Abstract
The persimmon leaf has been shown to improve cerebral ischemic outcomes; however, its mechanism of action remains unclear. In this study, mice were subjected to 10 minutes of ischemic preconditioning, and persimmon leaf flavonoid was orally administered for 5 days. Results showed that the persimmon leaf flavonoid significantly improved the content of tissue type plasminogen activator and 6-keto prostaglandin-F1 α in the cerebral cortex, decreased the content of thromboxane B2, and reduced the content of plasminogen activator inhibitor-1 in mice. Following optical microscopy, persimmon leaf flavonoid was also shown to reduce cell swelling and nuclear hyperchromatism in the cerebral cortex and hippocampus of mice. These results suggested that persimmon leaf flavonoid can effectively inhibit brain thrombosis, improve blood supply to the brain, and relieve ischemia-induced pathological damage, resulting in brain ischemic tolerance.
Keywords: neural regeneration, traditional Chinese medicine, brain injury, persimmon leaf flavonoid, brain ischemic tolerance, ischemic preconditioning, mice, cortex, hippocampus, pathology, tissue type plasminogen activator, plasminogen activator inhibitor-1, 6-keto prostaglandin-F1 α, thromboxane B2, grants-supported paper, neuroregeneration
Research Highlights
(1) This study verified that persimmon leaf flavonoid can induce brain ischemic tolerance.
(2) The mechanism underlying the neuroprotective effect of persimmon leaf flavonoid against cerebral ischemia injury involved dissolving the thrombus, reducing endothelial cell damage, increasing brain ischemic tolerance and reducing cerebral infarction.
INTRODUCTION
Transient cerebral ischemia preconditioning can induce an endogenous protective effect (effectively reducing serious, even fatal cerebral ischemic events), relieve ischemic tissue lesions, and induce cerebral ischemic tolerance[1]. Clinical data have shown that when cerebral infarction occurs, the infarct area is smaller and the prognosis is better in patients who have suffered with transient cerebral ischemic attacks previously when compared with those without non-cerebral ischemia[2]. These findings indicate the cerebral protective effects of ischemic preconditioning. Endogenous protective mechanisms of cerebral ischemic tolerance and preconditioning involve a wide range of mechanisms that have not been fully elucidated[3,4,5,6]. A previous study has shown that ischemic preconditioning improves the regulatory function of microcirculation[7], promotes opening of blood capillaries and microvascular blood flow, reduces tissue blood flow hypoperfusion during ischemia and no-reflow during reperfusion, promotes effective tissue perfusion and recovery, prevents further injury to tissue cells, reduces tissue damage, and promotes recovery after injury.
Kitagawa et al[8] were the first to observe brain ischemic tolerance, and believed that transient sublethal ischemia induced increased tolerance of hippocampal neurons to subsequent lethal ischemia, which is now known as brain ischemic tolerance. A variety of protective interventions prior to severe ischemia are known as pretreatments, of which ischemic preconditioning is a commonly used method. In modern medicine, physical pretreatment and pharmacological preconditioning are methods to improve brain ischemic tolerance, in which pharmacological preconditioning in the clinic is more practical. Pharmacological preconditioning can induce better ischemic tolerance, but the cost is higher, the mode of administration is limited, and there is the possibility of adverse reactions. Traditional Chinese medicine has more advantages in improving brain ischemic tolerance. New drugs for the prevention of cerebral ischemic diseases may be developed by further studying brain ischemic tolerance mechanisms, which have practical values for high-risk populations. Traditional Chinese medicines have few adverse reactions and multiple targets. Studies have reported that persimmon leaf extract can significantly improve electrophoretic mobility of rabbit erythrocytes, decrease blood and plasma viscosity, reduce the amount of fibrinogen, and have certain effects on activating blood circulation to dissipate blood stasis[9], as well as increase asphyxial anoxia endurance[10]. However, few studies have investigated the precise effect and mechanism of action of persimmon leaf extract in the treatment of ischemic tolerance. Some studies have shown that flavonoids from the leaves of Diospyros kaki increased brain ischemic tolerance. The persimmon leaf flavonoid is an effective constituent of traditional Chinese medicinal leaves. This study explored the effect and mechanism of persimmon leaf flavonoids on improving brain ischemic tolerance by observing the effects of persimmon leaf flavonoids on tissue type plasminogen activator, plasminogen activator inhibitor-1, 6-keto prostaglandin-F1 α and thromboxane B2 in a mouse brain ischemic tolerance model.
