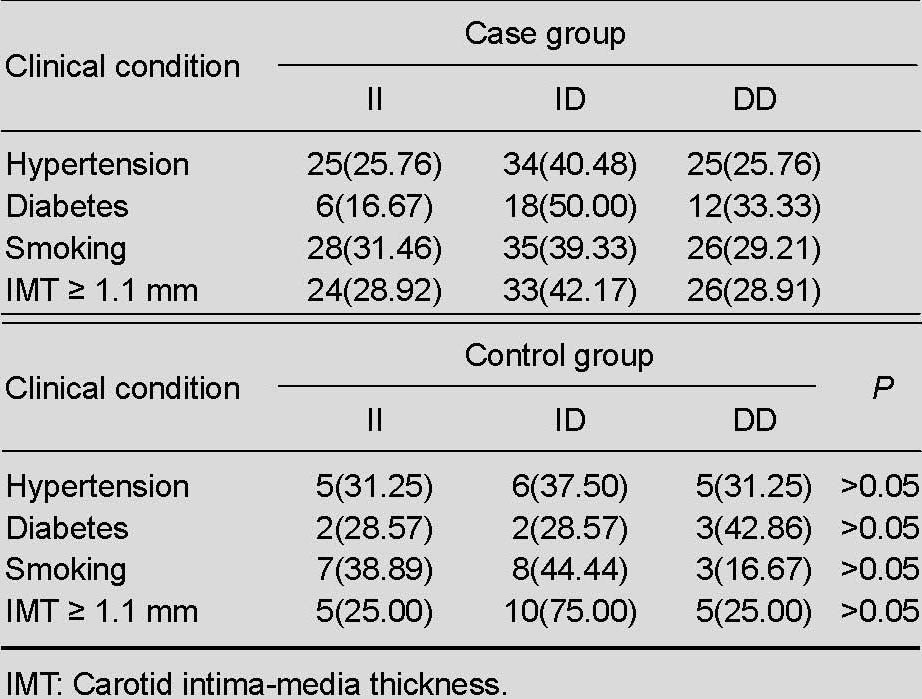
One hundred and forty-eight patients with middle cerebral artery stenosis from North China, as confirmed by brain color ultrasound, were selected as the case group, and 62 healthy volunteers with normal brain color ultrasound results were selected as controls. No participant withdrew from the study. There were no significant differences between the two groups in terms of mean age and gender. The number of patients with a history of hypertension, diabetes, smoking, and increased carotid intima-media thickness (≥1.1 mm) was significantly greater in the case group than in the control group (P < 0.05), but patients with a history of hyperlipidemia and drinking were similar between the two groups (P > 0.05; Table 1).
Table 1.
Clinical data of patients with middle cerebral artery stenosis and normal controls
Angiotensin-converting enzyme gene polymorphism in patients with middle cerebral artery stenosis
The angiotensin-converting enzyme gene is associated with three genotypes due to Alu insertion/deletion, insertion (II), deletion (DD), and heterozygote (ID)[9]. The angiotensin-converting enzyme gene electrophoresis results are shown in Figure 1.
Figure 1.
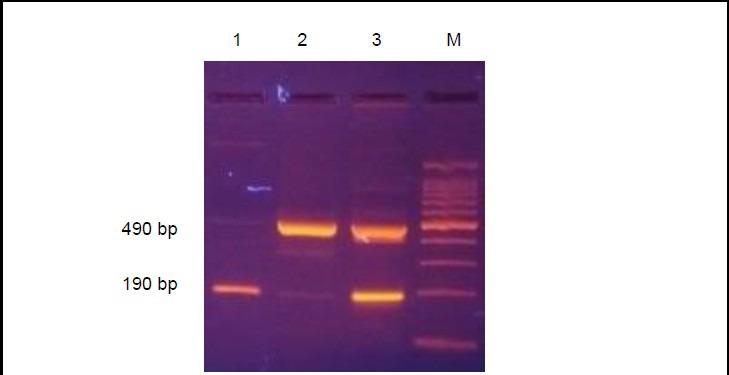
Three genotypes of angiotensin-converting enzyme gene due to Alu insertion/deletion
M: Molecular weight standard; 1: DD genotype, 190 bp, homozygous deletion; 2: 490 bp, homozygous insertion; 3: ID genotype, 490 and 190 bp, heterozygous.
