Abstract
Insular lesions remain surgically challenging because of the need to balance aggressive resection and functional protection. Motor function deficits due to corticospinal tract injury are a common complication of surgery for lesions adjacent to the internal capsule and it is therefore essential to evaluate the corticospinal tract adjacent to the lesion. We used diffusion tensor imaging to evaluate the corticospinal tract in 89 patients with insular lobe lesions who underwent surgery in Chinese PLA General Hospital from February 2009 to May 2011. Postoperative motor function evaluation revealed that 57 patients had no changes in motor function, and 32 patients suffered motor dysfunction or aggravated motor dysfunction. Of the affected patients, 20 recovered motor function during the 6–12-month follow-up, and an additional 12 patients did not recover over more than 12 months of follow-up. Following reconstruction of the corticospinal tract, fractional anisotropy comparison demonstrated that preoperative, intraoperative and follow-up normalized fractional anisotropy in the stable group was higher than in the transient deficits group or the long-term deficits group. Compared with the transient deficits group, intraoperative normalized fractional anisotropy significantly decreased in the long-term deficits group. We conclude that intraoperative fractional anisotropy values of the corticospinal tracts can be used as a prognostic indicator of motor function outcome.
Keywords: neural regeneration, neuroimaging, magnetic resonance imaging, diffusion tensor imaging, fractional anisotropy, insular lobe, corticospinal tracts, motor function, grants-supported paper, neuroregeneration
Research Highlights
(1) Diffusion tensor imaging could evaluate the integrity and function of the conduction system. There are few previous studies of the relationship between diffusion tensor imaging results and clinical prognosis.
(2) This study analyzed the relationship between diffusion tensor imaging results and motor function by assessing the integrity of the corticospinal tracts in patients with insular lesions.
(3) Intraoperative diffusion tensor imaging predicted motor function in patients with insular lesions. Acquiring diffusion tensor imaging early postoperatively helps to predict motor function. Continued follow-up of diffusion tensor imaging is warranted to explore the tendency of postoperative rehabilitation.
INTRODUCTION
Aggressive resection of tumors may result in longer patient survival. Volumetric assessment of the extent of resection found that gross total resection of tumors is associated with a low recurrence rate, and long-term tumor control[1,2,3,4]. The goal in caring for patients with lesions adjacent to the internal capsule is not only to maximize resection of the tumor, but also to maximize the duration of high-quality life by optimally balancing the risks of recurrence or progression of the tumor with surgically related adverse outcomes. Aggressive surgical strategies should be attempted only if the associated risk of new neurologic deficits can be minimized, and if methods are used to achieve maximal and safe resections. The use of intraoperative imaging systems and neuronavigation has made aggressive resection of tumors safer than ever[5,6,7]. However, lesions involving the insular region remain surgically challenging due to the close proximity to the internal capsule, complex surrounding functional structures, including arteries and centers critical for language. Given the potential involvement of essential neural networks, controversy persists as to which strategy is appropriate for patients with insular lesions and how interventions can affect patient outcome. Several prognostic factors have been identified, including extent of resection, pathological diagnosis of lesions, patient age and functional status[8,9]. However, motor function deficits due to corticospinal tract impairment are a common complication of lesions adjacent to the internal capsule[10]. Surgical manipulation close to the internal capsule frequently leads to impairment of the corticospinal tract. Moreover, it is not easy to evaluate whether the motor function deficit is long-term or transient. For these reasons, it is essential to evaluate the corticospinal tract adjacent to the lesion for prognostic evaluation.
Diffusion tensor imaging evaluates the integrity of white matter tracts by imaging water diffusion characteristics[11]. Diffusion tensor imaging and diffusion tensor tractography are the current methods used to visualize specific white matter tracts in vivo[12]. Many authors have reported encouraging results in corticospinal tract protection during surgery by integrating tractography of the corticospinal tract into a neuronavigation system[13,14]. Previous studies have demonstrated that diffusion tensor tractography is a useful technique for evaluating injuries of neural tracts in patients with traumatic axonal injury and stroke[15,16,17,18]. Diffusion tensor metrics, such as fractional anisotropy and apparent diffusion coefficient, have also been widely studied in neurological disease[14,15,16,17]. Previous studies found a positive correlation between the clinical presentation and fractional anisotropy values and abnormal fractional anisotropy values have also been reported as predictive indicators for brain injury[16,19]. Diffusion tensor imaging-based fiber tracking and metrics have confirmed that a tract in question remains intact, and in this state may facilitate the fiber tracking preoperatively and postoperatively[19]. Compared with the normalized fractional anisotropy values for patients with normal motor function, patients with abnormal motor function demonstrated significantly decreased fractional anisotropy. However, studies of correlations between intraoperative diffusion tensor imaging findings and clinical outcomes are lacking. For these reasons, we retrospectively analyzed the preoperative, postoperative and follow-up diffusion tensor images of the corticospinal tract in a series of patients and compared them with the patients’ motor function before and after surgical resection.
RESULTS
Quantitative analysis of subjects
We included 91 patients undergoing surgery for benign insular lobe lesions and low-grade tumors. Two patients were lost to follow-up and one refused continuous follow-up. An additional patient visited a local hospital where the MR imaging did not match our protocol. A total of 89 patients were included in the final analysis.