RESULTS
Quantitative analysis of experimental animals
Mice (n = 126) were randomly divided into seven groups: sham surgery group (intragastric administration of saline), ischemia/reperfusion group (ischemia/reperfusion + intragastric administration of saline), preprocessing model group (ischemic preconditioning 10 minutes + ischemia/reperfusion + intragastric administration of saline), high-, moderate- and low-dose persimmon leaf flavonoid groups (ischemia/reperfusion + 400, 200 and 100 mg/kg persimmon leaf flavonoid), and ginaton group (ischemia/reperfusion + 40 mg/kg ginaton). Each group contained 18 mice. Because 36 mice died during the operation, there were 18 mice in the sham surgery group, 12 mice in the ischemia/reperfusion group, 10 mice in the preprocessing model group, 12 mice in the high- and moderate-dose persimmon leaf flavonoid groups, 11 mice in the low-dose persimmon leaf flavonoid groups, and 15 mice in the ginaton group. A total of 90 mice were included in the final analysis.
Effect of persimmon leaf flavonoid on pathological damage to brain tissue in the mouse model of cerebral ischemia
Compared with the sham surgery group, pathological injury to nerve cells in the mouse brain was significant in the ischemia/reperfusion and preprocessing model groups (P < 0.01). Compared with the preprocessing model group, pathological injury was markedly reduced in the different doses of persimmon leaf flavonoid groups (P < 0.05 or P < 0.01), particularly in the high-dose persimmon leaf flavonoid group and ginaton group (Table 1).
Table 1.
Effect of persimmon leaf flavonoid on pathological damage to brain tissue

The cell membrane and nucleoli of neurons in the cerebral cortex and hippocampus were clearly visible in the sham surgery group. Cell swelling, increases in cell volume, pyknosis, and blue-stained cytoplasm and nuclei were observed in the ischemia/reperfusion group. Cell swelling and lightly stained nuclei were detectable in the preprocessing model group (Figure 1).
Figure 1.
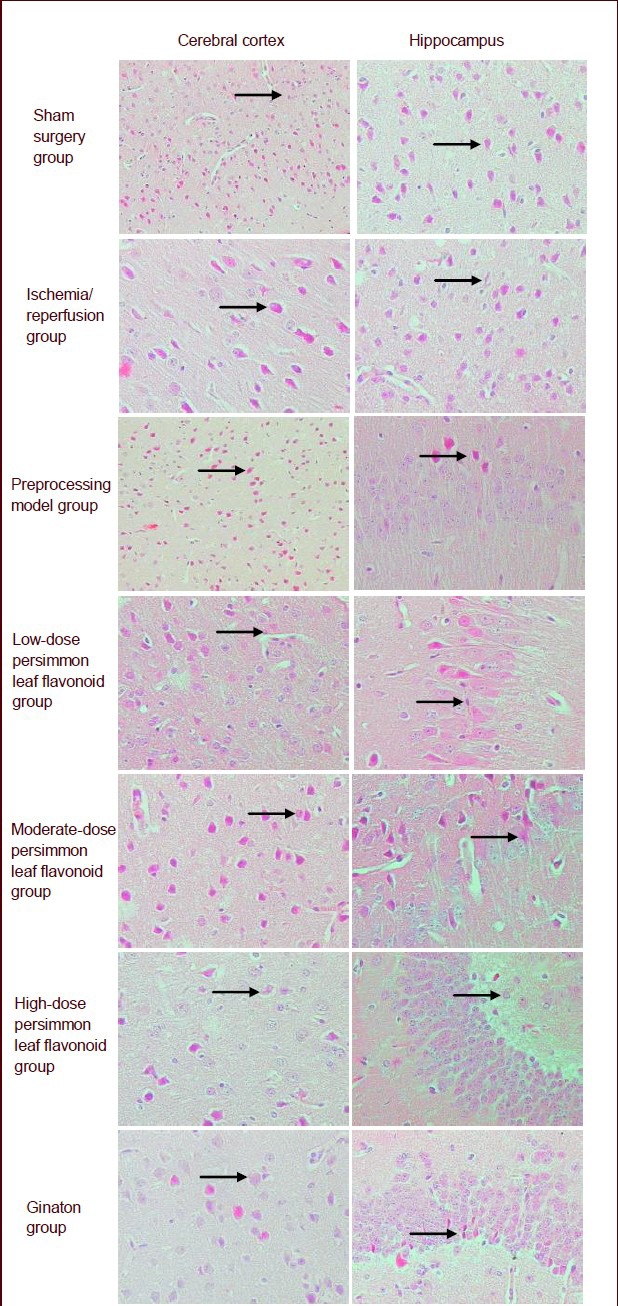
Effect of persimmon leaf flavonoid on pathological morphology in the cerebral cortex and hippocampus in the mouse ischemic model (hematoxylin-eosin staining, × 400). Arrows show nerve cells. Compared with the preprocessing model group, cell swelling and the number of lightly stained nuclei were reduced in the ginaton group, high-, moderate- and low-dose persimmon leaf flavonoid groups. Moreover, the ginaton group and high-dose persimmon leaf flavonoid group had a greater effect.