The frequencies of the DD genotype and D allele were increased in patients with middle cerebral artery stenosis, but the difference was not statistically significant compared with healthy controls (P > 0.05). There was no significant difference in the frequencies of genotypes and alleles between patients with a history of hypertension, diabetes, smoking, and increased carotid intima-media thickness (P > 0.05; Tables 2–4).
Table 2.
Angiotensin-converting enzyme genotype and allele distribution [n (%)] in patients with middle cerebral artery stenosis and normal controls
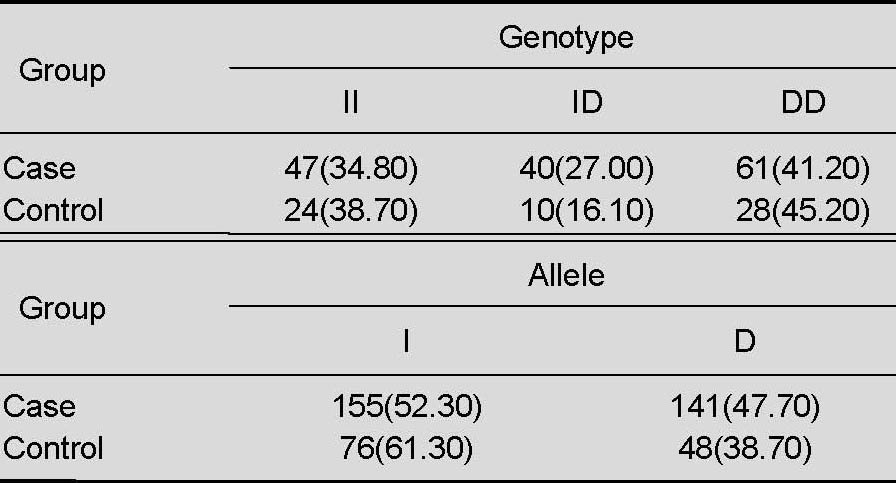
Table 4.
Angiotensin-converting enzyme allele frequency distribution [n (%)] in patients with middle cerebral artery stenosis and normal controls
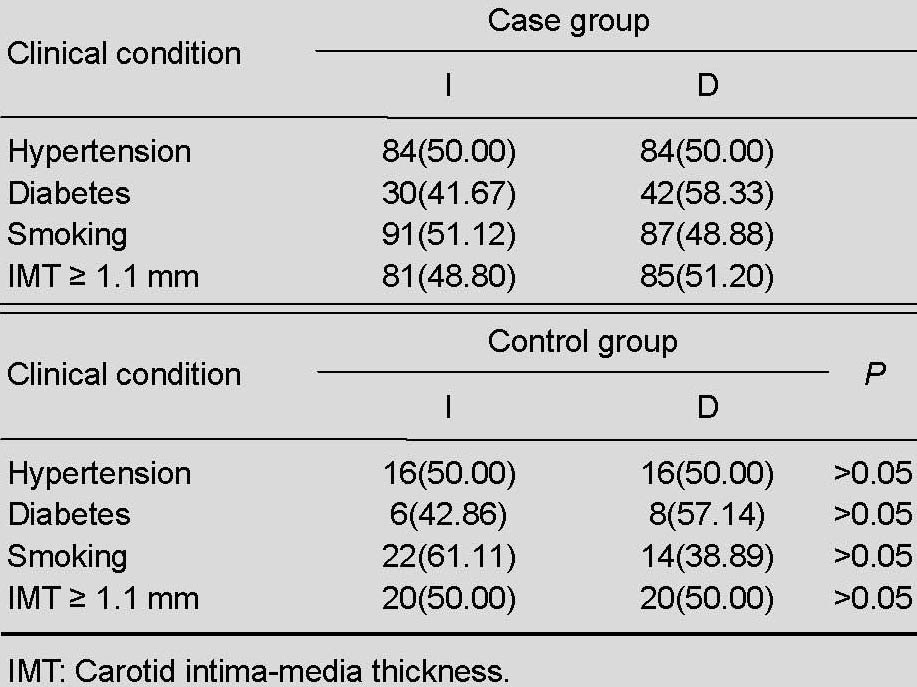
Table 3.
