Abstract
Traumatic spinal cord injury is often disabling and recovery of function is limited. As a consequence of damage, both spinal cord and brain undergo anatomical and functional changes. Besides clinical measures of recovery, biomarkers that can detect early anatomical and functional changes might be useful in determining clinical outcome—during the course of rehabilitation and recovery—as well as furnishing a tool to evaluate novel treatment interventions and their mechanisms of action. Recent evidence suggests an interesting three-way relationship between neurological deficit and changes in the spinal cord and of the brain and that, importantly, noninvasive magnetic resonance imaging techniques, both structural and functional, provide a sensitive tool to lay out these interactions. This review describes recent findings from multimodal imaging studies of remote anatomical changes (i.e., beyond the lesion site), cortical reorganization, and their relationship to clinical disability. These developments in this field may improve our understanding of effects on the nervous system that are attributable to the injury itself and will allow their distinction from changes that result from rehabilitation (i.e., functional retraining) and from interventions affecting the nervous system directly (i.e., neuroprotection or regeneration).
Keywords: neuroimaging, atrophy, cortical reorganization, disability
The estimate of people living with a spinal cord injury (SCI) has grown to more than 2 million people worldwide (Wyndaele and Wyndaele 2006). This high prevalence is in part due to an increase in the life expectancy of SCI patients—it is now just 10% lower than in the able-bodied population. This is due to improved acute care management, including safe rescue and transportation protocols from the scene of injury to hospital, provision of appropriate and timely spine and spinal cord imaging (i.e., CT and MRI on the same day of trauma), and surgical interventions with coordinated multidisciplinary care (Fehlings and others 2011). In patients with SCI, both voluntary (conscious sensory and motor functions) and involuntary (i.e., autonomic such as bladder and bowel control) control can be impaired. Although neurological deficits can recover to a certain degree within the first year (Fawcett and others 2007), the degree of recovery depends on the extent of the lesion. Whereas SCI patients with complete lesions show primarily segmental improvements close to the level of lesion, incomplete SCI patients have more substantial recovery of the upper and lower limbs (Curt and others 2008). However, about 50% of the subjects with SCI will be left with severe paralysis—for which there is no cure.
In the past decade, a number of agents focusing either on neuroprotection, axonal regeneration, or a combination of the two have emerged with the potential to translate into therapeutic opportunities (Cummings and others 2005; Freund and others 2006; Wang and others 2011). To enable efficient translation, these interventions require safety assessment and the establishment of proof of concept in phase I/II clinical trials (Barkhof and others 2009). To determine the efficacy of agents in a timely and economical manner, biomarkers are required that can be used as surrogate makers of outcome. Recent developments in quantitative neuroimaging of the spinal cord and brain have the potential to detect both anatomical changes and functional reorganization following SCI. There is now a pressing need to validate the accuracy and sensitivity of these MRI biomarkers: to increase our understanding of underlying mechanisms of damage and consequent functional reorganization, to identify potential therapeutic targets, and to track potential treatment-induced changes.
This review will outline the latest findings from neuroimaging studies of the spinal cord and brain following traumatic SCI. We first discuss anatomical changes in the spinal cord following trauma and then the distant changes seen in the brain. We will summarize current understanding of cortical reorganization and finally outline the neurophysiological mechanisms underlying the interrelationships between spinal and brain atrophy, cortical reorganization, and clinical disability.
Imaging the Injured Spinal Cord
Clinical Perspectives
It is essential for therapeutic planning to carefully investigate the level and extent of the lesion and the evolution of secondary processes following traumatic injury. MRI, in conjunction with clinical features, is now the gold standard to guide optimum management following spinal cord injury (Fehlings and others 2011). It provides a clear picture of the often devastating morphological sequelae of a typical spinal cord lesion (Fig. 1). At the epicenter of the injury—reflected by a hyperintense MR signal change that corresponds to edema and hemorrhage—a cyst forms, as early as three weeks after injury. By about six months, the widespread edema, above and below the lesion site, resolves and a well-demarcated cyst appears—representing the final morphological stage of SCI, as detected with conventional MRI.
Figure 1.
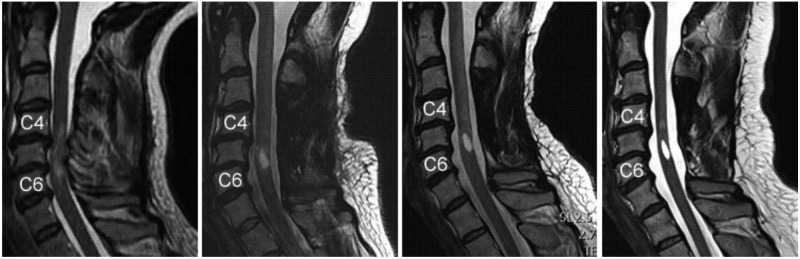
Morphological changes in the human spinal cord can be examined serially by clinical MRI. In this example, the characteristic stages of morphological changes in the spinal cord are apparent based on T2-weighted anatomical images, during the transition from acute to chronic stages of injury. Note the rather diffuse damage seen during the acute phase of spinal cord injury (SCI) (A) with extensive edema spreading from the lesion area, followed by the shrinking of the rostral-caudal boundary (B and C; 3–6 months after SCI), and the eventual formation of a posttraumatic cyst and focal spinal cord atrophy (D; 6–24 months after SCI). Figures modified from Curt (2012) with permission
The impact of cyst formation on neurological status is difficult to evaluate as no serial MRI studies have compared their evolution. Besides looking at the MRI characteristics from a clinical perspective (Curt 2011), there are also substantial caveats from a translational perspective (e.g., instrumentation artifacts, time delay between injury and treatment, etc.). Given that the extent and size of the cord injury is up to 10 times larger (absolute dimensions) than in rodent models of SCI, the challenges for bridging such damaged regions in animal models mimicking human SCI are considerable.
