Abstract
Emodin, an extract of dried rhizomes and the root of the Rhizoma Polygoni Cuspidati, can protect neurons from hypoxic-ischemic brain damage. This study aimed to verify the underlying mechanism. After PC12 cells had differentiated into neuron-like cells under the induction of mouse nerve growth factor, cells were subjected to oxygen-glucose deprivation and treated with emodin. Results showed that the viability of neuron-like cells cultured under an ischemia-hypoxia environment decreased, while the expression of activin A and caspase-3 in cells increased. Emodin raised the survival rate of oxygen-glucose deprived neuron-like cells, increased activin A expression, and decreased caspase-3 expression. Experimental findings indicate that emodin can inhibit neuronal apoptosis and alleviate the injury of nerve cells after oxygen-glucose deprivation through the activin A pathway.
Keywords: neural regeneration, traditional Chinese medicine, emodin, oxygen-glucose deprivation, activin A, apoptosis, caspase-3, neuroprotection, grants-supported paper, neuroregeneration
Research Highlights
(1) Emodin can improve the viability of neurons following ischemic-hypoxic injury, upregulate the expression of activin A, and downregulate the expression of caspase-3, thus inhibiting neuronal apoptosis and playing a neuroprotective role.
(2) The oxygen-glucose deprived PC12 model was established using the combined method of physical chemistry with some modifications.
(3) Unlike other studies, we aimed to observe changes in activin A expression and the signaling pathway involved following ischemic injury in neurons subjected to emodin intervention.
INTRODUCTION
The pathogenesis and mechanism underlying hypoxic-ischemic brain damage remains unclear, and there are few clinical preventive measures at present. The existing protective agents are citicoline and edaravone. Activin is widely distributed in vivo and exerts great biological activity. In addition, activin A can regulate cell repair, differentiation and apoptosis, promote tissue regeneration, participate in many pathological processes, and has apparent tissue-specific features[1].
Interest in activin A as a strong protective factor in the nervous system has recently emerged[2], and its mechanism of action has been suggested to depend on the activin A/Samds signal transduction system[3]. The activin A signal system is a member of the transforming growth factor β1 signal transduction system superfamily[4], and growing evidence supports the neuroprotective effect of activin A[5,6]. Activin binds to serine/threonine phosphatase receptors (type 1 or 2) on the cell membrane to transfer signals[7]. Activin receptors may bind with a variety of transforming growth factor ligands, such as tubocurarine chloride, growth differentiation factor 11, and nodal. Once the ligand of activin A is activated in vivo, it produces different physiological and pathological events. Activin receptors activated by ligands can modulate the phosphorylation of Smads, such as Smad2 and Smad3. Receptor-regulated Smad protein is attributable to Smad4 protein multimerization. This polymer then transfers to the nucleus where it has a synergic effect on intranuclear cofactors to regulate the transcription of target genes[8,9]. The effects may affect intracellular protein stability, localization, and transcriptional activity, which is also regulated by a variety of mechanisms through receptors and Smads signal transduction, such as phosphorylation and other post-translational modifications[10].
Emodin, extracted from the dried rhizome and the root of Rhizoma Polygoni Cuspidati, has been shown to have antioxidant properties[11] and an antitumor[12] function. Growing evidence has shown that emodin interacts with transforming growth factor beta 1[13], Rho kinase[14] and other signal transduction pathways. In addition, emodin has neuroprotective effects and may prevent the formation of atherosclerotic plaques[15,16] by reducing the productions of photo-induced reactive oxygen species, interleukin 8, and monocyte chemoattractant protein 1. Because of its anti-oxidative effect and inhibitory effect on glutamate toxicity, emodin plays a neuroprotective role, and can antagonize ischemia-reperfusion brain injury, as well as the resultant glutamate toxicity[17,18]. Similar effects were also found against cerebral ischemia[16]. Emodin leads to an apparent decline in cell apoptosis[19]. Because of this protective effect of emodin in the nervous system, we speculate that the effect may be associated with the activin A signaling system. Therefore, in this study, we aimed to investigate the protective role of emodin and activin A.
RESULTS
Morphology of PC12 cells
Under the inverted microscope, PC12 cells cultured for 12 hours were observed to be freely floating; after 36 hours of culture, PC12 cells began to adhere to the wall of culture flasks; after 72 hours of culture, PC12 cells proliferated rapidly and covered the bottom of the culture flask (Figure 1).
