Abstract
Novel microcarriers consisting of calcium phosphate cement and alginate were prepared for use as three-dimensional scaffolds for the culture and expansion of cells that are effective for bone tissue engineering. The calcium phosphate cement-alginate composite microcarriers were produced by an emulsification of the composite aqueous solutions mixed at varying ratios (calcium phosphate cement powder/alginate solution = 0.8–1.2) in an oil bath and the subsequent in situ hardening of the compositions during spherodization. Moreover, a porous structure could be easily created in the solid microcarriers by soaking the produced microcarriers in water and a subsequent freeze-drying process. Bone mineral-like apatite nanocrystallites were shown to rapidly develop on the calcium phosphate cement–alginate microcarriers under moist conditions due to the conversion of the α-tricalcium phosphate phase in the calcium phosphate cement into a carbonate–hydroxyapatite. Osteoblastic cells cultured on the microspherical scaffolds were proven to be viable, with an active proliferative potential during 14 days of culture, and their osteogenic differentiation was confirmed by the determination of alkaline phosphatase activity. The in situ hardening calcium phosphate cement–alginate microcarriers developed herein may be used as potential three-dimensional scaffolds for cell delivery and tissue engineering of bone.
Keywords: Microcarriers, calcium phosphate cements, alginate, cell culture, bone regeneration
Introduction
Spherical particles are gaining increasing interest as the scaffolding matrices for the culture of tissue cells and for the direct filling of defective bone tissues.1–3 Particulates with sizes of hundreds of micrometers can provide good substrate conditions for tissue cells to adhere to and populate on, and further them to develop into specific tissue forms, consequently healing the defects.2,3 Biological molecules can also be incorporated within the spheres to elicit therapeutic efficacy.4,5 Among the compositions developed, bioactive ceramics have proven to be potential candidate substrates for bone regeneration. However, those ceramics generally require high-temperature processing to consolidate and maintain structural integrity, which, however, is detrimental to the incorporation of bioactive molecules and therapeutics.6,7
In contrast, calcium phosphate cements (CPCs) self-set to form hardened materials, and the compositions are also biocompatible and effective for bone-cell functions.8,9 We herein used the CPC as the precursor for the preparation of microspheres. Moreover, alginate was combined with CPC in order to form a sphere particle more effectively. The CPC and alginate composite system has been demonstrated to be a useful matrix with its improved mechanical integrity and bone-cell differentiation.10,11 The CPC–alginate microsphere is considered to be properly applied as injectable bone fillers either directly or with incorporating bioactive therapeutics. Moreover, bone progenitor or stem cells can be cultivated on the spherical scaffolds for the purpose of bone tissue engineering.
While CPC with its in situ hardening property is considered to solidify at room temperature, the alginate used together with CPC composition can improve the CPC’s setting ability and, ultimately, the mechanical integrity of the microcarriers.12 Alginate is a natural polymer derived from algae, is non-toxic and tissue-compatible, and thus has been widely used as a biomaterial for wound repair and cell encapsulation.13 In particular, the CPC–alginate microcarriers have also been explored to allow the development of macropores within the structure to retain large spaces for effective culture of tissue cells. The processing route to prepare CPC–alginate microcarriers is described, and the in vitro bioactive responses are also addressed for use as bone regeneration materials.
Materials and methods
Preparation and characterization of microcarriers
As the precursor of CPC powder, α-tricalcium phosphate was prepared, as described in a previous work.9 In brief, calculated amounts of calcium carbonate and dicalcium phosphate anhydrous were put in a platinum crucible and heat-treated at 1400℃ for 5 h and then quenched. About 5% Na2HPO4, either with or without alginate (Na-alginate, Aldrich), was used as the liquid phase. The concentration of alginate was 2% or 3%. The CPC powder and alginate liquid phases were mixed at varying ratios (CPC powder/alginate liquid = 0.8, 0.9, 1.0, 1.1, and 1.2 by weight).
Microcarriers of the CPC–alginate were produced by means of a water-in-oil emulsification method. The CPC–alginate mixture of 1 ml was dropped into a 60-ml olive oil bath, while stirring at 380 rpm for about 60 min. The stirring speed was determined to produce spherical microcarriers with sizes in the hundreds of micrometers range that are appropriate for cell culture. Furthermore, the stirring time was optimized to enable the in situ hardening process to form stable microcarriers. After stirring, the microcarriers were washed with ethanol and lyophilized at −60℃ using a freeze-dryer. In order to provide a macroporous structure within the spheres, the ethanol-treated spheres were soaked in distilled water for 1 min and frozen at −20℃ and then lyophilized at −60℃ using a freeze-dryer.