Cross-Sectional Spinal Cord Area Change
Moving beyond routine clinical applications, it is important to quantify the extent and impact on neurological impairment of trauma-induced disruption of the microstructure and macrostructure within the spinal cord. Unlike those of the brain, imaging pathological processes in the injured spinal cord is technically challenging due to the small size of the spinal cord, the inhomogeneous anatomy of the spine, and the difference in magnetic susceptibility between adjacent air- and fluid-filled structures (Andre and Bammer 2010). Orthopedic fixative screws and bolts, which are often used to stabilize the fractured vertebra following traumatic injury, cause a significant MR signal drop out. One strategy to overcome problems caused by fixative artifacts is to assess trauma-induced anatomical changes in spinal segments above the site of trauma.
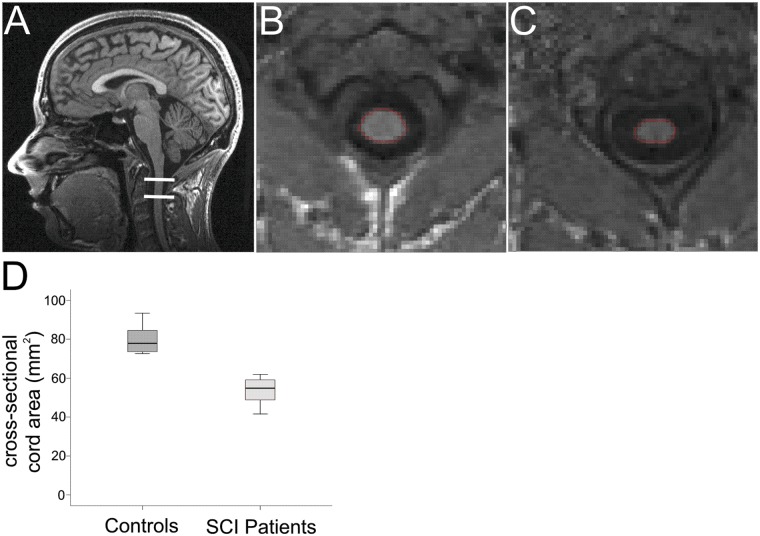
In the field of multiple sclerosis (MS), where the entire spinal cord can be involved, imaging outcomes such as lesion volume, lesion number, and cross-sectional spinal cord area are assessed routinely (Miller and others 2002). The methodology developed to measure cross-sectional spinal cord area in MS at cervical level C2/C3 (Horsfield and others 2010; Losseff and others 1996) has now been applied to SCI (Freund and others 2010b). In brief, the method exploits the regionally specific contrast between the CSF and spinal cord at C2/C3 on conventional three-dimensional T1-weighted anatomical scans. This enables one to calculate the cross-sectional spinal cord area, accurately and automatically (Fig. 2).
Figure 2.
T1-weighted anatomical image covering the brain and cervical spinal cord to simultaneously assess the cross-sectional spinal cord area and cortical gray and white matter volume using voxel-based morphometry (Freund and others 2010b) (A). (B) and (C) show a reformatted axial slice of the cervical cord (C2/C3 level) in one control and one chronic tetraplegic patient, respectively. (D) Cross-sectional spinal cord area comparison between 16 healthy controls and 10 chronic tetraplegic patients. Note significant cord area shrinkage of more than 30% in SCI patients (Freund and others 2011b). Figures modified from Freund and others (2011b) with permission
In chronic SCI patients, a decline of cross-sectional spinal cord area, of between 11% and 30%, has been demonstrated repeatedly (Fig. 2) (Cohen-Adad and others 2011; Freund and others 2011b; Lundell and others 2011a). Given the extensive disease duration of the patients studied (≥14 years), these volumetric changes are likely to represent spinal atrophy—the presumed endpoint of neurodegeneration. Atrophy of the CNS develops as a result of an accumulation of multiple pathophysiological insults over time at and rostral to the lesion (Dusart and Schwab 1994), also involving the brain (Felix and others 2012), which are nonreversible, partly due to the inability of axons to regenerate in the adult CNS (Schwab, 2002). Consequently, information flow between the spinal cord and the brain is impaired, with ensuing clinical disability.
To fully understand this relationship between trauma-induced spinal changes and neurological function, essential for any surrogate marker, longitudinal MRI and clinical studies from the onset of trauma to the chronic stage are required to quantify the spatial and temporal patterns of the underlying pathological processes. Improved MRI techniques to image the spinal cord, with increasingly high anatomical resolution of the cervical spinal cord, now make this possible (Fig. 3). Besides cross-sectional assessment of the spinal cord, these imaging protocols will eventually enable the segmentation of gray and white matter that can be characterized using voxel-based morphometry (VBM) (Ashburner and Friston 2000). Moreover, the link between cord atrophy and disability speak to future assessments in longitudinal studies and the validation of clinical measures within carefully selected cohorts of acute SCI patients (such as the European Multicenter Study in SCI cohort; http://www.emsci.org).
Figure 3.
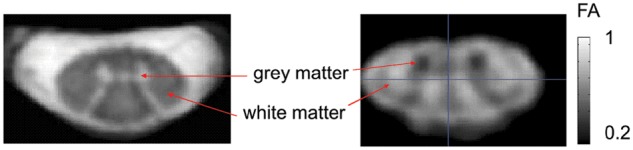
Axial high-resolution images of the human cervical spinal cord. (Left panel) Proton density weighted structural image with 0.25 × 0.25 × 2.5 mm3 resolution was acquired using a multi-echo gradient echo sequence (3D MEDIC [Schmid and others 2005], effective TE = 19 ms, TR = 44 ms, number of averaged acquisitions = 7, total acquisition time = 14.5 min). Spinal gray matter and cerebral spinal fluid appear hyper- and white matter hypo-intense. (Right panel) Fractional anisotropy (FA) map with 0.5 × 0.5 × 5 mm3 resolution was estimated from four multidirectional diffusion tensor imaging scans (TE = 73 ms, b = 500 s/mm2, cardiac gated, total acquisition time 5.8 min on average, depending on the heart rate). To achieve the high resolution while maintaining small image encoding matrices and readout times, the imaged field of view was restricted to the spinal cord by ventral and dorsal saturation bands (Morelli and others 2010). The quality of the estimated FA map was improved by a weighted average of the four scans after correcting for eddy current distortions and subject’s motion (Mohammadi and others 2011; Mohammadi and others 2012)
Axonal Degeneration Assessed with Diffusion Tensor Imaging in the Cervical Cord
Characterizing microstructural abnormality of white matter integrity with diffusion tensor imaging (DTI) can shed light on the relationship between tract-specific changes and clinical status. As with T1-weighted imaging, application of DTI to the cord is technically challenging—due to the small size, physiological motion, and the rapid spatial changes in the spinal column (Andre and Bammer 2010). DTI quantifies the in vivo signal attenuation of water molecules that diffuse in an axial or radial direction, relative to the fibers of the spinal pathways (Basser and Pierpaoli 1996). DTI provides several indices, such as fractional anisotropy (FA), which has been related to axonal count and myelin content (Schmierer and others 2004; Schmierer and others 2007). Moreover, axial diffusivity (AD) and radial diffusivity (RD), which represent the prevalence of water diffusion along or across axons, are thought to reflect the integrity of axons and myelin, respectively (Budde and others 2008). Crucially, AD and RD measured after the onset of an acute event in the spinal cord can help predict functional recovery (Freund and others 2010a; Kim and others 2010).