Figure 1.
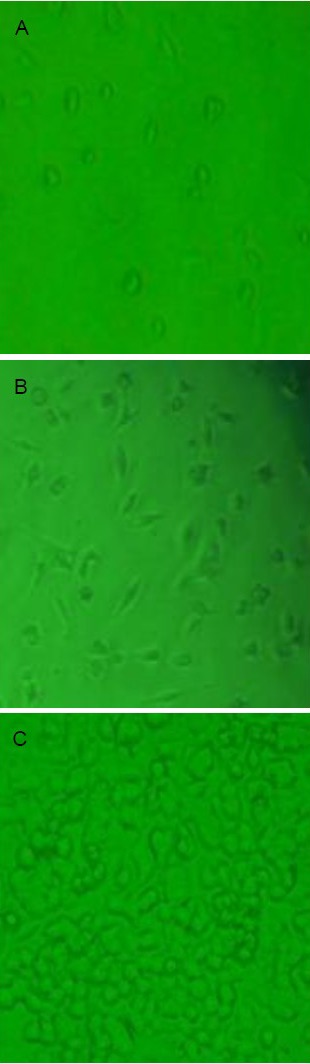
Morphology of cultured PC12 cells (inverted microscopy, × 100).
(A) After 12 hours of culture, PC12 cells had not adhered to the flask and were prone to clustering when in suspension.
(B) After 36 hours of culture, PC12 cells in culture medium were oval or polygonal shaped, and aggregated into clusters.
(C) After 72 hours of culture, PC12 cells entered the logarithmic growth phase, proliferated rapidly, with strong refraction and significantly increased number.
PC12 cells differentiated into neuron-like cells under stimulation of mouse nerve growth factor
After PC12 cells were induced with mouse nerve growth factor for 24 hours, cells formed processes; 36 hours later, cell processes became longer and thicker; 72 hours later, the length of processes were five times longer than the cell body, and synapses and neurite networks formed, all of which are morphological characteristics of neurons. Cells with apparent neuronal characteristics accounted for 95% of the total cell number (Figure 2).
Figure 2.
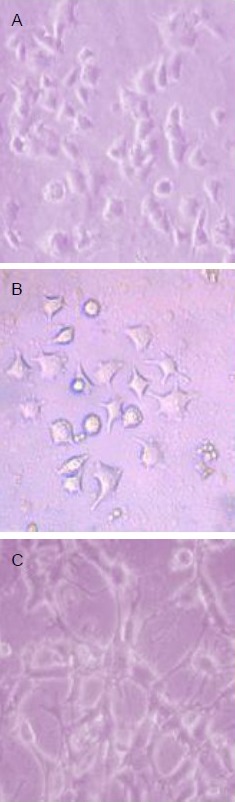
Mouse nerve growth factor induced PC12 cell differentiation into neuron-like cells (inverted microscopy, × 100).
(A) Induced for 24 hours, cells extended short processes.
(B) Induced for 36 hours, cell processes thickened and the number increased.
(C) Induced for 72 hours, synaptic length was five times that of the cell body, and networks formed, showing typical neuronal morphology.
After PC12 cells were induced with mouse nerve growth factor for 72 hours, immunofluorescence cytochemical staining revealed the expression of microtubule-associated protein 2 within cells (Figure 3).
Figure 3.
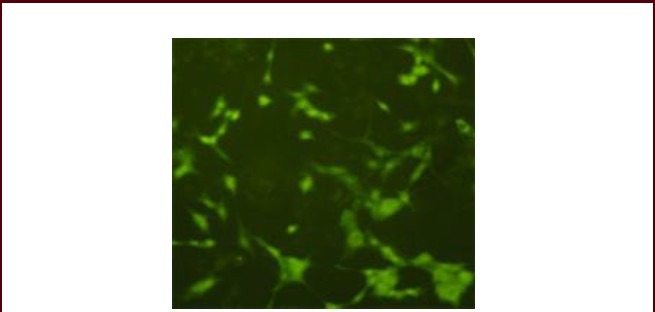
PC12 cells expressed microtubule-associated protein 2 after induction with mouse nerve growth factor for 72 hours (immunofluorescence cytochemical staining, × 400).