The morphology of the microcarriers was examined with scanning electron microscopy (SEM, Hitachi S-3000H), and the size of the microcarriers was evaluated from the images. The microcarriers were soaked in a simulated body fluid (which contains 2.5 mM Ca2+, 142 mM Na+, 1.5 mM Mg2+, 5.0 mM K+, 125.0 mM Cl−, 27.0 mM HCO3−, 1.0 mM HPO42−, and 0.5 mM SO42−) for different time periods to examine any changes in the phase, and the surface morphology was observed using SEM.14
In vitro cell culture on the microcarriers
The biocompatibility of the CPC–alginate microcarriers was briefly examined by the proliferative potential of the pre-osteoblast cell line (MC3T3-E1).15 The microcarriers of 30 mg were contained in each well of a 96-well plate, and the cells were seeded at a density of 5 × 103 cells/well and then cultured in α-minimal essential medium supplemented with fetal bovine serum containing antibiotics in a humidified atmosphere of 95% CO2 at 37℃.
The cell morphology was examined with SEM after fixing the cells with glutaraldehyde (2.5%), dehydrating with a graded series of ethanol, and treating with hexamethyldisilazane and gold coating. Cell proliferation was measured by means of the MTS assay (CellTiter 96 Aqueous One Solution, Promega, USA).15 For the differentiation study, osteogenic factors involving 10 mM β-glycerol phosphate and 50 µg/ml l-ascorbic acid were added to the culture medium. The alkaline phosphatase activity (ALP), an early osteogenic marker, was determined, as described in our previous study.16 Experiments were conducted on three replicate samples. Statistical analysis was performed using ANOVA. A significant level was considered at p < 0.05.
Results and discussion
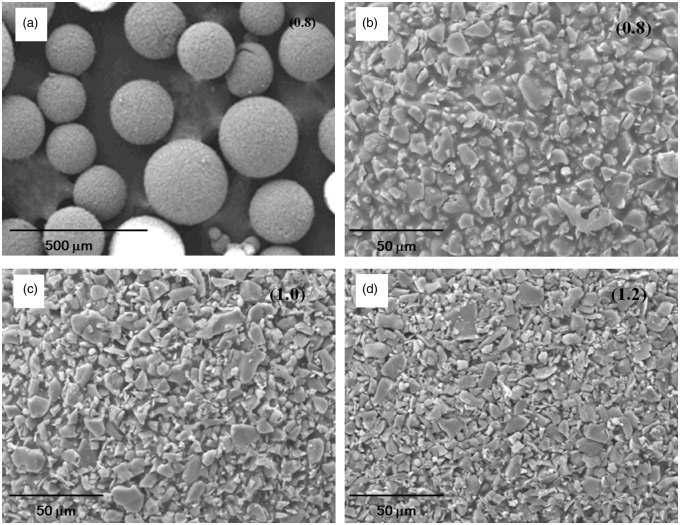
The CPC–alginate solutions prepared at different ratios (CPC/alginate = 0.8–1.2) were demonstrated to be well-developed into a spherical form by the water-in-oil emulsification method. Figure 1(a) shows the SEM images of the representative CPC–alginate microcarriers (CPC/alginate = 0.8) at low magnification. Spherical-shaped particles were well formed, and the surface was shown to reveal that the CPC particles were several micrometers in size, which were surrounded with alginate polymeric phase. At high magnification of the representative CPC–alginate microcarriers (CPC/alginate = 0.8, 1.0, and 1.2), as the powder content (CPC/alginate ratio) increased, the surface morphology became less dense (Figure 1(b–d)). For all cases, the microcarriers were well-integrated, which is appropriate for handling in other assays, such as cell tests.
Figure 1.
SEM morphologies of the CPC/alginate microcarriers. Images were taken at different magnifications (CPC/alginate ratios of 0.8, 1.0, and 1.2 are marked in each image). Microspherical particles with hundreds of micrometers in size were well generated at low magnification (a), and a few micrometer-sized CPC particles and surrounding alginate are revealed at high magnification (b–d).