In acute (Cheran and others 2011) and chronic SCI (Cohen-Adad and others 2011; Petersen and others 2012), AD and RD are altered both at the level and rostral to the lesion—suggesting both axonal degeneration and demyelination of descending and ascending central pathways. Importantly, reduced white matter integrity of specific spinal pathways has been linked to clinical disability (Cohen-Adad and others 2011; Petersen and others 2012) and cortical reorganization (Freund and others 2012). Improving MR pulse sequence design (Thurnher and Law 2009; Wilm and others 2009) and correcting for physiological artifacts (Mohammadi and others 2011; Mohammadi and others 2012) may further improve the quality of these imaging markers (Mohammadi and others 2011; Mohammadi and others 2012). Thus, DTI holds promise to quantify the degree of white matter integrity, to predict recovery, and to monitor the effects of therapeutic interventions.
In summary, there is increasing evidence that the application of structural spinal cord MRI in SCI patients might be at a turning point. Moreover, the continued development of spinal cord imaging with increasing resolution and optimized signal to noise holds great promise to improve our understanding of the relationship between degeneration and reorganization in the spinal cord and, crucially, to clinical recovery.
The Neurobiology Underlying Plasticity in the Injured Spinal Cord
Despite the detrimental impact of trauma, the axonal architecture of the spinal cord undergoes a cascade of dynamic (short term and long term) regenerative mechanisms that have been linked to spontaneous functional recovery (Schwab 2002). An essential anatomical feature—underlying this recovery from SCI—is synaptic plasticity of preexisting connections (Jacobs and Donoghue 1991), rewiring of injured fiber tracts (Ghosh and others 2010), transient down-regulation of the Nogoreceptor-1 signaling cascade (Endo and others 2009), and the formation of new “detour circuits” (Lang and others 2012). Experimental evidence suggests that axonal remodeling and plasticity occurs not only within the spinal cord below and above the lesion but also within the brain both subcortically and cortically. At the cervical level, collateral corticospinal sprouts emerge about 10 days after injury and may connect to interneurons within 3 months (Lang and others 2012). The early formation of corticospinal sprouts is in line with the acute effects on brain activation changes in complete thoracic “lesioned” rodents, where corticospinal hindlimb fibers are rewired—changing into forelimb fibers. Here the cortical representation of the unimpaired, overused forepaw in the ipsilesional cortex was enhanced at 3 months (Ghosh and others 2010). The formation of detour circuits—which encompass the lesion in spared tissue—could reconnect to locomotor circuits, thus enabling afferent input to be processed and conveyed to the cortex (Courtine and others 2008). Indeed, clinical findings suggest that these detour formations could be the substrate for improved spinal reflexes, even below the injury in incomplete SCI patients (Hubli and others 2012).
Promoting regenerative sprouting, to restore function, has been a major goal over decades. Recently, several interventions have entered clinical trials that either aim to protect neurons (e.g., minocycline; Casha and others 2012) or to foster regeneration (e.g., anti-Nogo-A antibody treatment; Freund and others 2006). Treatment-induced evidence for functionally meaningful connectivity is sparse but has been established for regenerating sensory axons (Ramer and others 2000) that reconnect with dorsal horn neurons and for corticospinal axons (Bradbury and others 2002) regenerating below the level of lesion. Moreover, no adverse effects were observed following the anti-Nogo-A antibody treatment in nonhuman primates (Freund and others 2006) and in a clinical phase I trial in SCI patients (http://www.clinicaltrials.gov, NCT00406016).
Imaging Remote Changes in the Brain
Volumetric Changes of White and Gray Matter in the Brain
In contrast to the spinal cord, imaging sequences and analysis software are well established for the analysis of brain structure and function. In the context of SCI, it is important to understand the impact of trauma on remote (central) white and gray matter changes, secondary to those at the local (spinal) level. Voxel-based analysis, such as VBM (Ashburner and Friston 2000) and voxel-based cortical thickness (VBCT) (Hutton and others 2008) are valid and sensitive (and spatially unbiased) automated methods that quantify changes in white and gray matter volume and cortical thinning, respectively. VBM detects significant changes in cortical surface area and thickness, whereas VBCT measures cortical thinning in particular. As a result, the conjoint application of both techniques can provide complementary results in aging (Hutton and others 2009) and disease (Freund and others 2011b).