General data
Among the 89 patients, 51 were male and 38 were female; the mean age was 43 ± 13 years (range, 16–68 years). The most common pathological diagnosis was astrocytoma (37 patients, 42%), followed by oligodendroglioma (26 patients, 29%), oligoastrocytoma (13 patients, 14%), cavernoma (nine patients, 10%) and other (four patients, 5%, including two cases of neuronal and mixed neuronal/glial tumors, and two cases of arteriovenous malformation). The majority of the lesions involved the temporal lobe (57 cases, 64%); 47 lesions (53%) involved the frontal lobe; and 21 lesions (24%) were located only in the insular lobe. Patients with no recurrence were censored as of their last imaging data. At admission, the majority of patients presented with headache (61 patients, 68%), while 15 cases (17%) presented with motor function deficits; one presented with facial muscle deficits; other symptoms included seizure, sensory deficit, and language deficit. No adverse intraoperative events or death occurred.
Motor function examination results
At the postoperative motor function evaluation, 32 patients (36%) had new or worsened scores. Twenty-nine (33%) of the 32 patients had new or worsened weakness in the contralateral extremities. Seven of these 29 patients had complete recovery when discharged from the hospital, and eight patients (9%) had new or worsened facial weakness. Twelve months later, motor function examination follow-up showed that 20 of the 32 patients who had motor function deterioration postoperatively achieved complete functional recovery. In the remaining 12 cases, only one suffered from continued mild facial palsy.
We divided the 89 patients into three subgroups based on the results of the motor function examination as follows: 1) stable group: 57 patients (64%) displayed no postoperative motor function grade decrease; 2) transient deficits group: 20 patients (22%) had new motor function deficits or worsened extremities and/or facial weakness after operation, and achieved a complete recovery by the time of their last follow-up visit after 6–12 months; 3) long-term deficits group: 12 (14%) patients had new motor function deficits and/or weakness postoperatively and over a 12-month follow-up obtained incomplete recovery of their motor function compared to the preoperative status.
Changes in fractional anisotropy at various time periods in patients with different prognosis
Twenty patients from the stable group were selected for the subsequent analyses. Normalized fractional anisotropy values from the preoperative, intraoperative and 6 to 12 months follow-up data are shown in Table 1.
Table 1.
Normalized fractional anisotropy (FA) in each group at different time periods
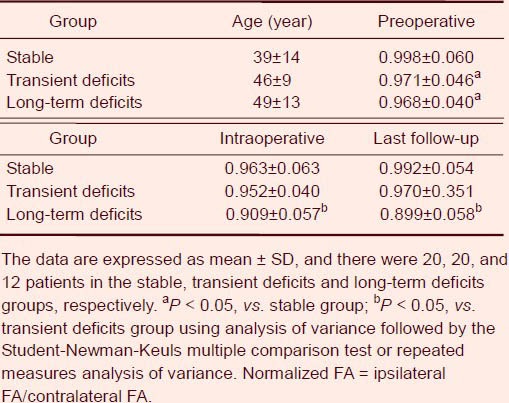
Univariate analysis of variance showed no significant difference in age in the three subgroups, but a significant difference in normalized fractional anisotropy among the groups was found (P < 0.05). Multiple comparisons demonstrated that the normalized fractional anisotropy preoperatively in the stable group was higher than in the transient deficits group or in the long-term deficits group (P < 0.05). However, no significant difference was found between the transient deficits group and the long-term deficits group (P > 0.05). Considering the different growth patterns of the lesions, we calculated the change rate compared to the preoperative normalized fractional anisotropy. Repeated measures analysis of variance revealed a significant difference in change ratio of normalized fractional anisotropy values between the transient deficits group and the long-term deficits group (P < 0.05), and a difference between stages (P < 0.05). Compared with the transient deficits group, intraoperative fractional anisotropy significantly decreased in the long-term deficits group.
Illustrative cases
Case 1: A 48-year-old male (Figure 1) experienced headaches for 5 days, and was pathologically diagnosed as ganglioglioma. Preoperative T2-weighted imaging revealed a diffuse lesion extending from the right temporal lobe to the insular lobe (Figure 1A). We performed fiber tracking based on preoperative, intraoperative and 12-month follow-up diffusion tensor imaging. Preoperative, intraoperative and follow-up normalized fractional anisotropy values were 0.986, 0.921 and 0.952, respectively. Postoperative and follow-up examination found no weakness.
Figure 1.
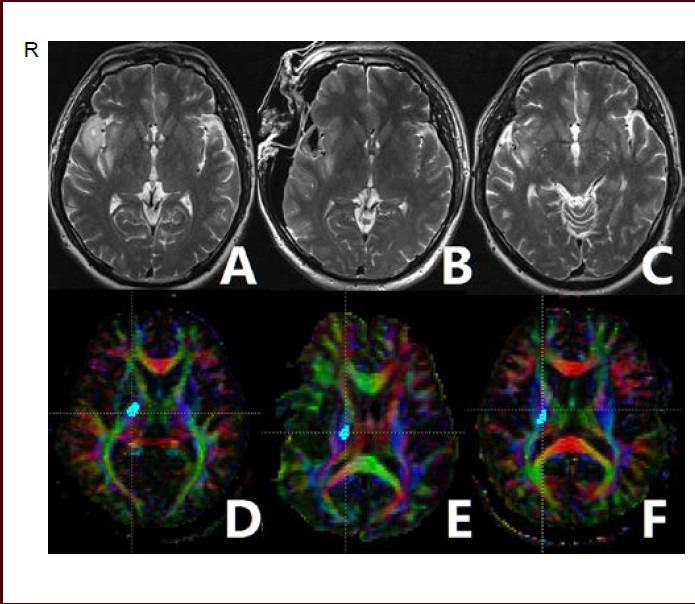
A 48-year-old male patient with right temporal and insular lobe ganglioglioma.