The size distributions of the CPC–alginate microcarriers were assessed from the SEM images and are shown in Figure 2(a). Microcarriers had sizes of about 100–500 µm, and the increase in CPC powder content increased the microcarrier size (from 183 to 288 µm, on average), as shown in Figure 2(b). This size range is considered to be appropriate for the culture of tissue cells upon the spherical substrates targeting tissue regeneration purposes.2–4
Figure 2.
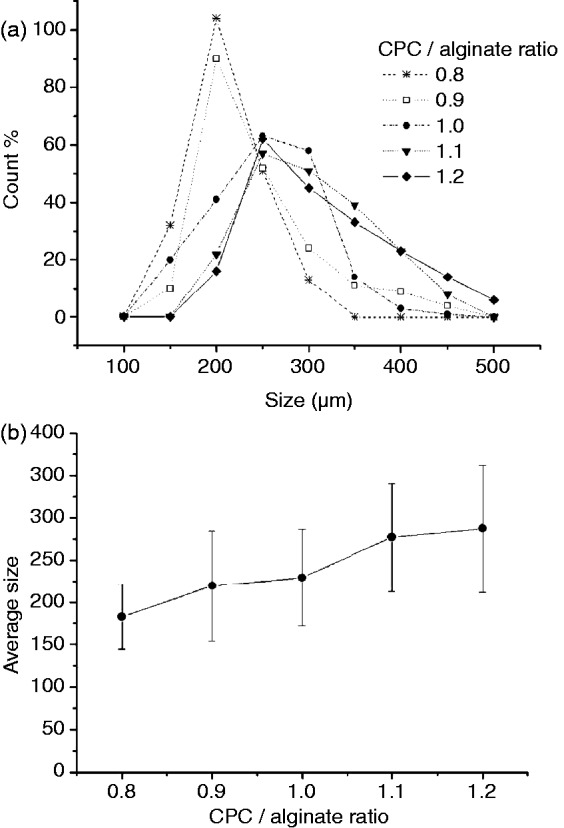
Measurement of sizes of microcarriers with different CPC/alginate compositions: (a) size distribution and (b) average size.
The process of this sphere formation was designed to last for 60 min, which was based on our previous work on the observation of setting time of CPC (within an hour) for the powder-to-liquid ratios used herein.10 Specifically, the self-setting reaction of the CPC is considered to occur with the alginate solution within the droplets contained in an oil bath. Because the alginate becomes cross-linked by the exchange with calcium ions, the alginate should play some role in the hardening of the CPC-based spheres through reaction with calcium ions released from the CPC composition.10,11 Therefore, when compared to the pure CPC, the alginate-added CPC has been shown to have better setting properties, and this positively affects the resultant mechanical integrity.
The obtained CPC–alginate microcarriers exhibited the phase transformation of the initial CPC composition into a hydroxyapatite (HA) phase, a general reaction in the α-TCP-based cement under moisture conditions. Figure 3 shows the representative microstructural change of the CPC–alginate microcarriers during immersion in a fluid for 7 days. Low-magnification image appeared to show a similar morphological feature to that without immersion (Figure 3(a)). At higher magnification, small nanocrystallites completely covered the surface of the microcarriers (Figure 3(b)). As shown in the XRD data (Figure 3(c)), the initial α-TCP phase was transformed into the HA phase after 7 days of immersion. The nanocrystalline HA phase produced on the CPC–alginate spheres is considered to be favorable for the cell and tissue responses.
Figure 3.