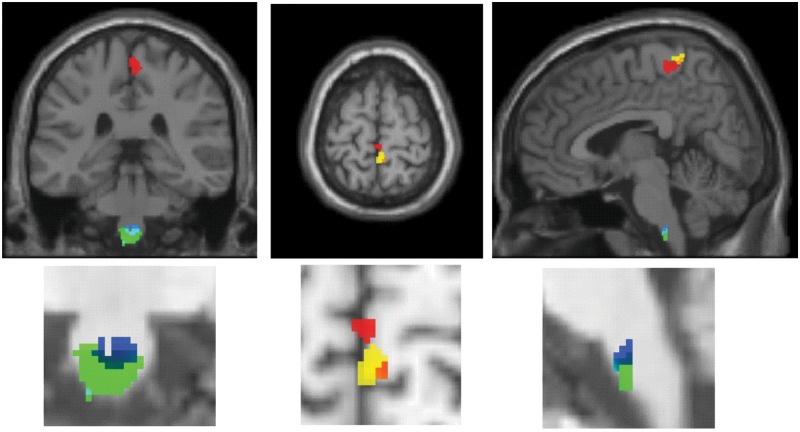
VBM in chronic SCI patients revealed white matter volume decline in spatially distinct areas of the corticospinal tract (CST), such as the bilateral pyramids and the cerebral peduncle (Freund and others 2011b). In line with the decline in white matter volume, volumetric changes of gray matter have been observed specifically in the denervated leg area of the primary motor (M1) and sensory cortex (S1) (Fig. 4) (Freund and others 2011b; Jurkiewicz and others 2006).
Figure 4.
Voxel-based morphometry (VBM) and diffusion tensor imaging detects volumetric (green) and microstructural (cyan) changes in the white matter at the level of the pyramids in chronic spinal cord injury when compared with controls. VBM of the gray matter and cortical thickness reveal changes in the denervated leg area of M1 (red) and S1 (yellow) in the same tetraplegic cohort. Figures modified from Freund and others (2011b) and Freund and others (2012) with permission
However, the latter findings have not been confirmed in all studies (Crawley and others 2004; Lundell and others 2011b). Possible explanations for a failure to reproduce these findings might be the nature of underlying volumetric changes assessed by VBM—the changes might not only represent degeneration but also activity dependent changes that are dynamic and follow distinct temporal patterns. Therefore, confounds such as rehabilitative strategies, which differ between centers and groups, might have increased afferent inputs in specific patient cohorts. Also, the effect of the lesion extent—on remote degenerative changes—has not been established but could have also had a significant influence on local volume changes in the different cohorts. Longitudinal studies that start immediately after the injury might elucidate and disentangle changes that are trauma induced, compared with activity-dependent changes due to rehabilitation.
Over and above the observed changes within the motor systems, gray matter volume loss in the medial prefrontal and anterior cingulate cortex has been detected (Wrigley and others 2009). Interestingly, a functional MRI study also found increased brain activations in the subgenual anterior cingulate cortex during emotional processing in SCI subjects (Nicotra and others 2006). The latter areas are known to be crucial for the processing of emotional information. Therefore, decoupling of the brain from the body—leading to changes in brain anatomy—may, besides inducing motor and sensory impairment, also modify emotional processing in SCI patients.
Axonal Degeneration Assessed with DTI in the Brain
As in the spinal cord, DTI measured in the brain allows one to quantify remote changes in the white matter integrity of central pathways (Basser and Pierpaoli 1996) following SCI. Several studies have shown that, besides volumetric changes, alterations of white matter microstructure occur in motor regions and their descending pathways (Fig. 4) (Freund and others 2012; Guleria and others 2008; Henderson and others 2011; Wrigley and others 2009). Using tractography, which enables the graphic reconstruction of white matter fiber pathways (Ciccarelli and others 2008), Wrigley and others showed that, besides a reduction in the integrity of the CST, the corticopontine tract (which is not directly connected to the spinal cord) also shows trauma-related changes. The most likely interpretation for altered DTI indices in descending pathways, such as the CST, is axonal degeneration and demyelination (Budde and others 2008).
Most central pathways are organized bilaterally, yet findings often show asymmetric changes (Freund and others 2012). This asymmetry might relate to compensatory overuse (Elbert and others 1994) of the less affected limb, following SCI, thus contributing to increased integrity in the intact side of the tract. Future studies could address this asymmetry by comparing patients with Brown-Sequard syndrome and patients with an anatomical complete lesion.
In summary, subcortical and cortical degenerative changes can be detected reliably with valid and sensitive neuroimaging techniques. These structural changes are predominantly located in denervated areas of the cortical sensorimotor regions and descending motor tracts. Crucially, the emergent relationships between gray matter volume and disability indicate that treatment strategies should target both the injured spinal cord and the brain.
The Neurobiology Underlying Volumetric Cerebral Cortex Changes
Although VBM, VBCT, and DTI are very sensitive to trauma-induced changes, they are unable to provide information about the biological events leading to atrophy in the brain. Experimental evidence suggests that trauma causes the disintegration of axons, thereby disrupting information flow in central pathways between the injured spinal cord and brain. The majority of axotomized neurons survive (Hains and others 2003; Wannier and others 2005), albeit with significant somatic shrinkage of the cell body and loss of dendritic spines (Ghosh and others 2010). Despite these substantial anatomical changes, axotomized pyramidal neurons remain receptive to synaptic input, even a year after injury (Tseng and Prince 1996). Whether changes in white matter precede or parallel changes in gray matter is unknown. For example, in MS, changes in normal white matter are adjacent to changes in the gray matter, suggesting, at least in MS, that degenerative events occur simultaneously (Bodini and others 2009). Similar processes could occur in SCI, even though the disease etiology is quite different: the degree of microstructural change within the pyramids has been linked to cortical reorganization, suggesting that subcortical remodeling predicts the degree of cortical reorganization (Freund and others 2012). However, the time-dependent association between white and gray matter degeneration has not been established in SCI. Therefore, further investigations assessing white and gray matter microstructural changes along the neuroaxis are required. These might establish a relationship between the spatiotemporal pattern of axonal degeneration (and demyelination) to somatic neuronal changes.