Preoperative T2-weighted imaging (T2WI) (A) showing that the tumor involved the right insular lobe. Intraoperative (B) and 12-month follow-up (C) T2WI showing that the tumor was resected. Fiber tracking based on preoperative (D), intraoperative (E), and follow-up (F) diffusion tensor imaging was performed.
Red indicates a predominant left-right anisotropic diffusion gradient; green represents an anterior-posterior gradient; and blue represents a superior-inferior gradient orientation. R: Right.
Case 2: A 39-year-old male (Figure 2) suffering from chronic headaches for 1 year with no other neurological deficits, pathologically diagnosed as having oligodendroglioma. Preoperative MRI revealed that the lesion was located in the left insular lobe causing ipsilateral internal capsule compression. Corticospinal tract reconstruction revealed compression and displacement of the preoperative corticospinal tract. Preoperative, intraoperative and follow-up normalized fractional anisotropy values were 1.024, 0.933 and 0.912, respectively. Postoperative and 12-month follow-up motor function examinations revealed modest weakness in the contralateral extremities (muscle test 3/5).
Figure 2.
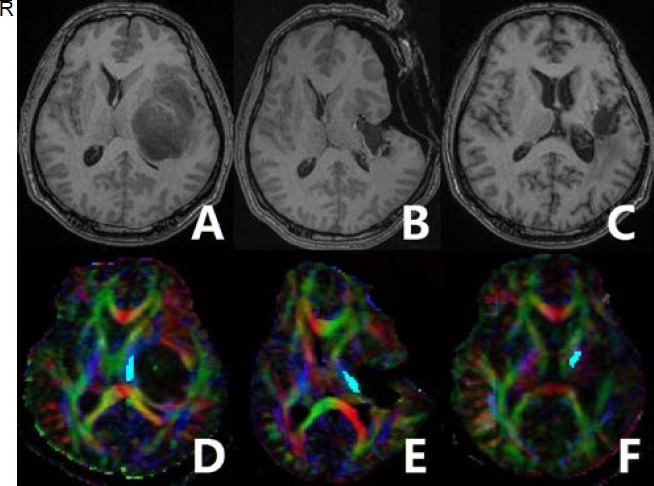
A 39-year-old male olibgodendroglioma patient with long-term motor function deficits.
Preoperative T1-weighted imaging (T1WI) (A) showing a lesion located in the left insular lobe. The left internal capsule was compressed. Intraoperative (B) and 12-month follow-up (C) T1WI showing that the tumor was resected. Preoperative diffusion tensor imaging-based fiber tracking showing that the corticospinal tract was markedly displaced to the contralateral side (D). Fiber tracking based on preoperative (D), intraoperative (E), and follow-up (F) diffusion tensor imaging was performed.
Red represents a predominant left-right anisotropic diffusion gradient; green represents an anterior-posterior gradient; and blue represents a superior-inferior gradient orientation. R: Right.
DISCUSSION
Surgical treatment of lesions adjacent to the internal capsule carries significant risks of extremity paresis or speech disturbances because of the location.
Aggressive resection cannot be advocated without evidence of an improved outcome. The development of intraoperative techniques that involve tractography and functional magnetic resonance imaging in conjunction with neuronavigation resulted in a significant increase in the extent of resection[20]. High-field intraoperative MRI-guided neurosurgery provides high-quality anatomic and functional images for interpretation. During the resection of intracranial lesions involving eloquent areas, intraoperative judgment regarding extended resection and the visualization of these areas are crucial. To overcome this problem, immediate MRI resection control procedures have been devised[21,22,23]. The implementation of immediate MRI in standard neurosurgical procedures has been widely accepted because of the benefit of immediate confirmation of the extent of resection and the location of major fiber tracts, making additional tumor resection possible. Nimsky et al[5] reported, with this system, that the extent of resection at the completion of the surgery was significantly increased from 76% to 96%. Using this system, safe resection and precise evaluation of the risk of postoperative complications can be accomplished. However, evaluating an individual's prognosis after neurosurgery remains difficult. Therefore, we sought to evaluate the integrity of corticospinal tracts in patients with insular lesions and to measure the related parameters based on diffusion tensor imaging, and to analyze whether the image results related to the motor function prognosis.
The role of diffusion tensor imaging in neurosurgical planning and postoperative follow-up is currently being defined. We used fractional anisotropy, which represents the ratio of the anisotropic component of the diffusion tensor to the whole diffusion tensor because it has been reported to be the best rotationally invariant scalar metric for measuring diffusion anisotropy[24]. Basser and Pierpaoli[24] attempted to validate preoperative and postoperative diffusion tensor imaging findings by comparing them to physical examination findings. The relationship between fractional anisotropy and apparent diffusion coefficient values and preoperative deficits in patients with brain tumors adjacent to the main white matter tracts has been reported. The appearance of corticospinal tracts on diffusion tensor imaging demonstrated excellent correlation with findings on motor examination[18,25,26,27]. In all patients, preoperative corticospinal tract involvement determined using diffusion tensor imaging was predictive of the presence of motor deficits, and postoperative corticospinal tract normalization on diffusion tensor imaging was predictive of clinical improvement[28]. Fractional anisotropy values have been studied as an early stage indicator in evaluation of motor function deficits in many neurological diseases[29,30,31]. Despite these findings, there are no reports on the clinical utility of intraoperative diffusion tensor imaging in neurosurgical patients for the evaluation of motor function.