Compared with the preprocessing model group, cell swelling and the number of lightly stained nuclei were reduced in the ginaton group, and high-, moderate- and low-dose persimmon leaf flavonoid groups. However, the effects were greatest in the ginaton and high-dose persimmon leaf flavonoid group (Figure 1).
Effect of persimmon leaf flavonoid on plasma tissue type plasminogen activator and plasminogen activator inhibitor-1 levels
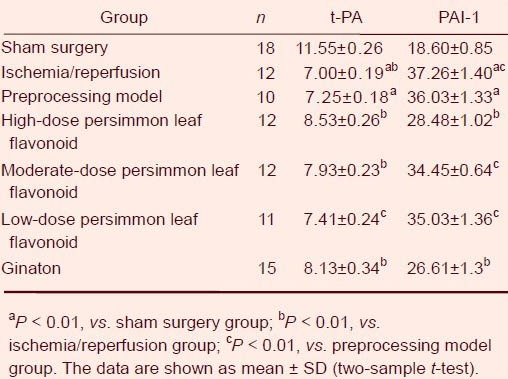
Compared with the sham surgery group, plasma tissue type plasminogen activator content was significantly lower, but plasminogen activator inhibitor-1 content was significantly greater in the ischemia/reperfusion group and preprocessing model group (P < 0.01). Compared with the ischemia/reperfusion group, tissue type plasminogen activator content was higher (P < 0.01), but plasminogen activator inhibitor-1 content was significantly lower (P < 0.05) in the preprocessing model group. Compared with the preprocessing model group, tissue type plasminogen activator content was significantly greater (P < 0.01 or P < 0.05), but plasminogen activator inhibitor-1 content was significantly lower (P < 0.01 or P < 0.05) in the high-, moderate- and low-dose persimmon leaf flavonoid groups and ginaton group (Table 2).
Table 2.
Effect of persimmon leaf flavonoid on plasma tissue type plasminogen activator (t-PA; ng/mL) and plasminogen activator inhibitor-1 (PAI-1; ng/mL)
Effect of persimmon leaf flavonoid on the levels of thromboxane B2 and 6-keto prostaglandin-F1 α
Compared with the sham surgery group, the content of 6-keto prostaglandin-F1 α reduced significantly (P < 0.01), and the content of thromboxane B2 was significantly increased (P < 0.01) in the ischemia/reperfusion group and preprocessing model group (Table 3).
Table 3.
Effect of persimmon leaf flavonoid on thromboxane B2 (TXB2; pg/mL), 6-keto prostaglandin-F1 α (6-Keto-PGF1α; pg/mL) and TXB2/6-Keto-PGF1α levels in mice with cerebral ischemia

Compared with the ischemia/reperfusion group, 6-keto prostaglandin-F1 α content in the cerebral cortex increased significantly (P < 0.01), while levels of thromboxane B2 significantly reduced (P < 0.01) in the preprocessing model group. Compared with the preprocessing model group, levels of 6-keto prostaglandin-F1 α in the cerebral cortex increased significantly (P < 0.01), and thromboxane B2 content was significantly diminished (P < 0.01) in the high-, moderate- and low-dose persimmon leaf flavonoid groups and ginaton group (Table 3).