Studies regarding cell survival are still controversial (Hains and others 2003; Wannier and others 2005), and alternative explanations for reduced gray matter volume have been discussed. For example, decreased cortical connectivity, due to a reduction in dendritic spine density of axotomized (Kim and others 2006) and nonaxotomized pyramidal neurons (Ghosh and others 2011) or a reduction in angiogenesis (Fields 2008) could result in a decline in gray matter volume. Accordingly, changes in cortical gray matter of M1 and S1 have been observed in conditions without any neural damage, due to intensive training (e.g., learning joggling) (Draganski and others 2004), spatial learning (Woollett and Maguire 2011), or immobilization (Langer and others 2012). The latter changes were evident in neurologically intact patients who, due to a fracture of the upper limb, were treated with a cast to immobilize the arm. Whereas the cortical thickness of M1 contralateral to the fixation declined, cortical thickness of M1 cortex ipsilateral to the cast (e.g., controlling nonimmobilized hand) increased during a time period of less than 2 weeks (Langer and others 2012). Crucially, the increase in cortical thickness was associated with improved manual dexterity of the nonimmobilized hand (Langer and others 2012). Similar effects have to be considered in acute SCI patients who, in addition to the focal lesion, are immobilized for a period during the acute management (surgery and acute care at intensive care unit with weaning from ventilation that can last up to several weeks). With subsequent rehabilitation training, afferent inputs may also increase, providing neurons with trophic support. Once trophic factor loss is minimized, these neurons could then reintegrate into existing circuits (Ghosh and others 2011).
Imaging Cortical Reorganization of the Sensorimotor Systems in the Brain
Functional MRI to Study Cortical Reorganization
Understanding and quantifying the impact of cortical reorganization of motor and sensory systems on the recovery of function is a challenging area in neurorehabilitation, especially as trauma to the spinal cord is heterogeneous (i.e., the size of damaged area and completeness or density of axial cord damage). Functional MRI (fMRI) reflects blood oxygen level dependent signal (BOLD) (Friston and others 1995; Friston and others 1996), an indirect marker of neuronal activation, from which one can evaluate the interaction between tissue damage and repair in SCI patients. This allows one to study the role of cortical reorganization in relation to functional recovery. Increases in task-related brain activation and its spread into adjacent denervated areas, such as the leg area, have been detected in SCI patients. These have been established (in relation to control subjects) by comparing activations during movement and/or sensory stimulation of both paralyzed (i.e., lower limbs) and clinically nonaffected limbs (upper limbs in paraplegic subjects) in M1 and S1 (Curt and others 2002; Freund and others 2011b; Jurkiewicz and others 2007; Jurkiewicz and others 2010; Lundell and others 2011b).
The key question here is whether remapping of cortical areas, through changes in ascending and descending pathways, translates into functional gain or further impairment (Freund and others 2011; Freund and others 2012, Henderson and others 2011). Therefore, it is important to study the mechanisms underlying cortical reorganization and its contribution to disability.
Relationship between CNS Atrophy, Cortical Reorganization, and Disability
Given that a traumatic injury to the spinal cord disrupts axons to cortical neurons, one would expect a relationship between the severity of damage and the compensatory response of the cortex to the deafferentation. Indeed, lower cross-sectional spinal cord area (e.g., greater spinal atrophy) is associated with the degree of cortical reorganization during an upper (Freund and others 2011b) and lower limb task in M1 (Lundell and others 2011b). Put simply, the greater the spinal damage, the more the cortex reorganized—possibly to maximize motor output via remaining fibers in cortical areas that are normally not recruited during that specific movement. Correspondingly greater damage to the spinal cord correlated with decreased corticospinal excitability and longer durations of cortical silent periods—as assessed by transcranial magnetic stimulation (Freund and others 2011a). In other words, greater spinal cord atrophy predicted changes at the cortical level that led to reduced excitability of corticospinal neurons and increased cortico-cortical inhibition. However, a link between the corticospinal output of M1 leg neurons to voluntary contraction of forearm muscles (in tetraplegic patients) could not be established (Freund and others 2011a). Instead, cortical forearm motor representations reorganized toward the intrinsic hand motor representation—possibly tapping into upper limb circuits to compensate for the functional deficit of the impaired forearm. Thus, it is debatable if the spread of activation during a handgrip results from cortical overflow, due to disinhibited connections that are dormant in healthy subjects. Alternatively, interlimb coupling (e.g., co-contraction of leg muscles during hand grip) in severe SCI could induce nonfunctional signal increases in the M1 leg area (Calancie and others 1996).
The link between central atrophy and the degree of cortical reorganization also holds true for S1 (Freund and others 2011b; Henderson and others 2011). That is, greater cord atrophy projects abnormal spread of activations elicited by median nerve stimulation in the face area of S1—in SCI patients with greater sensory impairment (Freund and others 2011b). Similar relationships are evident between cortical atrophy and cortical reorganization. Here, although the lower body representation of S1 was atrophic, its extent was minimized in patients with greater medial shifts of the little finger representation (Henderson and others 2011). Correspondingly, lower fractional anisotropy, a marker of axonal count and myelin content derived from DTI (Schmierer and others 2004; Schmierer and others 2007), of the corticospinal tract at the pyramidal level predicted greater reorganization during a hand grip task in the leg area of M1 (Freund and others 2012). The latter findings suggest that central changes in the architecture of white and gray matter relate directly to cortical reorganization.
The key factors in assessing atrophy are how well it relates to disability and whether its progression can predict neurological recovery. In chronic SCI, cord atrophy is significantly correlated with the total ASIA score and clinical measures of hand function (Freund and others 2011b; Lundell and others 2011a). Motor neuron pools, innervating upper limb muscles, such as those employed during manual dexterity, are located several segments below C2. Therefore, cross-sectional spinal cord area changes at C2 represent remote trauma-induced changes of spinal pathways, resulting from axonal dieback and demyelination rather than from direct trauma-induced neuronal changes in spinal gray matter. The onset and rate of atrophy is unknown. Moreover, it is unclear how cord atrophy is related to the etiology of lesion, lesion level, or time after injury. Lundell and others found a weak relationship between the lesion level and the amount of cervical atrophy but no clear relationship between disease duration and cord atrophy (Lundell and others 2011a).
Cortical atrophy measures are correlated to the degree of clinical impairment in M1 (Freund and others 2011b) and S1 (Jurkiewicz and others 2006). For example, changes in gray matter volume in the leg area of M1 were associated with impaired manual dexterity (Freund and others 2011b). This association suggests that cortical reorganization is restricted to areas with neuronal outputs responsible for voluntary control of upper limb muscles, if sufficient neuronal output to the impaired hand is preserved (Freund and others 2011a). In simple terms, SCI patients with greater impairment engage additional neuronal resources to maximize motor output relative to patients with less impairment (Ward and others 2004). Hence, the use-dependent integrity of rewired neurons might provide a sufficient level of neurotrophic support to prevent somatic change (Ghosh and others 2010). Thus, remote changes in cortical areas have a direct influence on brain motor and sensory function, and therefore, treatment strategies should address these associated and remote cortical changes.