Compared with previous similar studies focused on lesions adjacent to the internal capsule, clinical outcomes were unchanged in 72–85% of cases; worsened hemiparesis occurred in 15–28% and were long-term in 10–20%[10,32,33]. In our study, postoperative motor function examination revealed that 64% of the cases were unchanged or improved. A possible reason was the more aggressive surgical strategy we adopted using intraoperative MRI. However, we did not see a greater number of cases suffering from long-term motor function deficits than previous studies.
Using the intraoperative MRI system, we acquired the diffusion tensor images immediately. Therefore, we minimized blood flow disturbances and postoperative edema. Fractional anisotropy was measured after reconstruction of the corticospinal tracts and we divided patients into three subgroups according to the motor function changes. Univariate analysis of variance results confirmed that preoperative normalized fractional anisotropy in the stable group was higher than in the transient deficits and long-term deficits groups. Possible reasons are the differences in size, location, growth pattern or distance to the corticospinal tract[34]. Also, patients with high-grade histology and malignant tumors were excluded because the biological behavior of high-grade tumors is more aggressive and the natural history is shorter than for low-grade tumors. In a previous study, differences in diffusion tensor imaging fiber tracking in two pathological tumors showed how the pathological process affects the fibers[35]. Tumors produce a decrease in the fiber representation because of a decrease in the diffusivity[36,37]. In most cases, glioma cells invade normal tissue by migrating along the white matter fibers[36,37]. This type of tumor invasion affects water molecule diffusion and results in a mild decrease in the fractional anisotropy value and the volume of the reconstructed corticospinal tract[34]. Also, in some tumors and benign lesions, the fractional anisotropy value of the ipsilateral side is higher than the contralateral. This can be explained by the mass effect on the corticospinal tract from compression by the noninvasive tumor[26]. Tumor-affected corticospinal tracts may exhibit several different patterns of structural alteration on diffusion tensor imaging, and these patterns are not yet completely explained[34,38]. For these reasons, in this study, the fractional anisotropy values were transformed into a change ratio of intraoperative to follow-up normalized fractional anisotropy versus preoperative values. The stable group was not included in repeated measures analysis of variance. The results revealed that compared to the transient deficits group, patients in the long-term deficits group had a significant decrease in intraoperative normalized fractional anisotropy value and lower rebound. It appears that the reduction in the ratio of normalized fractional anisotropy reflected irreversible corticospinal tract impairment and presented as poor motor function outcome. However, the patients in the transient deficits group showed a mild reduction in the ratio of normalized fractional anisotropy and presented with immediate postoperative motor function deterioration, but had a complete recovery in 12 months. The reduction in fractional anisotropy in these patients might be due to reversible injury or disturbance related to the resection. These results implied that the change ratio of intraoperative normalized fractional anisotropy might be a predictive factor for motor function outcomes in patients with lesions adjacent to the corticospinal tract.
Diffusion tensor imaging allows noninvasive mapping of white matter tractography and has been widely used in neurology; however, some technical limitations remain. Using diffusion tensor imaging-based fiber tracking, we successfully reconstructed all corticospinal tracts in our patients. The accuracy of this technique remains controversial in the literature, because of the limitations inherent in diffusion tensor imaging and fiber tracking[39,40,41]. Some authors found that this method cannot precisely reflect the true boundary of corticospinal tracts, especially the corticospinal tract adjacent to the peritumoral area[42,43]. However, others confirmed that diffusion tensor imaging-based fiber tracking can reflect the course of the fiber tracts accurately. The densely packed pattern of the corticospinal tract at the internal capsule was the most suitable location for the deterministic fiber tracking algorithm and patients were excluded if their corticospinal tracts were affected excessively by the tumor or edema[33,44,45]. Diffusion tensor tractography is software- and operator-dependent. It is important to emphasize that a low fractional anisotropy value is not equal to the absence of corticospinal tracts; corticospinal tracts may be in their normal location, but not detected by diffusion tensor imaging techniques[45]. Also, diffusion tensor tractography represents the major trajectories only, and does not represent all of the fiber tracts[46]. It is difficult to solve the problem of crossing fibers[46]. The superior longitudinal fasciculus crosses the corticospinal tract and makes it difficult to track exactly. In fact, the results of the diffusion tensor imaging fiber tracking technique are a function of the fractional anisotropy cut-off value which is the result of a compromise between the ability to detect as many fibers as possible and still obtain a consistent fiber tracking[35]. The absence of a bundle in the tracking technique does not imply that the tract is anatomically destroyed. Another issue in the study of diffusion tensor imaging is whether the diffusion tensor imaging is performed in the late or immediate period. The quality of intraoperative diffusion tensor imaging in neurosurgical tumor resections has been widely discussed[47]. Echo planar imaging distortion is more obvious when approaching the cortex and brain stem. Given the high vulnerability of echo planar imaging to susceptibility-related artifacts and the frequent presence of both air and blood products in the resection cavity soon after surgery, later scanning might provide better results[28]. Additionally, the superficial portion of the tract leads to problems related to distortion, especially from air-related susceptibility artifacts[48]. Nevertheless, a previous study showed that an open skull did not interfere with visualization of the corticospinal tract[49]. These results are associated with the fact that deep brain structures near the tract location display fewer artifacts[20,48,50]. Therefore, a study of the corticospinal tract in this area should be reliable.