DISCUSSION
Recent studies have shown that timing and dose are the two main factors for effective cerebral ischemic tolerance[11]. Cerebral ischemic tolerance has been shown to be induced after 120 hours of ischemic preconditioning[12]. At present, there are few studies investigating cerebral ischemic tolerance, and even fewer studies using effective ingredients of Chinese herbal medicines to improve cerebral ischemic tolerance. This study observed that persimmon leaf flavonoid reduced brain thrombosis, improved blood supply, and relieved the injury caused by ischemia by improving cerebral ischemic tolerance.
The experimental results showed that the number of neural cells decreased, and some cells were markedly swollen after cerebral ischemia reperfusion injury. However, in the preprocessing model group, cell swelling was reduced significantly in the infarct area. The number of nerve cells with integrated structure significantly increased. In the persimmon leaf flavonoid groups, cell swelling reduced and there were abundant nerve cells with integrated structure. These results suggested that 10-minute ischemic preconditioning can promote the recovery of neurological function after cerebral ischemia, effectively reduce its severity, and induce the occurrence of ischemic tolerance.
Endothelial cells are important for the formation and release of tissue type plasminogen activator and plasminogen activator inhibitor-1. Vascular endothelial growth factor can stimulate the growth of new neurons, play a protective effect on the nervous system before neovascularization, and prolong cell survival time until the formation of new blood vessels[13,14,15]. Ischemia preconditioning can promote the growth of blood vessels, and increase local blood flow, which is one of the protective effects and mechanisms of ischemic preconditioning following cerebral ischemia[16]. The increase in plasminogen activator inhibitor-1 levels is one of the causes of thrombosis[17]. Blood levels of tissue type plasminogen activator can be a direct reflection of fibrinolytic system function, which can be a predictor of thrombosis[18]. The experimental results showed that persimmon leaf flavonoid can increase tissue type plasminogen activator levels, reduce plasminogen activator inhibitor-1 content, and decrease the formation of cerebral thrombosis. Because thromboxane A2 and prostaglandin I2 are not stable, they can easily be metabolized into the stable end products thromboxane B2 and 6-keto prostaglandin-F1 α. Therefore, measuring metabolic end products can indirectly infer levels of thromboxane A2 and prostaglandin I2[19]. 6-keto prostaglandin-F1 α originating from vascular endothelial cells plays an important role in the regulation of cerebral blood flow. Thromboxane A2 contributes to platelet aggregation, induces thrombosis[20] and has strong cerebral vasoconstrictive properties[21]. Our experimental results showed that 6-keto prostaglandin-F1 α content in the persimmon leaf flavonoid groups increased, which may regulate cerebral blood flow, reduce endothelial cell function, and decrease the rate of cerebral ischemic injury.
Recent pharmacological studies have shown that persimmon leaf flavonoid has wide pharmacological effects, including vasodilatation, lipid-lowering properties, a hypoglycemic effect[22], and an antioxidative effect[23].
Persimmon leaf flavonoid, an extract, and ginaton, the positive control drug, were both administered by gavage. Experiments were paralleled in each group. Animals subjected to cerebral ischemia had severe endothelial dysfunction, enhanced coagulation of blood, and a significant decrease in plasmin activity. These were the main reasons for thrombus formation[24]. Therefore, timely correction of coagulation and fibrinolysis are important measures for the improvement of endothelial function, inhibition of platelet aggregation and thrombosis, which play an important role in the prevention and treatment of cerebral ischemia. Persimmon leaf flavonoid maintains plasminogen activator inhibitor-1 and tissue type plasminogen activator in dynamic equilibrium so that blood fibrinolysis and the coagulation system in patients with cerebral ischemia are relatively balanced. Persimmon leaf flavonoids diminish endothelial cell injury. At the same time, 6-keto prostaglandin-F1 α content increases and thromboxane B2 content decreases in animals treated with persimmon leaf flavonoids, which indicates that decreased thromboxane A2 content promotes platelet aggregation, reduces thrombosis, causes cerebral blood flow fluency, relieves cerebral vascular endothelial cell damage, reduces thrombosis, improves cerebral ischemic tolerance, and reduces ischemia-induced brain injury. Therefore, these results lay the foundation for using persimmon leaf flavonoid to improve cerebral ischemic tolerance, and to prevent and treat cerebral ischemia.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were conducted in the Pharmacology Laboratory, Henan College of Traditional Chinese Medicine, China from November to December 2009.