Frequency of angiotensin-converting enzyme genotype [n (%)] in patients with middle cerebral artery stenosis and normal controls
Risk factors of middle cerebral artery stenosis
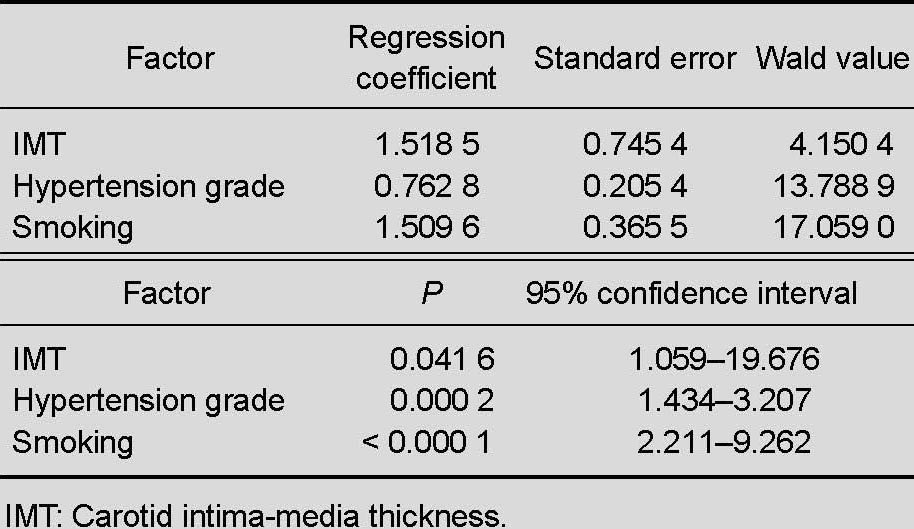
Multiple-factor logistic regression analysis including angiotensin-converting enzyme genotype and allele evaluation showed that the angiotensin-converting enzyme genotype and allele were not risk factors for middle cerebral artery stenosis, further confirming that hypertension, smoking, and increased carotid intima-media thickness are independent risk factors for middle cerebral artery stenosis (independent-variable quantization standard in Table 5, results in Table 6).
Table 5.
Quantization standard of risk factors for middle cerebral artery stenosis
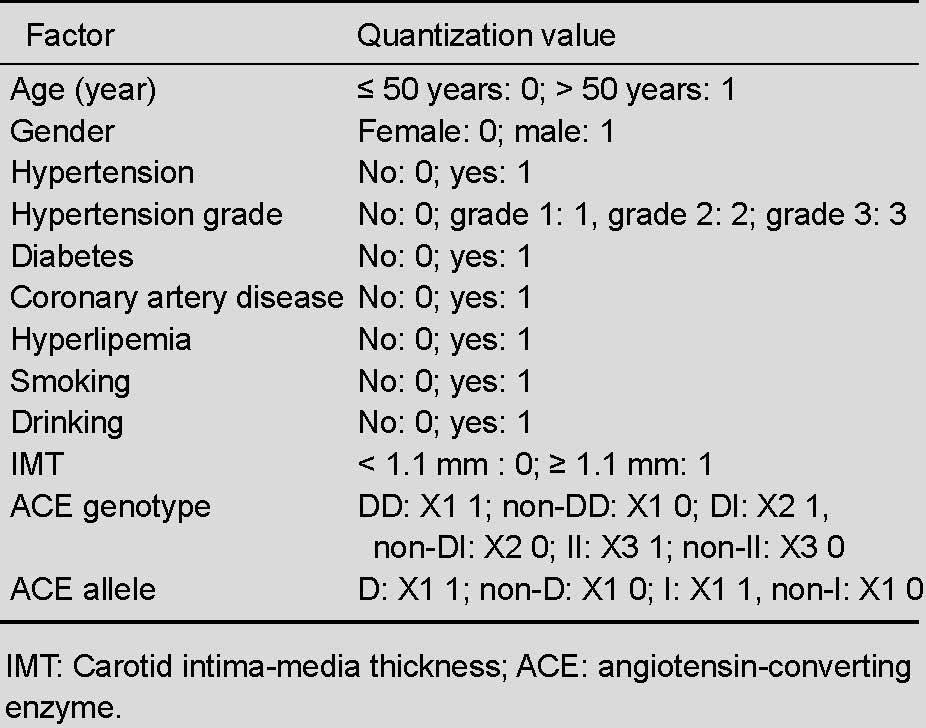
Table 6.