We used intraoperative diffusion tensor imaging to perform a prognosis evaluation of motor function; however, using diffusion tensor imaging early postoperatively is also helpful to predict motor function. Our study confirmed that follow-up diffusion tensor imaging could help explore the trends in recovery. Larger case numbers would be helpful to confirm our results and allow for multi-parameter correlation analysis.
We found significant differences in the change ratio of the intraoperative normalized fractional anisotropy in corticospinal tracts when we compared transient deficits patients and long-term deficits patients. However, the identification of universally applicable prognostic factors remains a challenge. Although there are many problems with the technique, the use of the fractional anisotropy value of intraoperative diffusion tensor imaging as a prognostic indicator of motor function is feasible.
SUBJECTS AND METHODS
Design
A retrospective study in radiology.
Time and setting
Experiments were performed from February 2009 to June 2012 in the Magnetic Resonance Laboratory, Department of Neurosurgery, Chinese PLA General Hospital, China.
Subjects
We collected data retrospectively on patients undergoing operation with benign lesions and low-grade tumors (World Health Organization grade I–II[51]) from February 2009 to May 2011 in the Chinese PLA General Hospital.
Inclusion criteria: patients undergoing surgery at an intraoperative MR imaging-guided therapy facility. The lesion was located in the insular lobe and seen on fluid attenuated inversion recovery images as an abnormal intensity signal. Low-grade tumors (World Health Organization grade I–II) and benign lesions were included. Pathological diagnosis was performed according to the World Health Organization guidelines[51]. Finally, patients were followed up for 6–12 months to observe their motor function and to acquire MRI data.
Exclusion criteria: World Health Organization grade III–IV gliomas and other malignant tumors were excluded because their biology and natural history differ substantially from low-grade tumors and benign lesions; their inclusion increased the cases lost to follow-up and therefore led to a selection bias. Cases with acute hemorrhage were excluded because of the serious progressive impairment of the corticospinal tracts which could not be distinguished from surgical disturbance. Noncooperative patients were also excluded. Functional outcomes were determined at least 6 months after operations, and deficits lasting for more than 12 months were considered a long-term deficit.
A total of 91 patients were involved. Informed consent was given by participants and family members, and the study was conducted in accordance with the Declaration of Helsinki.
Methods
Motor function examination
Preoperatively, postoperatively (72 hours after operation) and at each follow-up appointment, patients underwent neurological examination by two neurosurgeons. Motor function deterioration was defined as new-onset or worsening deficits related to the strength of the extremity muscles and facial muscles. Outpatient quantified neurological examinations were performed at 1–3 months, 6 months, and 12 months after surgery. Extremity motor function examination was conducted according to a muscle strength grading scale[52]. The patient's effort was graded on a scale of 0–5: Grade 5: Muscle contracts normally against full resistance. Grade 4: Muscle strength is reduced, but muscle contraction can still move the joint against resistance. Grade 3: Muscle strength is further reduced such that the joint can be moved only against gravity with the examiner's resistance completely removed. Grade 2: Muscle can move only if the resistance of gravity is removed. Grade 1: Only a trace or flicker of movement is seen or felt in the muscle or fasciculations are observed in the muscle. Grade 0: No contraction is observed (total paralysis). Pathological reflex was included in the evaluation. The National Institute of Health Stroke Scale was used in facial palsy examination[53]: 1) normal symmetrical movements; 2) minor paralysis: flattened nasolabial fold, asymmetry on smiling; 3) partial paralysis: total or near-total paralysis of the lower face; 4) complete paralysis of one or both sides (absence of facial movement in the upper and lower face).
Image acquisition
Images were acquired using a 1.5 T MR (Siemens Espree, Erlangen, Germany). All the MR images were collected using the same 1.5 T scanner and the same protocol. For diffusion tensor imaging, we applied a single-shot, spin-echo, diffusion-weighted echo planar imaging sequence (echo time, 147 ms; repetition time, 9 400 ms; matrix size, 128 × 128; field of view, 231 mm × 231 mm; slice thickness, 2.7 mm; bandwidth, 1 502 Hz per pixel; diffusion encoding gradients in 12 directions using b values of 0 and 1 000 s/mm2; and voxel size, 1.8 mm × 1.8 mm × 2.7 mm). We used 45 slices, no intersection gap, 45 continuous free interval collection slices, and four repetitions. This sequence was based on a balanced diffusion gradient design which strongly minimizes eddy current artifacts compared to a single-refocused design. Several preprocessing steps were initiated before fiber tracking including eddy current correction and the calculation of the apparent diffusion coefficient and fractional anisotropy maps. Co-registered Magnetization-Prepared Rapid Gradient Echo images were recorded for anatomical guidance[26].