Under blue light excitation, multiform green fluorescent areas with long processes were visible in a scattered distribution, with apparent contrast to the background. Stain is fluorescein isothiocyanate, and positive expression is green.
PC12 cell viability decreased following oxygen-glucose deprivation
Results from the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that the survival rate of PC12 cells decreased after 3 hours of oxygen-glucose deprivation, and the decrease was time-dependent (P < 0.05). Only 10% of cells survived compared with the control group at 24 hours (Figure 4).
Figure 4.
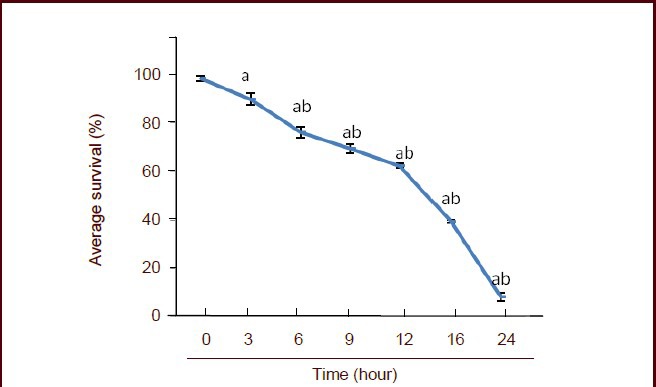
Effect of oxygen-glucose deprivation on the survival rate of PC12 cells.
The cell survival rate was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and the following formula: survival rate (%) = (absorbance at 490 nm in the experimental group / absorbance at 490 nm in the control group) × 100%. Data are expressed as mean ± SD, and multiple comparisons were performed using the Student-Newman-Keuls test. aP < 0.05, vs. 0 hour; bP < 0.05, vs. 3 hours.
Hoechst 33342 fluorescent staining showed that scattered apoptotic cells were visible, with bright blue nuclei after 3 and 6 hours of oxygen-glucose deprivation. The number of apoptotic cells increased after 9 hours of deprivation. The majority of cells were typically bright, showing pyknosis and hyperchromatic apoptosis morphology at 12 hours, while there were few normal cells. At 24 hours, most cells died, and healthy cells were rarely seen (Figure 5).
Figure 5.
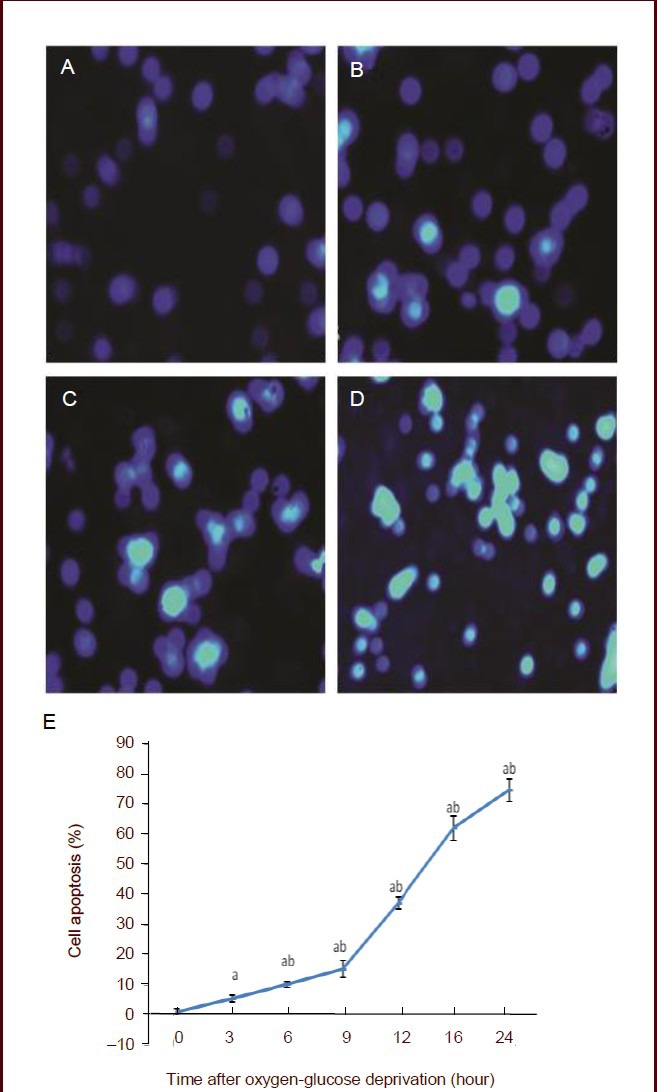
Effect of oxygen-glucose deprivation on PC12 cells apoptosis.