Multivariate logistic regression analysis of patients with middle cerebral artery stenosis
DISCUSSION
Increasing numbers of studies have demonstrated that the distribution of vessel damage differs among various ethnicities. For example, intracranial large artery stenosis is the main cause of ischemic cerebrovascular disease, especially middle cerebral artery stenosis[17,18]. Poor compensation of other vessels or absence of effective establishment of collateral circulation following middle cerebral artery stenosis may lead to cerebral vessel ischemic events. Kern et al[19] reported that symptomatic middle cerebral artery stenosis is an independent factor for entire and ipsilateral cerebral vessel events (entire, P < 0.01; ipsilateral, P = 0.02), and the recurrence rate of cerebral ischemia was higher in patients with symptomatic middle cerebral artery stenosis compared with those with asymptomatic stenosis, and was even higher than the incidence of extracranial internal carotid artery stenosis. Studies of the correlation between the condition of the middle cerebral artery and mortality and the recurrence rate of ischemic cerebrovascular disease indicate that middle cerebral artery occlusion has the highest risk of death and relapse (21.4%), followed by middle cerebral artery stenosis (16.6%) and a normal middle cerebral artery (12.2%)[13]. In addition, the preclinical phase of arterial stenosis is long prior to clinical symptoms of stroke[20]. Therefore, it is clinically important to investigate risk factors for middle cerebral artery stenosis. With developments in molecular biology, growing evidence has indicated that gene polymorphism plays a role in susceptibility to cerebrovascular disease, especially ischemic cerebrovascular disease. The human angiotensin-converting enzyme gene has aroused the most attention as a candidate stroke gene. In 1992, Cambin et al[21] first reported that I/D polymorphism is a potential risk factor for myocardial infarction. Subsequent studies suggested that this polymorphic site could increase the risk of cerebrovascular disease[22,23]. Studies of patients with symptomatic cerebral infarction and normal controls showed that the D/I allele frequency (0.59/0.41) in the patients was significantly different from that in the controls (0.48/0.52) and that the DD genotype was significantly greater than that in the controls, who showed a relative risk of 1.98 for cerebrovascular disease. These results indicate that the risk of cerebrovascular disease with the DD genotype is higher than that with the II and ID genotypes[24].
In the present study, the frequency of the I and D alleles in the control group was 61.30% and 38.70%, respectively, indicating that the percentage of the I allele was relatively higher, similar to previous results of the angiotensin-converting enzyme gene polymorphism distribution in a normal Han population[25]. However, the D allele frequency was relatively higher in Europeans, and the I/D frequency was about 0.44/0.55[26,27,28]. These results indicate significant race differences in the frequency of the I and D alleles in normal people. Results of the present study indicate no obvious correlation between I/D polymorphism and middle cerebral artery stenosis, consistent with previous results[29]. However, there was statistical significance between the case and control groups in terms of history of hypertension, diabetes, smoking, and increased carotid intima-media thickness. Multivariate logistic regression analysis further demonstrated that angiotensin-converting enzyme gene I/D polymorphism may not be an independent risk factor for middle cerebral artery stenosis and that a history of hypertension, diabetes, smoking, and increased carotid intima-media thickness play important roles in the development of middle cerebral artery stenosis. In addition, carotid intima-media thickness is a reliable index for early atherosclerosis detection[30]. Thus, carotid intima-media thickness of ≥ 1.1 mm can be used as a marker of middle cerebral artery stenosis.
The association between angiotensin-converting enzyme polymorphism and ischemic cerebrovascular disease remains controversial. This may result from: (1) different study methods: some retrospective studies of a case meta-analysis indicated a significant correlation between the angiotensin-converting enzyme gene D allele and ischemic cerebrovascular disease, but no correlation was found in prospective paired studies; (2) different case selection conditions: for example, the patients with cerebral infarction included those with lacunar infarction, large-sized infarction, or carotid artery blood supply region infarction, which led to different results; (3) sample sizes: investigations of gene interactions and environments require large sample sizes, and insufficient samples may result in different and even contrary results; (4) distribution of angiotensin-converting enzyme gene polymorphism: this is influenced by ethnic differences, and environmental and geographical variations. For example, the frequency of the D allele of the angiotensin-converting enzyme gene is significantly higher in Europeans than in Asians. No large-sample statistical data are available regarding the gene distributions in related populations. The number of cases in the present study was small, and the results could be regarded as a predication of one tendency; however, larger samples are needed for further validation.