Materials
Animals
Clean Kunming mice (n = 126), weighing 25–30 g, were provided by the Laboratory Animal Center of Hebei Province (license No. 911137). Animals were housed in separate cages according to gender at 25 ± 3°C, at a humidity of 55 ± 10%, and were allowed free access to food and water. The protocols were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[25].
Drugs
Persimmon leaf extract (persimmon leaf flavonoid; content: 68%, as measured by UV-Spectrophotometry; batch No. TY20080116) was provided by the Chemistry Room, Henan College of Traditional Chinese Medicine, China. Fresh or dried persimmon leaves of genus Diospyros kaki L.f., were purchased from Henan Medical Material Company, Zhengzhou, Henan Province, China. Persimmon leaves were weighed, immersed in 10 times the amount of 70% (v/v) ethanol for 2 hours, and extracted twice, each for 1 hour. After filtering, the filtrates were combined and the alcohol was removed. The extract was loaded on an AB-8 macroporous resin column, with a liquid concentration of 0.6 g/mL. The resin volume was 1:8; ratio of diameter to height of the resin was 1:10. The specimens were eluted using four times the column volume of water, followed by four times the column volume of 40% (v/v) ethanol. Ethanol (50% (v/v)) was used to elute the flavonoids. The ethanol eluting liquid was collected, followed by vacuum recovery. The specimens were dried at 50°C.
Methods
Establishment and intervention of ischemic tolerance model
Establishment of ischemia/reperfusion model: All mice were intraperitoneally anesthetized with 10% (w/v) chloral hydrate (0.03 mL/10 g). The bilateral common carotid arteries were exposed, but blood flow was not blocked. When animals were conscious, all mice were intragastrically administered saline (0.1 mL/10 g), once a day for 5 consecutive days. The dose of persimmon leaf flavonoid was determined from previous experiments[26]. At day 5, after fasting for 12 hours and 1 hour after administration, the mice were intraperitoneally anesthetized with 10% (w/v) chloral hydrate (0.03 mL/10 g). The bilateral common carotid arteries were occluded using a micro clip to block blood flow for 30 minutes, and then reperfusion was allowed[27].
Sham surgery group: Exposure of the bilateral common carotid arteries was performed, but blood flow was not blocked.
Preprocessing model group: After anesthesia, the bilateral common carotid arteries were occluded with a micro clip. Ischemic preconditioning was performed by blocking blood flow for 10 minutes. Subsequently, ischemia/reperfusion models were established[27].
High-, moderate- and low-dose persimmon leaf flavonoid groups and ginaton group: Ischemia/reperfusion mice were subjected to ischemic preconditioning for 10 minutes and given ginaton (40 mg/kg, 15 times the clinical dose), high- (400 mg/kg), moderate- (200 mg/kg), and low-dose (100 mg/kg) persimmon leaf flavonoid (delivery volume 4, 40, 20, 10 mg/mL, respectively), once per day, for 5 consecutive days.
Morphology of the cerebral cortex and hippocampus in ischemic mice
After blood was collected at 24 hours following the last operation, all mice were euthanized. Brain tissues were rapidly obtained, embedded in paraffin, sliced into 5 μm-thick serial sections, and stained with hematoxylin and eosin. The cerebral cortex and hippocampus of mice was observed under a 400 × optical microscope (Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay detection of fibrinolysis and thromboxane
Detection of tissue type plasminogen activator and plasminogen activator inhibitor-1[28,29]: Blood was collected at 24 hours following the last operation. After sodium citrate anticoagulation, blood plasma was separated. In accordance with the kit's instructions (tissue type plasminogen activator: Shanghai SUNBIO Co., Ltd., Shanghai, China; batch No. 41130; plasminogen activator inhibitor-1: Beijing Puerweiye Biological Technology Co., Ltd., Technology Development Center Radioimmunity Institute of General Hospital of Chinese PLA, Beijing, China; batch No. 20091125), the contents of tissue type plasminogen activator and plasminogen activator inhibitor-1 (ng/mL) were detected.