(A–D) Hoechst 33342 fluorescent staining, × 100. Apoptotic cells stained bright blue in the nucleus. (A) No apoptotic cells were found in the control group. (B) At 6 hours after oxygen-glucose deprivation, a few apoptotic cells were visible. (C) At 12 hours after oxygen-glucose deprivation, the number of apoptotic cells significantly increased. (D) At 24 hours after oxygen-glucose deprivation, the majority of cells died.
(E) The percentage of apoptotic cells was determined by the following equation: apoptotic rate (%) = (number of apoptotic cells/total number of cells counted) × 100%. Data are expressed as mean ± SD, and multiple comparisons were performed using the Student-Newman-Keuls test. The experiment was repeated three times. aP < 0.05, vs. 0 hour; bP < 0.05, vs. 3 hours.
Emodin increased PC12 cell viability following oxygen-glucose deprivation
MTT test results showed that emodin at 20 μmol/mL exerted the greatest protection against neuronal injury, with the rate of apoptosis reaching a minimum. Emodin at a lower or higher dose than 20 μmol/mL could not achieve a greater neuroprotective effect. Therefore, we selected 20 μmol/mL as the final concentration for intervention. Compared with the normal control group, cell viability decreased significantly after 6 hours of oxygen-glucose deprivation (P < 0.05); however, 20 μmol/mL emodin significantly improved cell viability (P < 0.05; Figure 6).
Figure 6.
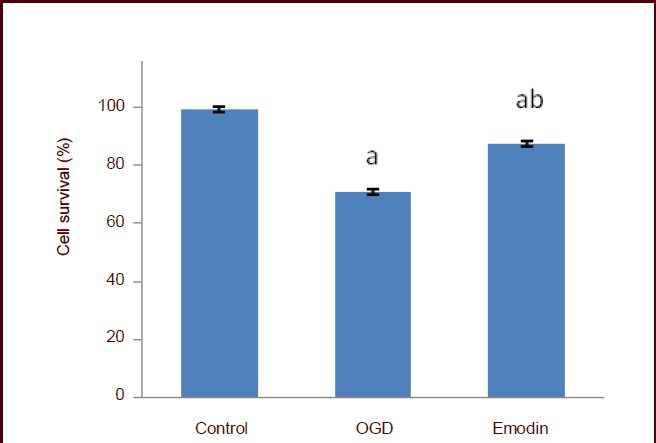
Effect of emodin on PC12 cell viability following oxygen-glucose deprivation (OGD).
The cell survival rate was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and the following formula: survival rate (%) = (absorbance at 490 nm in the experimental group / absorbance at 490 nm in the control group) × 100%. Data are expressed as mean ± SD, and multiple comparisons were performed using the Student-Newman-Keuls test. The experiment was repeated three times. aP < 0.05, vs. control group; bP < 0.05, vs. OGD group.
Emodin increased activin A content in PC12 cells following oxygen-glucose deprivation
After PC cells were cultured for 6 hours, a small amount of activin A was detected in the control group by enzyme linked immunosorbent assay. After oxygen-glucose deprivation, activin A levels in PC12 cell culture medium was significantly increased compared with the control group (P < 0.05), and was significantly increased in the emodin group compared with the oxygen-glucose deprivation group (P < 0.05). This evidence indicated that the emodin could stimulate the secretion of activin A (Figure 7).
Figure 7.
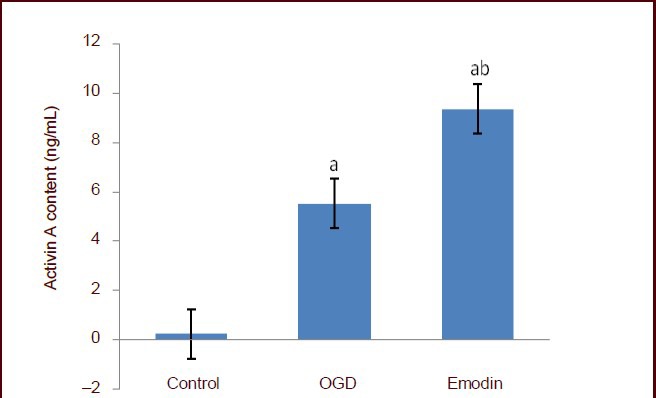
Effect of emodin on activin A content in PC12 cells following oxygen-glucose deprivation (OGD).