Image processing
The diffusion tensor imaging datasets were transferred to a PC with Windows platform. The Digital Imaging and Communications in Medicine files were converted to the 4D NifTI image format for analysis using the dcm2nii program (University of South Carolina, Columbia, SC, USA; www.mricro.com). We used the analysis software, diffusion tensor imaging Studio (Hangyi Jiang and Susumu Mori, Johns Hopkins University and Kennedy Krieger institute; http://godzilla.kennedykrieger.org or http://lbam.med.jhmi.edu) to process the data. Images were first realigned using the automatic image registration program to remove any potential small bulk motions that occurred during the scans. Subsequently, all diffusion-weighted images were visually inspected by the authors for apparent artifacts due to subject motion and instrumental malfunction. The six elements of the diffusion tensor were calculated for each pixel using multivariate linear fitting. After tensor diagonalization, three eigenvalues and eigenvectors were obtained and fractional anisotropy maps were calculated. The eigenvector associated with the largest eigenvalue was used as an indicator for fiber orientation. In the diffusion tensor imaging color maps, the spatial direction of the mean anisotropic diffusion gradient in each voxel was displayed graphically using different colors; red indicated a predominant left-right anisotropic diffusion gradient; green indicated an anterior-posterior gradient; and blue indicated a superior-inferior gradient orientation.
Tractography metrics
Fiber tracking was performed using diffusion tensor imaging Studio. To visualize and evaluate the tracks, we used images commonly referred to as fractional anisotropy maps which were displayed as gray-scale maps. Areas with high degrees of fractional anisotropy were bright and areas with low degrees of fractional anisotropy were dark. We placed the regions of interest according to previously proposed protocols as follows (Figure 3): one seed region included the ipsilateral cerebral peduncle in an axial plane at the level of the decussation of the superior cerebellar peduncle (Figure 3A), which was located using anatomical landmarks, and the second seed region was placed on the precentral gyrus (Figure 3B)[7,46]. The reconstructed tracts of interest were obtained by excluding the fibers, which were inappropriately identified based on anatomical knowledge of tract trajectories. We used a continuous tracking algorithm for fiber assignment. A minimum fractional anisotropy value of 0.2 and an inner product threshold of 0.70 were used as the fiber termination criteria, in agreement with generally accepted practice based on typical fractional anisotropy values observed in gray and white matter[46]. After the tracts were reconstructed, the mean fractional anisotropy was calculated over the tract[54]. Corticospinal tract reconstruction and fractional anisotropy measurements were performed for both hemispheres. The fractional anisotropy was normalized as a relative value compared with the contralateral normal side[36].
Figure 3.
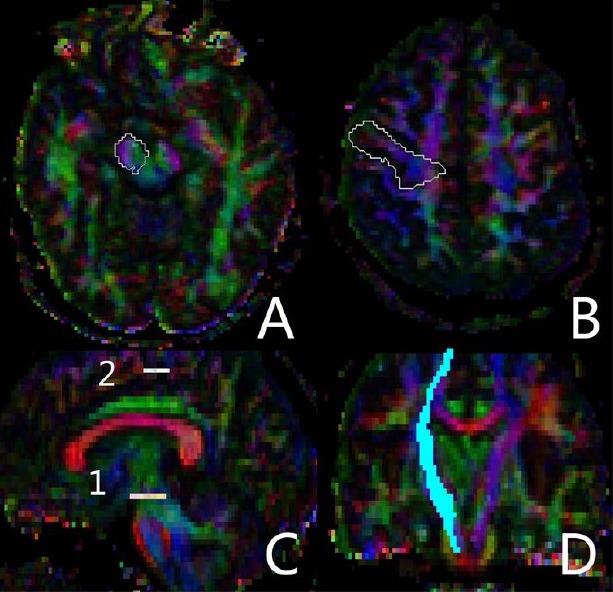
Identification of the region of interest in diffusion tensor images during fractional anisotropy measurement.
In the two regions of interest, one seed region included the ipsilateral cerebral peduncle in an axial plane at the level of the decussation of the superior cerebellar peduncle (A), and the second seed region was placed on the precentral gyrus (B); Sagittal view showing the level of the two regions of interest (C), and coronal view showing the reconstructed corticospinal tract (D).
Statistical analysis
Statistical analysis were performed using SPSS software (version 17.0, SPSS, Chicago, IL, USA). A univariate analysis of variance was used for the mean difference to test age and the normalized fractional anisotropy among groups with different motor function outcomes. When a significant difference was found, the means were compared by the Student-Newman-Keuls multiple comparison test. Repeated measures analysis of variance was used to detect significance when comparing changes in the normalized fractional anisotropy value in the transient deficits group and the long-term deficits group. A P value < 0.05 was considered statistically significant.
Acknowledgments:
We would like to thank Xinguang Yu, Jun Zhang, Zhenghui Sun, Jinli Jiang, Xiaodong Ma, Bo Bu and Ruyuan Zhu, from the Department of Neurosurgery, Chinese PLA General Hospital in China for their collaborative support.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by the National Natural Science Foundation of China, No. 30800349; and the Natural Science Foundation of Beijing, No. 7102145.
Ethical approval: This pilot project was approved by the Medical Ethics Committee of the Chinese PLA General Hospital, China.