A large number of epidemiologic studies and animal experiments have demonstrated that most ischemic cerebrovascular disease is controlled by genetic factors[31,32]. Synergistic effects of assortments of some genes on ischemic cerebrovascular disease have been found in addition to correlations between candidate gene polymorphism and ischemic cerebrovascular disease. Angiotensin-converting enzyme gene polymorphisms have synergistic effects with some other genes, such as the prehypertension[33,34] gene, methylene tetrahydrofolate reductase gene[35,36], apolipoprotein E gene[37,38], and 4G/5G polymorphism of the plasminogen activator inhibitor-1[39] during induction of ischemic cerebrovascular disease. Different genes have different clinical phenotypes. The risk for disease is increased when many predisposing genes function at the same time.
Studies of gene factors can further elucidate the pathogenesis of middle cerebral artery stenosis-induced ischemic cerebrovascular disease and screen susceptible populations, which can allow for primary and secondary prevention and be used in treatment. Studies have been conducted to investigate gene expression after middle cerebral artery occlusion in humans[40], regulation of gene expression in a rat model of brief middle cerebral artery occlusion[41], and accommodation of signal transducer and transcription factor activation pathways during regional cerebral ischemia using gene expression microarray[42,43].
In conclusion, angiotensin-converting enzyme gene I/D polymorphisms are not an independent risk factor for middle cerebral artery stenosis in the investigated Han population from North China. The present results further demonstrate that a history of hypertension, diabetes, smoking, and increased carotid intima-media thickness are risk factors for middle cerebral artery stenosis, indicating that primary and secondary prevention is important for cerebral vessel stenosis or cerebrovascular disease. Future studies on the association of angiotensin-converting enzyme gene polymorphisms with intracranial main vessel stenosis should focus on sample size, ethnicities of participants, and the synergistic effect of multiple stroke candidate genes to further elucidate the pathogenesis of stroke gene polymorphisms and intracranial main vessel stenosis-induced ischemic cerebrovascular disease, and to provide target sites for gene techniques in the treatment of ischemic cerebrovascular disease. With the development of life science and technology, research on the analysis and detection of disease-related genes has advanced to clinical levels and provides a scientific basis for diagnosis and differential diagnosis of diseases. Genetic testing is a means to confirm monogenic disease. For multigenic disease, gene mutation analysis helps to identify the etiology. The present study aimed to elucidate the correlation between stroke genes and middle cerebral artery stenosis to provide a reference for studies on the correlation between genetic synergy and middle cerebral artery stenosis.
SUBJECTS AND METHODS
Design
A case-control study of gene polymorphism.
Time and setting
Subject screening, clinical data collection, and blood sampling were performed in the Department of Neurology, Jilin University First Hospital, and DNA extraction, PCR amplification, and electrophoresis were conducted in the Laboratory of Biochemistry, Jilin University Basic Medicine College, China from January 2006 to April 2008.
Subjects
Participants were enrolled from the Out-patient Clinic and Admission Department, First Hospital of Jilin University from January 2006 to April 2008. Middle cerebral artery stenosis was diagnosed if the peak systolic velocity (Vs) was ≥ 160 cm/s or the mean blood flow rate (Vm) was ≥ 120 cm/s; if low-frequency components were increased; in the presence of a vortex and vascular murmur; and with reduction of blood flow in distal and proximal stenosis[44] as confirmed by a transcranial and peripheral vessel Doppler/monitor system (SciMed Ltd., Bristol, UK). The degree of vessel stenosis was classified as follows[45]: mild stenosis, 160 ≤ Vs ≤ 180 cm/s; moderate stenosis, 180 < Vs ≤ 220 cm/s; and severe stenosis, Vs > 220 cm/s.