Activin A content was determined using the enzyme-linked immunosorbent assay. Data are expressed as mean ± SD, and multiple comparisons were performed using the Student-Newman-Keuls test. The experiment was repeated three times. aP < 0.05, vs. control group; bP < 0.05, vs. OGD group.
Emodin inhibited caspase-3 expression in PC12 cells following oxygen-glucose deprivation
Western blot analysis showed that after 6 hours of oxygen-glucose deprivation, procaspase-3 and cleaved caspase-3 were highly expressed in cells (P < 0.05). However, emodin significantly inhibited cleaved caspase-3 expression (P < 0.05; Figure 8).
Figure 8.
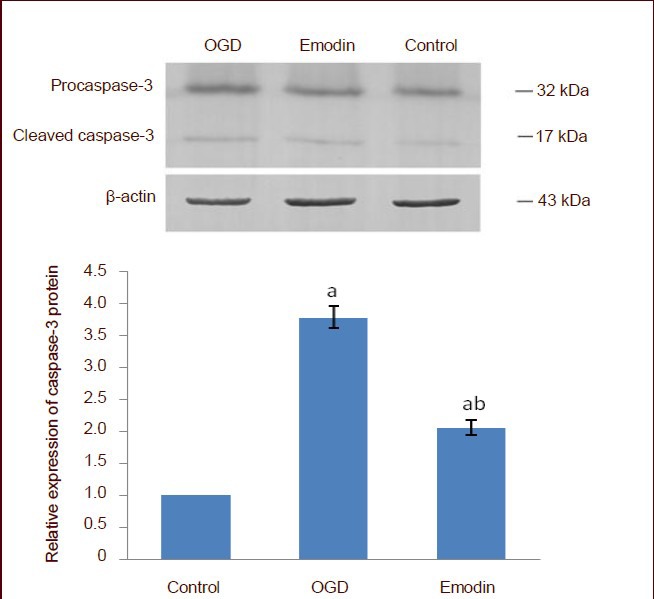
Effect of emodin on caspase-3 expression in PC12 cells following oxygen-glucose deprivation (OGD).
Caspase-3 expression is represented as the absorbance ratio of procaspase-3 and cleaved caspase-3 to β-actin. The expression of caspase-3 in the control group was 1. Data are expressed as mean ± SD, and multiple comparisons were performed using the Student-Newman-Keuls test. The experiment was repeated three times. aP < 0.05, vs. control group; bP < 0.05, vs. OGD group.
DISCUSSION
In this study, the rat PC12 cell line was shown to differentiate into neuron-like cells after induction with mouse nerve growth factor in vitro. The oxygen-glucose deprivation model was established using the method described by Guo et al[20] with some modifications, and the hypoxic-ischemic cell model was considered successful upon an increase in the rate of apoptosis.
Results of this study showed that emodin significantly increased the survival rate of oxygen-glucose-deprived PC12 cells, suggesting that emodin can improve the tolerance and viability of neurons after hypoxic-ischemic brain damage in vitro. Activin A was highly expressed in PC12 cells, which suggested that emodin activated the self-protection mechanism of neurons in advance, and upregulated the expression of activin. Physcione may induce mesenchymal progenitor cell transformation into osteoblasts through the activation of the Smad signal transduction system, which triggers bone morphogenetic protein expression and differentiation. Therefore, the Smad signal transduction system activates the protein kinase B/Akt and extracellular regulated protein kinases/microtubule-associated protein signal transduction system, and inhibits the phosphatidylinositol 3-kinase signal transduction system, which are all conducive to changing the biological activity of cells and to affecting the biological functions of tissues and organs. Emodin, one carbon atom less than physcion, can transform into physcion in vivo, so it is speculated that emodin may activate the activin A/Smads signal transduction system, which leads to the formation of autocrine activin A protein through a positive feedback mechanism that increases the anti-damage capacity of neuronal cells[21].