(Reviewed by Charbonneau J, Rave W, Miao YW, Yang JP)
(Edited by Wang LM, Qiu Y, Li CH, Song LP)
REFERENCES
- [1].Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome oflow-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- [2].Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109(5):835–841. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- [4].Bradley WG. Achieving gross total resection of brain tumors: intraoperative MR imaging can make a big difference. AJNR Am J Neuroradiol. 2002;23(3):348–349. [PMC free article] [PubMed] [Google Scholar]
- [5].Nimsky C, Kuhnt D, Ganslandt O, et al. Multimodal navigation integrated with imaging. Acta Neurochir Suppl. 2011;109:207–214. doi: 10.1007/978-3-211-99651-5_32. [DOI] [PubMed] [Google Scholar]
- [6].Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus. 2010;28(2):E1. doi: 10.3171/2009.12.FOCUS09266. [DOI] [PubMed] [Google Scholar]
- [7].Nossek E, Korn A, Shahar T, et al. Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. Clinical article. J Neurosurg. 2011;114(3):738–746. doi: 10.3171/2010.8.JNS10639. [DOI] [PubMed] [Google Scholar]
- [8].Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- [9].Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [10].Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112(1):1–9. doi: 10.3171/2009.6.JNS0952. [DOI] [PubMed] [Google Scholar]
- [11].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [12].Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [13].Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935–948. doi: 10.1227/01.neu.0000303189.80049.ab. [DOI] [PubMed] [Google Scholar]
- [14].Chen X, Xu BN, Meng X, et al. Dual-room 1.5-T intraoperative magnetic resonance imaging suite with a movable magnet: implementation and preliminary experience. Neurosurg Rev. 2012;35(1):95–110. doi: 10.1007/s10143-011-0336-3. [DOI] [PubMed] [Google Scholar]
- [15].Wang JY, Bakhadirov K, Devous MD, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65(5):619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- [16].Little DM, Kraus MF, Jiam C, et al. Neuroimaging of hypoxic-ischemic brain injury. NeuroRehabilitation. 2010;26(1):15–25. doi: 10.3233/NRE-2010-0532. [DOI] [PubMed] [Google Scholar]
- [17].Price SJ, Jena R, Burnet NG, et al. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol. 2007;17(7):1675–1684. doi: 10.1007/s00330-006-0561-2. [DOI] [PubMed] [Google Scholar]
- [18].Romano A, Fasoli F, Ferrante M, et al. Fiber density index, fractional anisotropy, adc and clinical motor findings in the white matter of patients with glioblastoma. Eur Radiol. 2008;18(2):331–336. doi: 10.1007/s00330-007-0740-9. [DOI] [PubMed] [Google Scholar]
- [19].Awasthi R, Verma SK, Haris M, et al. Comparative evaluation of dynamic contrast-enhanced perfusion with diffusion tensor imaging metrics in assessment of corticospinal tract infiltration in malignant glioma. J Comput Assist Tomogr. 2010;34(1):82–88. doi: 10.1097/RCT.0b013e3181ae29f0. [DOI] [PubMed] [Google Scholar]
- [20].Kleiser R, Staempfli P, Valavanis A, et al. Impact of fMRI-guided advanced DTI fiber tracking techniques on their clinical applications in patients with brain tumors. Neuroradiology. 2010;52(1):37–46. doi: 10.1007/s00234-009-0539-2. [DOI] [PubMed] [Google Scholar]
- [21].Gerlach R, du Mesnil de Rochemont R, Gasser T, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63(2):272–285. doi: 10.1227/01.NEU.0000312362.63693.78. [DOI] [PubMed] [Google Scholar]
- [22].Gasser T, Szelenyi A, Senft C, et al. Intraoperative MRI and functional mapping. Acta Neurochir Suppl. 2011;109:61–65. doi: 10.1007/978-3-211-99651-5_10. [DOI] [PubMed] [Google Scholar]
- [23].Forster MT, Hattingen E, Senft C, et al. Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery. 2011;68(5):1317–1325. doi: 10.1227/NEU.0b013e31820b528c. [DOI] [PubMed] [Google Scholar]
- [24].Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- [25].Bobek-Billewicz B, Stasik-Pres G, Majchrzak K, et al. Fibre integrity and diffusivity of the pyramidal tract and motor cortex within and adjacent to brain tumour in patients with or without neurological deficits. Folia Neuropathol. 2011;49(4):262–270. [PubMed] [Google Scholar]
- [26].Stadlbauer A, Nimsky C, Gruber S, et al. Changes in fiber integrity, diffusivity, and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2007;28(3):462–469. [PMC free article] [PubMed] [Google Scholar]
- [27].Yeo SS, Choi BY, Chang CH, et al. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke. 2012;43(8):2239–2241. doi: 10.1161/STROKEAHA.112.661116. [DOI] [PubMed] [Google Scholar]
- [28].Laundre BJ, Jellison BJ, Badie B, et al. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005;26(4):791–796. [PMC free article] [PubMed] [Google Scholar]
- [29].Kwak SY, Yeo SS, Choi BY, et al. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol. 2010;63(3):149–153. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- [30].Jang SH, Byun WM, Han BS, et al. Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: a diffusion tensor image study. Restor Neurol Neurosci. 2006;24(1):25–29. [PubMed] [Google Scholar]