In summary, the conjoint assessment of cross-sectional spinal cord area and cortical atrophy in combination with functional motor and sensory responses, using fMRI, allows one to evaluate the structural and functional integrity of spinal pathways along the entire neuroaxis. However, the application of serial fMRI in a clinical setting is currently limited, because this methodology is time consuming and difficult to evaluate longitudinally.
The Neurobiology Underlying Cortical Reorganization
There are multiple mechanisms that can explain the spatial temporal pattern of cortical reorganization, both in the motor and sensory cortex. For example, rapid unmasking of latent synapses could result in (sub-)cortical reorganization due to disinhibition or facilitation of existing subthreshold inputs onto adjacent cortical representations (Jacobs and Donoghue 1991). In addition, complete SCI can immediately change the state of the brain (Fig. 5), leading to changes of cortical somatosensory responses (Aguilar and others 2010). Conversely, partial lesions can produce cortical hyperexcitability, through preserved somatosensory pathways (Yague and others 2011). Over time, slower mechanisms such as subcortical rewiring (Ghosh and others 2010) and lateral sprouting of dendrites in subcortical nuclei (Jones 2000) or within the cortex itself (Henderson and others 2011) could reinnervate deprived cortical areas—engendering cortical reorganization as observed in chronic SCI patients (Kokotilo and others 2009). If this holds true, then one would expect to see a correlation between the size of cortical activations and clinical outcomes, similar to that seen after stroke (Ward and others 2004). Indeed, one study in human SCI found that there was a decrease in the anatomical extent of task-related activations in movement-associated cortical areas but an increase in M1 that was linked to improved outcome in acute SCI patients (Jurkiewicz and others 2010). A possible explanation for increased task-related activation might be the resumption of afferent feedback from muscles during impaired movements, following the resolution of edema and hemorrhage. Higher levels of afferent input might render the contribution of associated motor networks less important, thus explaining the reduced activations that are also seen following stroke. Moreover, in nonhuman primates, spared pathways, following a lesion of the corticospinal tract, can compensate for manual dexterity in a few weeks following injury (Nishimura and others 2007). Interestingly, early recovery of function is accompanied by bilateral activation of the M1, whereas at later stages the contralateral M1 and premotor cortex show increased activation (Nishimura and others 2007). In short, the contributions of different cortical regions show a distinct pattern of reorganization during the course of recovery. Therefore, the initial unmasking of existing connections might be replaced by the invasion of new collateral sprouting axons, from adjacent areas, which form new circuits with increased connectivity (Bareyre and others 2004).
Figure 5.
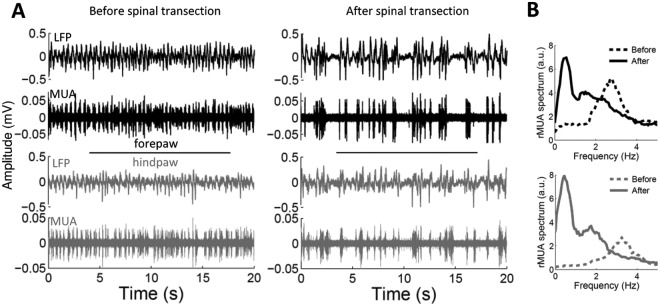
Changes of spontaneous cortical activity in spinalized rats. (A) Examples of 20 s spontaneous recordings of local field potentials and multiunit activity from the forepaw cortex (black) and the hindpaw cortex (gray) before (left) and immediately after (right) complete thoracic transection of the spinal cord in a representative animal. (B) Power spectrum of the multiunit activity corresponding to the recordings shown in “A.” Before spinal cord transection, the somatosensory cortex of this animal showed marked oscillations at delta frequencies (3 Hz), but the oscillation switched to slow-wave activity (0.5 Hz) after the spinal cord transection. Figure reproduced with permission Aguilar and others (2010)
Conclusions and Future Directions
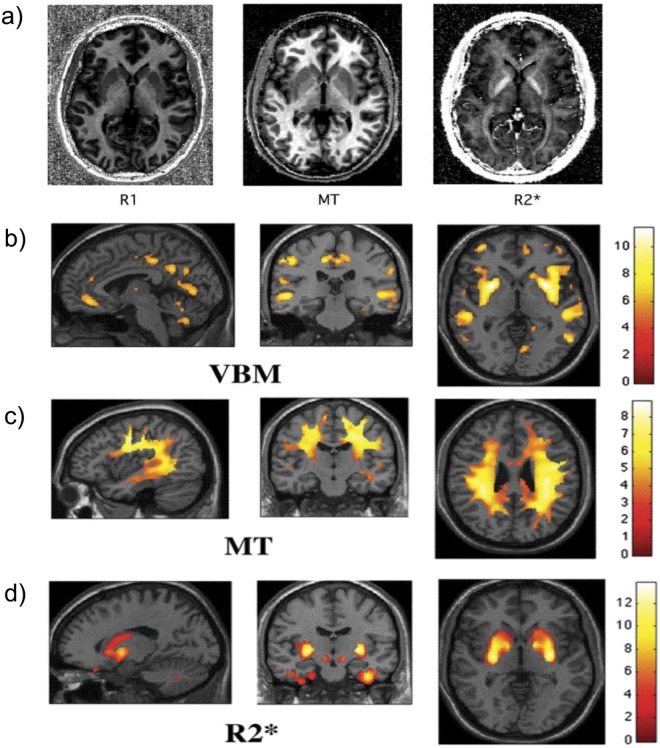
Although spatially distinct areas of the sensorimotor system in the spinal cord and brain become atrophic in chronic SCI, the onset and rate of these changes are unknown in the acute phase of injury. Moreover, although it is well established that cortical reorganization occurs, it remains unclear whether reorganization involves time-dependent anatomical changes. Improving our understanding of the neuronal mechanisms that subtend clinical recovery during the acute injury phase is key to develop evidence-based rehabilitation therapy and the tracking of treatment-induced changes. Quantitative neuroimaging of the spinal cord and brain is an advancing field that may increase our understanding of disease progression and facilitating the prediction and monitoring of individual patients following SCI. These new neuroimaging techniques exploit the physical water properties that define the MR contrasts, which provide multiple measures of underlying microstructural changes in myelin, iron deposits, and water (Fig. 6) (Draganski and others 2011).These measures can be assessed in spinal and cortical areas of volumetric decline following SCI, in order to further establish the correlates of central volumetric changes. Ultimately, quantitative and functional MRI in longitudinal multicenter assessments in acute SCI are required that measure central sequelae and their impact on cortical reorganization as SCI patients recover. This should allow the identification of the most sensitive imaging markers and their applicability in clinical trials.