After oxygen-glucose deprived PC12 cells were subjected to emodin intervention, the expression levels of procaspase-3 and cleaved caspase-3 were significantly decreased compared with the oxygen-glucose deprivation alone group. This is evidence that the neuroprotective effect of emodin can be achieved by preventing cell apoptosis. Heger et al[22] found that caspase-3 was closely associated with the Smads signal transduction system.
In summary, emodin can upregulate the expression of activin A, and activin A can downregulate caspase-3 expression through the activation of the activin A/Smads pathway, thus inhibiting neuronal apoptosis and playing a neuroprotective role.
MATERIALS AND METHODS
Design
A comparative observation focusing on cell biology.
Time and setting
Experiments were performed from January 2010 to December 2011 in the Animal Experimental Center of Capital Medical University, China.
Materials
Cells
PC12 cells were purchased from Beijing Golden Bauhinia Biological Co., Ltd., Beijing, China.
Drugs
Emodin (chemical name 6-methyl-1,3,8-trihydroxyanthraquinone, molecular formula C15H10O5, molecular weight 270.24, purity 98%) was provided by the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Emodin was dissolved in dimethyl sulfoxide solution and stored at –20°C.
Methods
PC12 cell culture
PC12 cells was incubated with Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA, USA) in polylysine-treated culture flasks at 37°C and maintained in a 5% (v/v) CO2 incubator. Two days later, culture medium was replenished. The cultured cells were used for the experiments if they were 85% confluent. After PC12 cells were cultured for 72 hours, cell morphology was observed using an inverted microscope (Olympus, Tokyo, Japan).
Induction of PC12 cell differentiation
PC12 cell coverslips, at a density of 5 × 104 cells/mL, were incubated with Dulbecco's modified Eagle's medium containing 0.05 mg/mL nerve growth factor in a 24-well plate for 24 hours. Cell morphology was observed under an inverted microscope at 0, 24, 36, and 72 hours after intervention with nerve growth factor. The neuron-like cells were identified upon the appearance of cell neurite lengths exceeding twice that of the cell body length, and cells with several processes were also regarded as neuron-like cells. The ratio of positive cells differentiating into neuron-like cells was calculated in five wells, and an average value was measured.
Expression of microtubule-associated protein 2 in neuron-like cells as detected by immunofluorescence cytochemical staining
Cell slides were removed from the 24-well culture plate, rinsed with 0.01 mol/L PBS, fixed with 4% (w/v) paraformaldehyde, and permeabilized with 0.1% (v/v) Triton X-100 for 10 minutes, and then blocked with 10% (v/v) sheep serum for 30 minutes. Cells were incubated with rabbit anti-rat microtubule-associated protein 2 (1 mg/mL; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) overnight at 4°C in a wet box, followed by incubation with fluorescein isothiocyanate-labeled goat anti-rabbit IgG (2 mg/mL; Beijing Biosynthesis Biotechnology Co., Ltd.) at 37°C for 20 minutes. Slides were mounted with Fluoromount-G. The negative control was treated with PBS instead of antibody. Cells were observed under a fluorescent microscope (Olympus).
Establishment of oxygen-glucose deprivation model
With some modifications based on the methods of Guo et al[20], cell culture flasks were fixed in sealed glassware with the addition of 100 mL sterile water and 40 g sodium hydrosulfite, to assist with deoxygenation. The sealed glassware was connected with a rubber pipe by two holes, to maintain CO2 at 5% (v/v) and N2 at 95% (v/v). PC12 cells were incubated in glucose-free culture medium containing 10% (v/v) fetal bovine serum and 1 mmol/L sodium hydrosulfite. The hypoxic tank (sealed glassware) was maintained at 37°C.
Emodin intervention and MTT detection of cell viability
At 3, 6, 9, 12, 16, and 24 hours of hypoxia, each well was added with 100 μL of cell suspension and cells at a density of 5 × 104 cells/mL were incubated with 5% (v/v) CO2 at 37°C until cells covered the bottom of culture flask. Cultured cells were then incubated with 2.5, 5, 10, 20, and 40 μmol/mL emodin for 48 hours, followed by the addition of 20 μL MTT solution (5 mg/mL) for an additional 4 hours. After the culture medium was discarded, 150 μL dimethyl sulfoxide was added for 10 minutes. The absorbance at 490 nm was measured using an enzyme linked immunoassay device (Beijing Prolong Science & Technology Co., Ltd., Beijing, China), and the survival rate of cells was calculated. The survival rate (%) = absorbance at 490 nm in the experimental group / absorbance at 490 nm in the control group × 100%. The experiment was performed in triplicate. Cells were incubated using Dulbecco's modified Eagle's medium culture solution at a concentration of 20 μmol/mL.