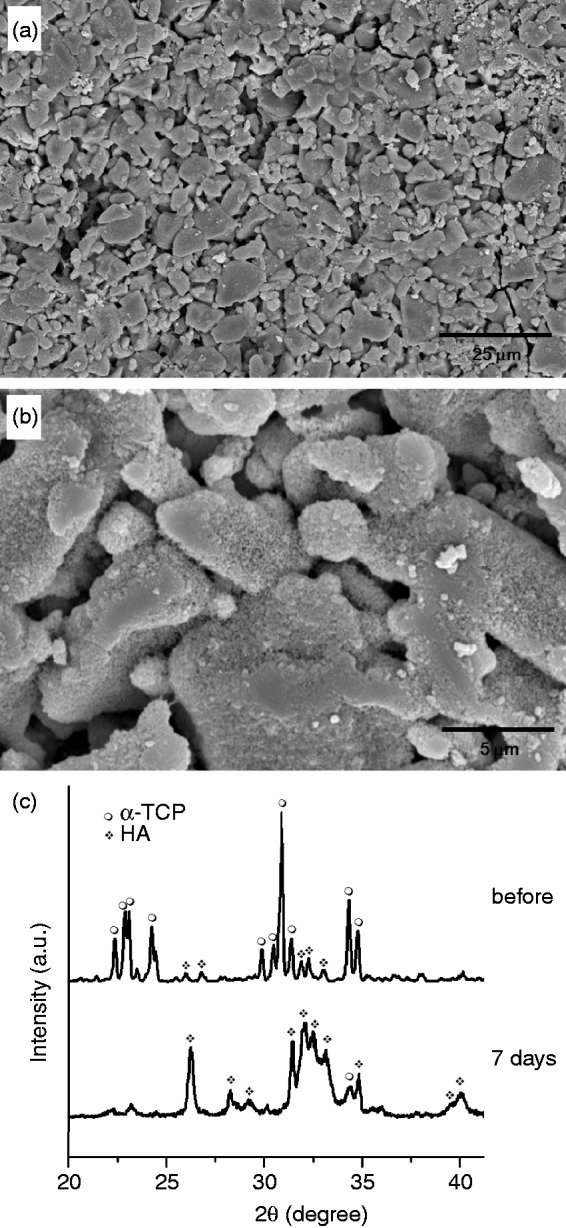
(a,b) Surface morphological change of microcarriers (CPC/alginate = 1.0) during immersion in the body simulating medium for 7 days shown at different magnifications (a,b) and the XRD patterns before and after immersion for 7 days, showing the development of HA phase, due to the transformation of α-TCP.
A feasibility study of the microcarriers was briefly performed by culturing pre-osteoblastic cells directly on the developed microcarriers. The SEM cell growth images on the solid microcarriers were taken at different positions and magnifications. Cells exhibited good adhesion and spreading, with active cytoskeletal processes on the surface of microcarriers (Figure 4(a)), and also accumulated at the contact points of microcarriers bridging them together (Figure 4(b) and (c)).
Figure 4.
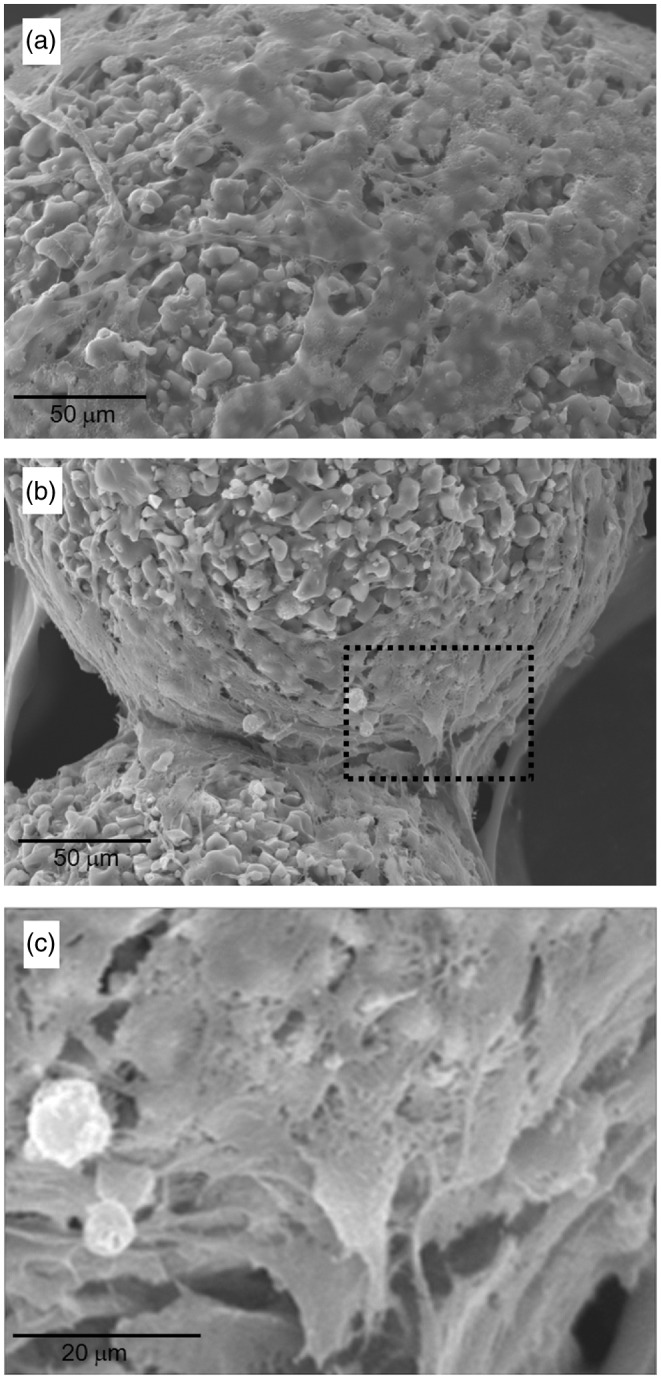
Cell growth morphology grown on the CPC/alginate microcarriers during culture for 3 days, taken from SEM.