Inclusion criteria for the (1) case group (asymptomatic Han population in North China) were the presence of middle cerebral artery stenosis as confirmed by transcranial Doppler ultrasound through a temporal window for evaluation of the blood flow of the bilateral middle cerebral artery; complete conduction of the questionnaire, genotyping, and biochemical detection; and acquisition of informed consent from patients and their families. Inclusion criteria for the (2) normal control group (healthy Han population in North China) were the absence of middle cerebral artery or other vessel stenosis as confirmed by transcranial Doppler ultrasound through a temporal window for evaluation of the blood flow of the bilateral middle cerebral artery; complete conduction of the questionnaire, genotyping, and biochemical detection; and acquisition of informed consent from patients and their families. Exclusion criteria were trauma, tumor, or other non-gene factors. Finally, 148 patients with middle cerebral artery stenosis and 62 individuals with no abnormalities as confirmed by transcranial Doppler ultrasound were included. The study was performed in accordance with the Declaration of Helsinki.
Methods
Collection of case histories
Case histories were inquired in detail, especially the risk factors of cerebrovascular disease, including hypertension, diabetes, coronary artery disease, hyperlipidemia, smoking, and drinking. In addition, patient age, gender, symptoms, and transcranial Doppler ultrasound results were recorded.
Carotid artery ultrasonography
Carotid artery ultrasonography was conducted to record intima thickness and carotid artery plaque formation.
PCR-restriction fragment length polymorphism analysis for angiotensin-converting enzyme gene polymorphism
After addition of ethylenediaminetetraacetic acid, 2 to 3 mL of blood was harvested from the antecubital vein and stored at –20 °C. DNA was extracted using a guanidinium isothiocyanate method and stored at –70°C. The angiotensin-converting enzyme forward primer was 5’-CTG GAG ACC ACT CCC CAT CCT TTC T-3’, and the reverse primer was 5’-GAT GTG GCC ATC ACA TTC GTC AGA T-3’. PCR was performed in a 50-μL solution containing 5 μL of 10 x PCR buffer, 4 μL of dNTP, 1 μL each of the forward and reverse primer, 0.25 μL of ExTaq enzyme (TaKaRa, Dalian, Liaoning Province, China), 3 to 5 μL of template, and sterilized double-distilled water. PCR was conducted for 35 cycles with predenaturation at 94°C for 3 minutes, denaturation at 94°C for 30 seconds, annealing at 56°C for 2 minutes, and extension at 72°C for 1 minute. Another extension at 72°C was performed for 10 minutes. Sterile distilled water replacing the template was used as a negative control. The appropriate amount of PCR products were mixed with corresponding amounts of 6 x loading buffer and electrophoresed in 3% agarose gel at 100 V for 20 minutes. Electrophoresis results were observed under an ultraviolet lamp. PCR product purification and DNA sequencing were performed by Dalian Takara, China.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software (SPSS, Chicago, IL, USA). Count data were analyzed using the chi-square test. Multivariate logistic regression analysis of risk factors was performed. The logistic regression model used middle cerebral artery stenosis as the dependent variable and gender, age, history of hypertension, hypertension grade, diabetes, coronary artery disease, hyperlipidemia, carotid intima-media thickness, smoking, drinking, angiotensin-converting enzyme genotype, and allele frequency as independent variables.
Acknowledgments:
We thank Guirong Zhang, Laboratory of Biochemistry, Jilin University Basic Medicine College in China for technical support. We also thank the other staff members at the Department of Neurology, Jilin University, First Hospital in China for their help.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Medical Ethics Committee of First Hospital, Jilin University, China.