Hoechst 33342 fluorescent staining detection of PC12 cell apoptosis
At 3, 6, 9, 12, 16, and 24 hours of cell culture, the cell suspension (5 × 104/mL) was added to the disposable cell slide in 12-well culture plates, and was subjected to oxygen-glucose deprivation. After the culture solution was discarded, cells were fixed with 0.5 mL of 95% (v/v) ethanol, rinsed twice with PBS, and stained with 0.5 mL Hoechst. Anti-fluorescence quenching mounting liquid was added to each slide and cells were observed under a fluorescence microscope. Apoptotic cells were stained bright blue in the nucleus. The apoptotic rate was measured as the percentage of apoptotic cells to the total number of cells under 100 × magnification. The experiment was performed in triplicate and the average value was calculated.
Enzyme linked immunosorbent assay for activin A content in PC12 cells
After cells were cultured for 6 hours, the supernatant was extracted and collected in a sterile tube, centrifuged at 2 000 r/min for 20 minutes, and preserved for further use. In strict accordance with the kit instructions (R&D, Minneapolis, MN, USA), activin A levels were determined using the enzyme linked immunosorbent assay and the linear regression equation involving the activin A standard curve. The experiment was performed in triplicate.
Western blot assay for caspase-3 protein expression in PC12 cells
After oxygen-glucose deprivation, PC12 cells were treated with emodin, culture medium was discarded, and cells were incubated with 1 × sodium dodecyl sulfate sample buffer. Cells were scraped and transferred to a centrifuge tube. DNA was sheared by sonication for 10–15 seconds, samples were boiled for 5 minutes and cells were centrifuged at 12 000 r/min for 5 minutes. After the supernatant was removed, cells were subjected to electrophoretic separation and transferred to a nitrocellulose membrane. The membrane was blocked with 5% (w/v) skim milk at room temperature for 1 hour, and incubated with rabbit anti-rat procaspase-3 and active caspase-3 monoclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-rat β-actin monoclonal antibody (200 μg/mL; Santa Cruz Biotechnology) at 4°C overnight. On the next day, the membrane was rinsed with blocking solution three times for 5 minutes each, and incubated with horseradish peroxidase-conjugated goat anti-rabbit/mouse antibody (1:1 000; Santa Cruz Biotechnology) at 37°C for 2 hours. The membrane was then washed with Tris buffered saline containing 0.1% (v/v) Tween 20 three times, for 5 minutes each. Protein was detected by chemiluminescence and the band intensities were quantified following scanning with a gel image analysis system (Olympus). The absorbance ratio of the protein band to the internal reference was calculated. The experiment was performed in triplicate.
Statistical analysis
Data are expressed as mean ± SD, and were statistically analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Differences between groups were compared by one-way analysis of variance, and multiple comparisons were performed using the Student-Newman-Keuls test. A P value less than 0.05 was regarded as a significant difference.
Footnotes
Conflicts of interest: None declared.
Funding: This study was financially supported by the National Natural Science Foundation of China (General Program), No. 30971037 and Postdoctoral Foundation of China (General Program), No. 2012MS10489.