The cell proliferation level, as assessed by the MTS method (Figure 5(a)), showed an ongoing increase in cell population with time. When grown on the microcarriers, cells were at the same level as the tissue culture plastic dish, which is used as a positive control. The results suggest that the developed CPC–alginate microcarriers are cell compatible and would be useful as a three-dimensional (3D) scaffold for bone regeneration. Furthermore, the effect of the composite microcarriers on osteogenic differentiation was assessed by means of ALP activity produced by the cells after culture for 7 and 14 days (Figure 5(b)). At day 7, the ALP level of cells grown on the microcarriers was comparable to that on the control. After 14 days, the ALP level on the microcarriers increased substantially, whereas that on the control only slightly increased. These results confirmed the potential role of the CPC–alginate composite microcarriers in providing appropriate scaffold conditions for cells to undergo osteogenic development under the osteogenic medium conditions. It is considered that the transformed bone-mineral like apatite phase may be an excellent substrate condition for the osteoblastic differentiation with respect to the culture dish. Moreover, the expected release of ions may also be possible stimulators of cell differentiation, and more in-depth studies will be needed as further work.
Figure 5.
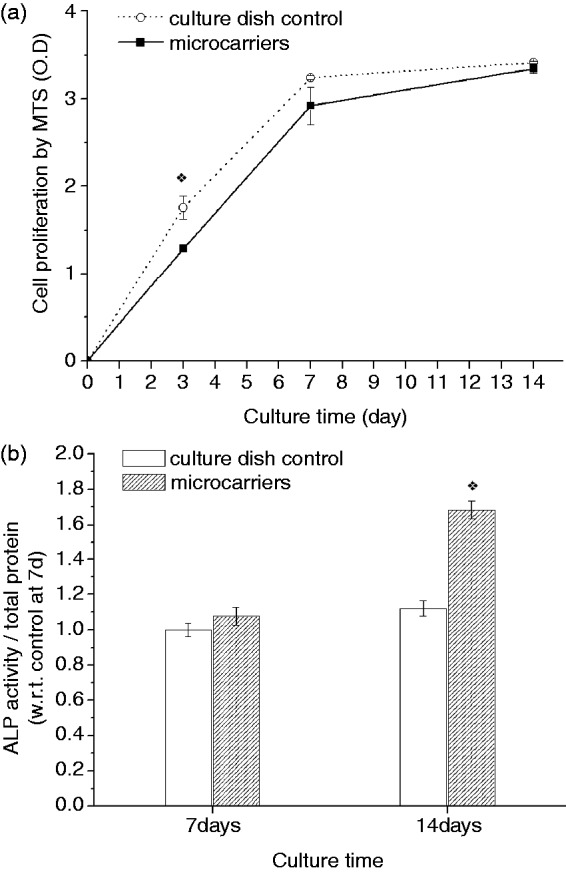
(a) Cell proliferation behavior on the spheres during 14 days, as compared with those on the culture dish control, showing an ongoing cell growth, and (b) ALP activity of the cells grown on the microcarriers significantly increased at 14 days, compared to control. (*p < 0.05 by ANOVA for n = 3).