Detection of the content of thromboxane B2 and 6-keto prostaglandin-F1 α in the ischemic mouse model: Mice were euthanized after blood sampling. Brain tissues were coronally sliced for hematoxylin-eosin staining. In addition, 15 mg of cerebral cortex was homogenated in nine times the volume of saline, followed by centrifugation at 3 500 r/min for 10 minutes. The supernatant was collected for measurement. According to the kit's instructions (thromboxane B2: Beijing Puerweiye Biological Technology Co., Ltd., Technology Development Center Radioimmunity Institute of General Hospital of Chinese PLA; batch No. 20091125), the samples were tested using the equilibrium method. After the sample or standard was added, the labeled antigen was added for the competitive binding reaction. After completion of the reaction, an immune separating agent was added to isolate the antigen-antibody complexes. The radioactivity (B) of complexes was determined, and the binding rate of the standard tube (B/B0%) was calculated. Relevant parameters, calibration curve, and sample concentration were directly obtained using a prefabricated program using a gamma counter[30].
Statistical analysis
Measurement data were expressed as mean ± SD, and analyzed using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). Paired intergroup comparisons were performed using the two-sample t-test. Rank data were compared using the Ridit test. A P < 0.05 level indicated statistical significance.
Acknowledgments:
We are grateful to Professor Jianguo Li for helping us to observe the pathological tissue sections.
Footnotes
Conflicts of interest: None declared.
Funding: This project was funded by the State “Major New Drug Creation” Science and Technology Major Special Project Foundation, No. 2009ZX09103-324; and a grant from the Henan Province Science and Technology Innovation Team in University, No. 2012IRTSTHN011.
Ethical approval: This study was approved by the Experimental Animal Ethics Committee, Henan University of Traditional Chinese Medicine, China.
(Reviewed by Diwakarla S, Haase R, Tu QY, Bai H)
(Edited by Wang J, Qiu Y, Li CH, Song LP)
REFERENCES
- [1].Blanco M, Lizasoain I, Sobrino T, et al. Ischemic preconditioning: a novel target for neuroprotective therapy. Cerebrovasc Dis. 2006;21(Suppl 2):38–47. doi: 10.1159/000091702. [DOI] [PubMed] [Google Scholar]
- [2].Moncayo J, de Freitas GR, Bogousslavsky J, et al. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54(11):2089–2094. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- [3].Zhou JY, Liu L, Luo ZM. Effect of focal ischemic preconditioning on expression of nerve groeth in rats with cerebral infarction. Nao yu Shenjing Jibing Zazhi. 2005;13(6):411–413. [Google Scholar]
- [4].Wang CH, Gao M, Han XM, et al. The expression change of GFAP after hypoxia preconditioning and focal cerebral ischemia in rat. Zhongguo Shiyan Zhenduanxue. 2005;9(6):884–886. [Google Scholar]
- [5].Li TH, Zhao Q, Qian JL, et al. The expression of GDNF after focal cerebral ischemic in rats. Zhongguo Laonianxue Zazhi. 2007;11(27):2063–2065. [Google Scholar]
- [6].Liu L, Liu XL, Luo ZM. Effect of focal cerebral ischemic pretreatment on expression of brain-derived neurotrophic factors in rats with cerebral ischemic tolerance. Zhongguo Linchuang Kangfu. 2005;9(5):84–86. [Google Scholar]
- [7].Wang JH, Liu XH, Liu FY, et al. Protective effect of ischemic preconditioning on rat brain with ischemia/reperfusion injury. Zhongguo Bingli Shengli Zazhi. 2003;19(4):533–536. [Google Scholar]
- [8].Kitagawa K, Matsumoto M, Tagaya M, et al. Ischemic tolerance henomenon found in the brain. Brain Res. 1990;528(1):21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- [9].Liang C, Fu FM, Zhang KS. The pharmacological effects of persimmon leaf on cardiovascular system. Yaoxue Tongbao. 1985;20(4):245. [Google Scholar]