(Reviewed by Diwakarla S, Raye Z, Su YH, Li ZQ)
(Edited by Yu J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Massagué J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tretter YP, Hertel M, Munz B, et al. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med. 2000;6(7):812–815. doi: 10.1038/77548. [DOI] [PubMed] [Google Scholar]
- [3].Lin X, Duan X, Liang YY, et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006;125(5):915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ling N, Ueno N, Ying SY, et al. Inhibins and activins. Vitam Horm. 1988;44:1–46. doi: 10.1016/s0083-6729(08)60692-5. [DOI] [PubMed] [Google Scholar]
- [5].Munz B, Tretter YP, Hertel M, et al. The roles of activins in repair processes of the skin and the brain. Mol Cell Endocrinol. 2001;180(1-2):169–177. doi: 10.1016/s0303-7207(01)00514-7. [DOI] [PubMed] [Google Scholar]
- [6].Ageta H, Murayama A, Migishima R, et al. Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS One. 2008;3(4):e1869. doi: 10.1371/journal.pone.0001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsuchida K, Nakatani M, Uezumi A, et al. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J. 2008;55(1):11–21. doi: 10.1507/endocrj.kr-110. [DOI] [PubMed] [Google Scholar]
- [8].Schneyer AL, Sidis Y, Gulati A, et al. Differential antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology. 2008;149(9):4589–4595. doi: 10.1210/en.2008-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou WJ, Hu ZP. Expression of Smad 2 and Smad 4 proteins in brain tissue following cerebral ischemia/reperfusion in gerbils. Guoji Shenjing Bing Xue Shenjing Waike Zazhi. 2008;35(2):112–114. [Google Scholar]
- [10].Tsuchida K, Nakatani M, Hitachi K, et al. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal. 2009;7:15. doi: 10.1186/1478-811X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li YP, Takamiyagi A, Ramzi ST, et al. Inhibitory effect of Rumex Japonicus Houtt on the porphyrin photooxidative reaction. J Dermatol. 2000;27(12):761–768. doi: 10.1111/j.1346-8138.2000.tb02278.x. [DOI] [PubMed] [Google Scholar]
- [12].Zeng Y, Liu A, Tong HF. Effect of emodin combined gemcitabine on the growth and apoptosis of pancreatic cancer cell line BxPC-3 in vitro. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(4):552–554. [PubMed] [Google Scholar]
- [13].Hu Q, Noor M, Wong YF, et al. In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol Dial Transplant. 2009;24(10):3033–3041. doi: 10.1093/ndt/gfp245. [DOI] [PubMed] [Google Scholar]
- [14].Cai J, Niu X, Chen Y, et al. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia. 2008;10(1):41–51. doi: 10.1593/neo.07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heo SK, Yun HJ, Noh EK, et al. Emodin and rhein inhibit LIGHT-induced monocytes migration by blocking of ROS production. Vascul Pharmacol. 2010;53(1-2):28–37. doi: 10.1016/j.vph.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [16].Li JS, Liu JX, Liang SW, et al. Effects of glucoside and aglycone parts of rhubarb on the metabolism of free radicals in rats with ischemic brain injury. Zhongguo Linchuang Kangfu. 2004;8(34):7748–7750. [Google Scholar]
- [17].Wang C, Zhang D, Ma H, et al. Neuroprotective effects of emodin-8-O-beta-D-glucoside in vivo and in vitro. Eur J Pharmacol. 2007;577(1-3):58–63. doi: 10.1016/j.ejphar.2007.08.033. [DOI] [PubMed] [Google Scholar]
- [18].Gu JW, Hasuo H, Takeya M, et al. Effects of emodin on synaptic transmission in rat hippocampal CA1 pyramidal neurons in vitro. Neuropharmacology. 2005;49(1):103–111. doi: 10.1016/j.neuropharm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [19].Huang Z, Chen G, Shi P. Emodin-induced apoptosis in human breast cancer BCap-37 cells through the mitochondrial signaling pathway. Arch Pharm Res. 2008;31(6):742–748. doi: 10.1007/s12272-001-1221-6. [DOI] [PubMed] [Google Scholar]
- [20].Guo HL, Xu ZX, Li XH, et al. Use of RNAi silencing to target preconditioned glial cell line-derived neurotrophic factor in neuronal apoptosis. Neural Regen Res. 2011;6(7):510–516. [Google Scholar]
- [21].Lee SU, Choi YH, Kim YS, et al. Physcion-8-O-beta-D-glucopyranoside enhances the commitment of mouse mesenchymal progenitors into osteoblasts and their differentiation: Possible involvement of signaling pathways to activate BMP gene expression. J Cell Biochem. 2010;109(6):1148–1157. doi: 10.1002/jcb.22494. [DOI] [PubMed] [Google Scholar]
- [22].Heger J, Abdallah Y, Shahzad T, et al. Transgenic overexpression of the adenine nucleotide translocase 1 protects cardiomyocytes against TGFβ1-induced apoptosis by stabilization of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2012;53(1):73–81. doi: 10.1016/j.yjmcc.2012.04.013. [DOI] [PubMed] [Google Scholar]