(Reviewed by Morben A, Wysong S, Wang YL, Zou LY)
(Edited by Yu J, Su LL, Li CH, Song LP)
REFERENCES
- [1].Allen AM, Moeller I, Jenkins TA, et al. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47(1):17–28. doi: 10.1016/s0361-9230(98)00039-2. [DOI] [PubMed] [Google Scholar]
- [2].Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst. 2006;7(1):3–14. doi: 10.3317/jraas.2006.003. [DOI] [PubMed] [Google Scholar]
- [3].Woods DR, Pollard AJ, Collier DJ, et al. Insertion/deletion polymorphism of the angiotensin I-converting enzyme gene and arterial oxygen saturation at high altitude. Am J Respir Crit Care Med. 2002;166(3):362–366. doi: 10.1164/rccm.2103060. [DOI] [PubMed] [Google Scholar]
- [4].Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol. 2003;35(6):855–866. doi: 10.1016/s1357-2725(02)00342-4. [DOI] [PubMed] [Google Scholar]
- [5].Jiang X, Sheng H, Li J, et al. Association between renin-angiotensin system gene polymorphism and essential hypertension: a community-based study. J Hum Hypertens. 2009;23(3):176–181. doi: 10.1038/jhh.2008.123. [DOI] [PubMed] [Google Scholar]
- [6].Morishita R, Gibbons GH, Ellison KE, et al. Evidence for direct local effect of angiotensin in vascular hypertrophy. In vivo gene transfer of angiotensin converting enzyme. J Clin Invest. 1994;94(3):978–984. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharma P. Meta-analysis of the ACE gene in ischaemic stroke. J Neurol Neurosurg Psychiatry. 1998;64(2):227–230. doi: 10.1136/jnnp.64.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lucarini L, Sticchi E, Sofi F, et al. ACE and TGFBR1 genes interact in influencing the susceptibility to abdominal aortic aneurysm. Atherosclerosis. 2009;202(1):205–210. doi: 10.1016/j.atherosclerosis.2008.04.038. [DOI] [PubMed] [Google Scholar]
- [9].Hubert C, Houot AM, Corvol P, et al. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991;266(23):15377–15383. [PubMed] [Google Scholar]
- [10].Rigat B, Hubert C, Corvol P, et al. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20(6):1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Catto A, Carter AM, Barrett JH, et al. Angiotensin-converting enzyme insertion/deletion polymorphism and cerebrovascular disease. Stroke. 1996;27(3):435–440. [PubMed] [Google Scholar]
- [12].Higashida RT, Meyers PM, Connors JJ, 3rd, et al. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. J Vasc Interv Radiol. 2009;20(7 Suppl):S312–316. doi: 10.1016/j.jvir.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [13].Wong KS, Li H, Lam WW, et al. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke. 2002;33(2):532–536. doi: 10.1161/hs0202.102602. [DOI] [PubMed] [Google Scholar]
- [14].Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000;31(11):2641–2647. doi: 10.1161/01.str.31.11.2641. [DOI] [PubMed] [Google Scholar]
- [15].Companioni Nápoles O, Sautié Castellanos M, Leal L, et al. ACE I/D polymorphism study in a Cuban hypertensive population. Clin Chim Acta. 2007;378(1-2):112–116. doi: 10.1016/j.cca.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [16].1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17(2):151–183. [PubMed] [Google Scholar]
- [17].Feldmann E, Daneault N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40(10):1541–1545. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- [18].Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- [19].Kern R, Steinke W, Daffertshofer M, et al. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65(6):859–864. doi: 10.1212/01.wnl.0000175983.76110.59. [DOI] [PubMed] [Google Scholar]
- [20].Sacco RL. The 2006 William Feinberg lecture: shifting the paradigm from stroke to global vascular risk estimation. Stroke. 2007;38(6):1980–1987. doi: 10.1161/STROKEAHA.106.481143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359(6396):641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- [22].Szolnoki Z, Somogyvári F, Kondacs A, et al. Evaluation of the interactions of common genetic mutations in stroke subtypes. J Neurol. 2002;249(10):1391–1397. doi: 10.1007/s00415-002-0848-4. [DOI] [PubMed] [Google Scholar]
- [23].del Ser T, Bornstein B, Barba R, et al. Relationship of angiotensin converting enzyme genotype with serum triglyceride concentration in stroke patients. Neurosci Lett. 2001;316(1):21–24. doi: 10.1016/s0304-3940(01)02345-x. [DOI] [PubMed] [Google Scholar]