- [31].Albrecht J, Dellani PR, Muller MJ, et al. Voxel based analyses of diffusion tensor imaging in Fabry disease. J Neurol Neurosurg Psychiatry. 2007;78(9):964–969. doi: 10.1136/jnnp.2006.112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neuloh G, Pechstein U, Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg. 2007;106(4):582–592. doi: 10.3171/jns.2007.106.4.582. [DOI] [PubMed] [Google Scholar]
- [33].Talacchi A, Turazzi S, Locatelli F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010;100(3):417–426. doi: 10.1007/s11060-010-0193-x. [DOI] [PubMed] [Google Scholar]
- [34].Stadlbauer A, Polking E, Prante O, et al. Detection of tumour invasion into the pyramidal tract in glioma patients with sensorimotor deficits by correlation of (18)F-fluoroethyl-L-tyrosine PET and magnetic resonance diffusion tensor imaging. Acta Neurochir (Wien) 2009;151(9):1061–1069. doi: 10.1007/s00701-009-0378-2. [DOI] [PubMed] [Google Scholar]
- [35].Giussani C, Poliakov A, Ferri RT, et al. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. Neuroimage. 2010;52(1):217–223. doi: 10.1016/j.neuroimage.2010.03.079. [DOI] [PubMed] [Google Scholar]
- [36].Bello L, Giussani C, Carrabba G, et al. Angiogenesis and invasion in gliomas. Cancer Treat Res. 2004;117:263–284. doi: 10.1007/978-1-4419-8871-3_16. [DOI] [PubMed] [Google Scholar]
- [37].Morita N, Wang S, Kadakia P, et al. Diffusion tensor imaging of the corticospinal tract in patients with brain neoplasms. Magn Reson Med Sci. 2011;10(4):239–243. doi: 10.2463/mrms.10.239. [DOI] [PubMed] [Google Scholar]
- [38].Field AS, Alexander AL, Wu YC, et al. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging. 2004;20(4):555–562. doi: 10.1002/jmri.20169. [DOI] [PubMed] [Google Scholar]
- [39].Berman JI, Berger MS, Chung SW, et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107(3):488–494. doi: 10.3171/JNS-07/09/0488. [DOI] [PubMed] [Google Scholar]
- [40].Burgel U, Madler B, Honey CR, et al. Fiber tracking with distinct software tools results in a clear diversity in anatomical fiber tract portrayal. Cent Eur Neurosurg. 2009;70(1):27–35. doi: 10.1055/s-0028-1087212. [DOI] [PubMed] [Google Scholar]
- [41].Hahn HK, Klein J, Nimsky C, et al. Uncertainty in diffusion tensor based fibre tracking. Acta Neurochir Suppl. 2006;98:33–41. doi: 10.1007/978-3-211-33303-7_6. [DOI] [PubMed] [Google Scholar]
- [42].Kinoshita M, Yamada K, Hashimoto N, et al. Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage. 2005;25(2):424–429. doi: 10.1016/j.neuroimage.2004.07.076. [DOI] [PubMed] [Google Scholar]
- [43].Byrnes TJ, Barrick TR, Bell BA, et al. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed. 2011;24(1):54–60. doi: 10.1002/nbm.1555. [DOI] [PubMed] [Google Scholar]
- [44].Lazar M, Alexander AL. An error analysis of white matter tractography methods: synthetic diffusion tensor field simulations. Neuroimage. 2003;20(2):1140–1153. doi: 10.1016/S1053-8119(03)00277-5. [DOI] [PubMed] [Google Scholar]
- [45].Hou Y, Chen X, Xu B. Prediction of the location of the pyramidal tract in patients with thalamic or Basal Ganglia tumors. PLoS One. 2012;7(11):e48585. doi: 10.1371/journal.pone.0048585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mori S, van Zijl PC. Fiber tracking: principles and strategies-a technical review. NMR Biomed. 2002;15(7-8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- [47].Nimsky C, Ganslandt O, Kober H, et al. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery. 2001;48(5):1082–1091. doi: 10.1097/00006123-200105000-00023. [DOI] [PubMed] [Google Scholar]
- [48].Nimsky C, Ganslandt O, Hastreiter P, et al. Intraoperative diffusion-tensor MR imaging: shifting of white matter tracts during neurosurgical procedures--initial experience. Radiology. 2005;234(1):218–225. doi: 10.1148/radiol.2341031984. [DOI] [PubMed] [Google Scholar]
- [49].Nimsky C, Grummich P, Sorensen AG, et al. Visualization of the pyramidal tract in glioma surgery by integrating diffusion tensor imaging in functional neuronavigation. Zentralbl Neurochir. 2005;66(3):133–141. doi: 10.1055/s-2005-836606. [DOI] [PubMed] [Google Scholar]
- [50].Bozzao A, Romano A, Angelini A, et al. Identification of the pyramidal tract by neuronavigation based on intraoperative magnetic resonance tractography: correlation with subcortical stimulation. Eur Radiol. 2010;20(10):2475–2481. doi: 10.1007/s00330-010-1806-7. [DOI] [PubMed] [Google Scholar]
- [51].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gonnella C. The manual muscle test in the patient's evaluation and program for treatment. Phys Ther Rev. 1954;34(1):16–18. doi: 10.1093/ptj/34.1.16. [DOI] [PubMed] [Google Scholar]
- [53].Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53(1):126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- [54].Lee AY, Shin DG, Park JS, et al. Neural tracts injuries in patients with hypoxic ischemic brain injury: Diffusion tensor imaging study. Neurosci Lett. 2012;528(1):16–21. doi: 10.1016/j.neulet.2012.08.053. [DOI] [PubMed] [Google Scholar]