Figure 6.
Ageing of the brain studied with quantitative multiparameter mapping: assessment of brain macro- and microstructure. (a) Longitudinal relaxation rate (R1 = 1/T1), magnetization transfer saturation (MT), and apparent transverse relaxation rate (R2 = 1/T2*) were estimated from three optimized multi-echo fast low angle shot (FLASH) acquisitions (total acquisition time ~20 min; 1 mm isotropic resolution (Draganski and others 2011; Weiskopf and others 2011). Different parameters are sensitive to different tissue components such as iron in the basal ganglia (see R2* map) or myelin in white matter tracts (see R1 and MT maps). (b) Changes in local gray matter volume due to normal ageing were detected using voxel-based morphometry (VBM) in a cross-sectional design (Draganski and others 2011) in line with previous VBM studies. (c-d) Parameter maps reflecting tissue characteristics revealed accompanying tissue changes using voxel-based quantification (VBQ) (Draganski and others 2011). (c) Widespread reduction in MT suggests changes in myelination. (d) The increase of R2* in the basal ganglia is in line with an increased iron content observed with histological methods. Figures modified from Draganski and others (2011) with permission by Elsevier
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Swiss National Science Foundation (Grant No. PBFR33-120920), Schweizerische Stiftung für medizinische und biologische Stipendien (Grant No. PASMP3-124194), and the Wellcome Trust in the United Kingdom.
References
- Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, and others. 2010. Spinal cord injury immediately changes the state of the brain. J Neurosci 30:7528–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre JB, Bammer R. 2010. Advanced diffusion-weighted magnetic resonance imaging techniques of the human spinal cord. Top Magn Reson Imaging 21:367–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2000. Voxel-based morphometry—the methods. Neuroimage 11:805–21 [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. 2004. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7:269–77 [DOI] [PubMed] [Google Scholar]
- Barkhof F, Calabresi PA, Miller DH, Reingold SC. 2009. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5:256–66 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. 1996. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–19 [DOI] [PubMed] [Google Scholar]
- Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O. 2009. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp 30:2852–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, and others. 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–40 [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Russell JH, Cross AH, Song SK. 2008. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed 21:589–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Lutton S, Broton JG. 1996. Central nervous system plasticity after spinal cord injury in man: interlimb reflexes and the influence of cutaneous stimulation. Electroencephalogr Clin Neurophysiol 101:304–15 [DOI] [PubMed] [Google Scholar]
- Casha S, Zygun D, McGowan MD, Bains I, Yong VW, John HR. 2012. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135:1224–36 [DOI] [PubMed] [Google Scholar]
- Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, and others. 2011. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma 28:1881–92 [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. 2008. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol 7:715–27 [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, and others. 2011. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55:1024–33 [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, and others. 2008. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley AP, Jurkiewicz MT, Yim A, Heyn S, Verrier MC, Fehlings MG, and others. 2004. Absence of localized grey matter volume changes in the motor cortex following spinal cord injury. Brain Res 1028:19–25 [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, and others. 2005. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 102:14069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt A. 2012. The translational dialogue in spinal cord injury research. Spinal Cord 50:352–7 [DOI] [PubMed] [Google Scholar]
- Curt A, Alkadhi H, Crelier GR, Boendermaker SH, Hepp-Reymond MC, Kollias SS. 2002. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain 125:2567–78 [DOI] [PubMed] [Google Scholar]
- Curt A, van Hedel HJ, Klaus D, Dietz V. 2008. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 25:677–85 [DOI] [PubMed] [Google Scholar]
- Draganski B, Ashburner J, Hutton C, Kherif F, Frackowiak RS, Helms G, and others. 2011. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 55: 1423–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. 2004. Neuroplasticity: changes in grey matter induced by training. Nature 427:311–2 [DOI] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. 1994. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci 6:712–24 [DOI] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, and others. 1994. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport 5:2593–7 [DOI] [PubMed] [Google Scholar]
- Endo T, Tominaga T, Olson L. 2009. Cortical changes following spinal cord injury with emphasis on the Nogo signaling system. Neuroscientist 15:291–9 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, and others. 2007. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45:190–205 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Cadotte DW, Fehlings LN. 2011. A series of systematic reviews on the treatment of acute spinal cord injury: a foundation for best medical practice. J Neurotrauma 28:1329–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MS, Popa N, Djelloul M, Boucraut J, Gauthier P, Bauer S, and others. 2012. Alteration of forebrain neurogenesis after cervical spinal cord injury in the adult rat. Front Neurosci 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Rothwell J, Craggs M, Thompson AJ, Bestmann S. 2011a. Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury. Eur J Neurosci 34:1839–46 [DOI] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, and others. 2006. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med 12:790–2 [DOI] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, and others. 2011b. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134:1610–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler-Kingshott C, Jackson J, Miller D, Thompson A, Ciccarelli O. 2010a. Recovery after spinal cord relapse in multiple sclerosis is predicted by radial diffusivity. Mult Scler 16:1193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler-Kingshott CA, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, and others. 2012. Axonal integrity predicts cortical reorganisation following cervical injury. J Neurol Neurosurg Psychiatry 83:629–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund PA, Dalton C, Wheeler-Kingshott CA, Glensman J, Bradbury D, Thompson AJ, and others. 2010b. Method for simultaneous voxel-based morphometry of the brain and cervical spinal cord area measurements using 3D-MDEFT. J Magn Reson Imaging 32:1242–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. 1996. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4:223–35 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. 1995. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210 [Google Scholar]
- Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, and others. 2010. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 13:97–104 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Peduzzi S, Snyder M, Schneider R, Starkey M, Schwab ME. 2011. Heterogeneous spine loss in layer 5 cortical neurons after spinal cord injury. Cereb Cortex. 10.1093/cercor/bhr191 [DOI] [PubMed] [Google Scholar]
- Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, and others. 2008. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res 86:2271–80 [DOI] [PubMed] [Google Scholar]
- Hains BC, Black JA, Waxman SG. 2003. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 462:328–41 [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ. 2011. Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J Neurosci 31:2630–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield MA, Sala S, Neema M, Absinta M, Bakshi A, Sormani MP, and others. 2010. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. Neuroimage 50:446–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubli M, Dietz V, Bolliger M. 2012. Spinal reflex activity: a marker for neuronal functionality after spinal cord injury. Neurorehabil Neural Repair 26:188–96 [DOI] [PubMed] [Google Scholar]
- Hutton C, De Vita E, Ashburner J, Deichmann R, Turner R. 2008. Voxel-based cortical thickness measurements in MRI. Neuroimage 40:1701–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. 2009. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48:371–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. 1991. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251:944–7 [DOI] [PubMed] [Google Scholar]
- Jones EG. 2000. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci 23:1–37 [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Crawley AP, Verrier MC, Fehlings MG, Mikulis DJ. 2006. Somatosensory cortical atrophy after spinal cord injury: a voxel-based morphometry study. Neurology 66:762–4 [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, Fehlings MG, Verrier MC. 2010. Sensorimotor cortical activation in patients with cervical spinal cord injury with persisting paralysis. Neurorehabil Neural Repair 24:136–40 [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. 2007. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair 21:527–38 [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. 2006. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198:401–15 [DOI] [PubMed] [Google Scholar]
- Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K, and others. 2010. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma 27:587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ, Curt A. 2009. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma 26:2113–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Guo X, Kerschensteiner M, Bareyre FM. 2012. Single collateral reconstructions reveal distinct phases of corticospinal remodeling after spinal cord injury. PLoS One 7:e30461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Hanggi J, Muller NA, Simmen HP, Jancke L. 2012. Effects of limb immobilization on brain plasticity. Neurology 78:182–8 [DOI] [PubMed] [Google Scholar]
- Losseff NA, Webb SL, O’Riordan JI, Page R, Wang L, Barker GJ, and others. 1996. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119:701–8 [DOI] [PubMed] [Google Scholar]
- Lundell H, Barthelemy D, Skimminge A, Dyrby TB, Biering-Sorensen F, Nielsen JB. 2011a. Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord 49:70–5 [DOI] [PubMed] [Google Scholar]
- Lundell H, Christensen MS, Barthelemy D, Willerslev-Olsen M, Biering-Sorensen F, Nielsen JB. 2011b. Cerebral activation is correlated to regional atrophy of the spinal cord and functional motor disability in spinal cord injured individuals. Neuroimage 54:1254–61 [DOI] [PubMed] [Google Scholar]
- Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ. 2002. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125:1676–95 [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Nagy Z, Hutton C, Josephs O, Weiskopf N. 2011. Correction of vibration artifacts in DTI using phase-encoding reversal (COVIPER). Magn Reson Med. 10.1002/mrm.23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Nagy Z, Moller HE, Symms MR, Carmichael DW, Josephs O, and others. 2012. The effect of local perturbation fields on human DTI: characterisation, measurement and correction. Neuroimage 60:562–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli JN, Runge VM, Feiweier T, Kirsch JE, Williams KW, Attenberger UI. 2010. Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest Radiol 45:29–35 [DOI] [PubMed] [Google Scholar]
- Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. 2006. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain 129:718–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. 2007. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science 318:1150–5 [DOI] [PubMed] [Google Scholar]
- Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, and others. 2012. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 29:1556–66 [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. 2000. Functional regeneration of sensory axons into the adult spinal cord. Nature 403:312–6 [DOI] [PubMed] [Google Scholar]
- Schmid MR, Pfirrmann CW, Koch P, Zanetti M, Kuehn B, Hodler J. 2005. Imaging of patellar cartilage with a 2D multiple-echo data image combination sequence. AJR Am J Roentgenol 184:1744–8 [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. 2004. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–15 [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Boulby PA, Scaravilli F, Altmann DR, Barker GJ, and others. 2007. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage 35:467–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME. 2002. Repairing the injured spinal cord. Science 295:1029–31 [DOI] [PubMed] [Google Scholar]
- Thurnher MM, Law M. 2009. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am 17:225–44 [DOI] [PubMed] [Google Scholar]
- Tseng GF, Prince DA. 1996. Structural and functional alterations in rat corticospinal neurons after axotomy. J Neurophysiol 75:248–67 [DOI] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. 2011. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31:9332–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannier T, Schmidlin E, Bloch J, Rouiller EM. 2005. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma 22:703–17 [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. 2004. The influence of time after stroke on brain activations during a motor task. Ann Neurol 55:829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Lutti A, Helms G, Novak M, Ashburner J, Hutton C. 2011. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT). Neuroimage 54:2116–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm BJ, Gamper U, Henning A, Pruessmann KP, Kollias SS, Boesiger P. 2009. Diffusion-weighted imaging of the entire spinal cord. NMR Biomed 22:174–81 [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. 2011. Acquiring “the knowledge” of London’s layout drives structural brain changes. Curr Biol 21:2109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, and others. 2009. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19:224–32 [DOI] [PubMed] [Google Scholar]
- Wyndaele M, Wyndaele JJ. 2006. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 44:523–29 [DOI] [PubMed] [Google Scholar]
- Yague JG, Foffani G, Aguilar J. 2011. Cortical hyperexcitability in response to preserved spinothalamic inputs immediately after spinal cord hemisection. Exp Neurol 227:252–63 [DOI] [PubMed] [Google Scholar]