- [10].Yu YZ, Yu ZY, Guo J. Clinical observation and experiment of Naoxinning in treating ischemic cerebrovascular disease. Jiefangjun Yixue Zazhi. 1988;13(1):30. [Google Scholar]
- [11].Hao ML. Transient cerebral ischemia and ischemic tolerance. Zhongguo Shiyong Shenjing Jibing Zazhi. 2006;9(4):166. [Google Scholar]
- [12].Wang ZH, Yan GH, Ma X, et al. Induction time window of mice brain ischemic tolerance. Heilongjiang Keji Xinxi. 2011;6(34):80–81. [Google Scholar]
- [13].Yao X, Miao W, Li M, et al. Protective effect of albumin on VEGF and brain edema in acute ischemia in rats. Neurosci Lett. 2010;472(3):179–183. doi: 10.1016/j.neulet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [14].Wang YQ, Cui HR, Yang SZ, et al. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem Int. 2009;55(7):629–636. doi: 10.1016/j.neuint.2009.06.007. [DOI] [PubMed] [Google Scholar]
- [15].Li B, Piao CS, Liu XY, et al. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res. 2010;1327:91–102. doi: 10.1016/j.brainres.2010.02.030. [DOI] [PubMed] [Google Scholar]
- [16].Hoyte LC, Papadakis M, Barber PA, et al. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res. 2006;1121(1):231–237. doi: 10.1016/j.brainres.2006.08.107. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Q, Zhang YD. PAI-1 and thrombosis in rats. Guowai Yixue: Naoxueguan Jibing Fence. 1999;7(4):218–221. [Google Scholar]
- [18].Yarnell JW, Sweetnam PM, Rumley A, et al. Lifestyle and hemostatie risk factors for isehemie heart disease: the Caerphilly Study. Arterioscler Thromb Vasc Biol. 2000;20(3):271–279. doi: 10.1161/01.atv.20.1.271. [DOI] [PubMed] [Google Scholar]
- [19].Qin QY, Chen JJ, Zhang QF, et al. Alteration of thromboxane A2 and prostacyclin levels in rats with obstructive jaundice and the effect of saiviae miltiorrhizae on them. Zhongguo Zhongxiyi Jiehe Zazhi. 2010;3(3):283–285. [PubMed] [Google Scholar]
- [20].Wang XD, Zhao YD. Beijing: People's Military Medical Press; 2001. Neurology, Nervous System Injury. [Google Scholar]
- [21].Xiong SQ, Lian QZ, Chen J, et al. Research on the related factors at different stages of blood stasis syndrome of tcm for cerebrovascular disease. Zhongguo Weixunhuan. 2002;6(4):234–237. [Google Scholar]
- [22].Xin N, Feng J, Yao B. Persimmon leaf flavones extraction separation and pharmacological actions. Zhongyiyao Xuebao. 2007;2(35):49–51. [Google Scholar]
- [23].Yang JX, Yuan JF, Li FR. The anti-oxidative effect of persimmon leaf flavonoid in vitro. Yingyang Xuebao. 2003;25(2):215–217. [Google Scholar]
- [24].Wang GX, Liang CJ, Yao JY, et al. Effect of a novel fibrinolytic enzyme FA-I on thrombosis and thrombolysis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40(2):288–291. [PubMed] [Google Scholar]
- [25].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [26].Gao YF, Gao YS, Zhang YL. Protective effect of. Persimmon leaf flavonoids on the liver in diabetic mice. Zhongguo Shiyong Yixue. 2009;4(27):12–13. [Google Scholar]
- [27].Fang XY, Sun JN, Miao MS. Influences of cerebral ischemic preconditioning on content of brain IL-1β,TNF-α and IL-10 in rats with second cerebral ischemic lesion at different time points. Beijing Zhongyiyao Daxue Xuebao. 2010;8(33):537–540. [Google Scholar]
- [28].Dawson SJ, Wiman B, Hamsten A, et al. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1)gene responsed differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268(15):10739–10745. [PubMed] [Google Scholar]
- [29].Chmielewska J, Ranby M. Evidence for a rapid inhibitor to tpainplasma. Throm Res. 1983;31(5):427. doi: 10.1016/0049-3848(83)90407-3. [DOI] [PubMed] [Google Scholar]
- [30].Gao DY. Suzhou: Soochow University; 2004. The Study of the changes and their significances of GMP140, TXB2, 6-keto-PGF1α plasma concentrations of dog models with carotid atherosclerosis. [Google Scholar]