- [24].Markus HS, Barley J, Lunt R, et al. Angiotensin-converting enzyme gene deletion polymorphism. A new risk factor for lacunar stroke but not carotid atheroma. Stroke. 1995;26(8):1329–1333. doi: 10.1161/01.str.26.8.1329. [DOI] [PubMed] [Google Scholar]
- [25].Gao C, Gu GH, Xia Z. Genetic polymorphism of angiotensin-converting enzyme gene insertion/deletion in Chinese Han population. Zhongguo Linchuang Kangfu. 2006;10(44):188–190. [Google Scholar]
- [26].Arenillas JF, Molina CA, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32(12):2898–2904. doi: 10.1161/hs1201.099652. [DOI] [PubMed] [Google Scholar]
- [27].Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schunkert H, Hense HW, Holmer SR, et al. Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertrophy. N Engl J Med. 1994;330(23):1634–1638. doi: 10.1056/NEJM199406093302302. [DOI] [PubMed] [Google Scholar]
- [29].Thomas GN, Lin JW, Lam WW, et al. Middle cerebral artery stenosis in type II diabetic Chinese patients is associated with conventional risk factors but not with polymorphisms of the renin-angiotensin system genes. Cerebrovasc Dis. 2003;16(3):217–223. doi: 10.1159/000071119. [DOI] [PubMed] [Google Scholar]
- [30].Brenner D, Labreuche J, Touboul PJ, et al. Cytokine polymorphisms associated with carotid intima-media thickness in stroke patients. Stroke. 2006;37(7):1691–1696. doi: 10.1161/01.STR.0000226565.76113.6c. [DOI] [PubMed] [Google Scholar]
- [31].Venti M, Parnetti L, Gallai V. Genetics of ischemic stroke. Clin Exp Hypertens. 2002;24(7-8):531–534. doi: 10.1081/ceh-120015329. [DOI] [PubMed] [Google Scholar]
- [32].Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123(Pt 9):1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- [33].Cui JG, Yang G, Su XK, et al. Study on gene polymorphism of AGT, ACE and MTHFR in cerebral infarction patients. Yixue Linchuang Yanjiu. 2008;25(10):1752–1754. [Google Scholar]
- [34].Du MY, Feng J, Du HS, et al. Association between the gene polymorphism of ACE and AGT in hypertension disease complicated with cerebral infraction. Jichu Yixue yu Linchuang. 2011;31(11):1189–1193. [Google Scholar]
- [35].Huang HW, Fu X, Tan SQ, et al. ACE I/D with MTHFR 677CC genotype is an independent genetic factor that protects against middle cerebral artery stenosis:a community study in Foshan of China. Zhonghua Shenjing Yixue Zazhi. 2008;7(10):1019–1022. [Google Scholar]
- [36].Lu XL, Mo ZL, Yao XL, et al. ACE gene and MTHFR gene polymorphism and genetic susceptibility of in Hainan Li ischemic stroke patients. Zhonghua Yixue Yichuan Xue Zazhi. 2007;24(5):605–606. [Google Scholar]
- [37].Sun HY, He JL, Jiang CC. Relationship between the ACE gene and ApoE gene polymorphisms and cerebral infarction. Inner Mongolia Yixue Zazhi. 2008;40(1):4–6. [Google Scholar]
- [38].Zhao YN, Guo X, Gao JY, et al. Influence of ApoEε4 and ACE DD allele genes on the executive function of patients with ischemic stroke. Zhongguo Quanke Yixue. 2011;14(32):3711–3714. [Google Scholar]
- [39].Guo X, Shi XM, Zhao XY, et al. The relationship between PAI-1 promoter region 4G/5G gene polymorphism, ACE I/D gene polymorphism and cerebral stroke. Jichu Yixue yu Linchuang. 2011;31(11):1238–1241. [Google Scholar]
- [40].Vikman P, Edvinsson L. Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur J Neurol. 2006;13(12):1324–1332. doi: 10.1111/j.1468-1331.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- [41].Ryang YM, Dang J, Kipp M, et al. Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci. 2011;12:113. doi: 10.1186/1471-2202-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sun SL, Li TJ, Yang PY, et al. Modulation of signal transducers and activators of transcription (STAT) factor pathways during focal cerebral ischaemia: a gene expression array study in rat hippocampus after middle cerebral artery occlusion. Clin Exp Pharmacol Physiol. 2007;34(11):1097–1101. doi: 10.1111/j.1440-1681.2007.04679.x. [DOI] [PubMed] [Google Scholar]
- [43].Mitsios N, Saka M, Krupinski J, et al. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007;8:93. doi: 10.1186/1471-2202-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang YN, Gao S, Wang LJ, et al. Occlusive cerebrovascular disease diagnosed by transcranial Doppler ultrasound and cerebral angiography. Zhonghua Shenjing Ke Zazhi. 1997;30(2):98–101. [Google Scholar]
- [45].Sliwka U, Klötzsch C, Popescu O, et al. Do chronic middle cerebral artery stenoses represent an embolic focus? A multirange transcranial Doppler study. Stroke. 1997;28(7):1324–1327. doi: 10.1161/01.str.28.7.1324. [DOI] [PubMed] [Google Scholar]