Apart from the compositional merit of the CPC–alginate solid microcarriers, we aimed to develop the spheres to have a porous structure, which is considered to be effective to hold tissue cells within the spheres and support their multiplication for tissue engineering due to their large surface area to volume ratio. The solidified microcarriers were frozen and lyophilized after being soaked in water. Figure 6 shows the SEM image of the porous microcarriers. Particulates of a few hundreds of micrometers were obtained after the lyophilization process; however, the shape was not isotropic, but somewhat irregular. More noticeable was the formation of a large amount of macropores throughout the spheres, with pore sizes of 20–50 µm, which also appeared interconnected, as shown in the morphology of the fragmented sphere. By adopting a simple process, that is, soaking in water followed by lyophilization, the dense microcarriers could be developed with a porous structure. The frozen ice crystals were sublimated to leave interconnected pores throughout the spheres. The size of the pores can be tuned by varying the freezing temperature, i.e., with increasing temperature, the pore size increases.17–19 Herein, the developed pore sizes were about 20–50 µm at the freezing temperature of −20℃. The beneficial effects of the pore structure generated in the microcarriers are obvious based on previous works that report a high-loading capacity of cells for use as tissue engineering scaffold.2,6,20,21 Further work is needed regarding the applications of this porous microcarrier.
Figure 6.
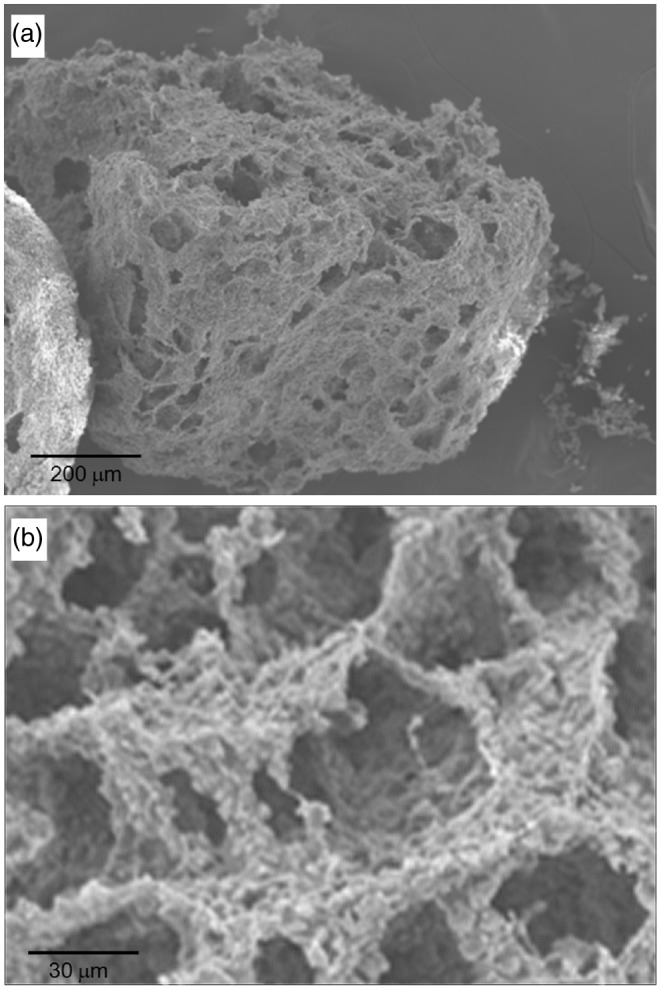
SEM images at different magnifications, showing the morphology of the porous-structured CPC/alginate microcarriers produced by immersing the solidified CPC/alginate spheres in water, followed by freeze-drying to create the pores.
Conclusions
CPC–alginate microcarriers were newly developed as a 3D matrix for bone repair. The self-setting reaction of the composition facilitated ease-of-sphere formation at mild conditions. The resultant bone-mineral-like apatite phase transformed from the initial CPC powder is considered to provide excellent substrate conditions for bone-associated cells. Preliminary cellular study proved that the spheres were favorable for cells to adhere, spread, and proliferate on, and to undergo osteogenic differentiation. Taken together, the newly developed CPC–alginate microcarriers are considered to be useful as a 3D matrix for bone regeneration.
Acknowledgments
This work was supported by Priority Research Centers Program (No. 2009-0093829) and WCU Program (No. R31-10069) through the National Research Foundation (NRF), funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea.
References
- 1.Borden M, Attawia M, Khan Y, et al. Tissue engineered microsphere-based matrices for bone repair: design and evaluation Biomaterials 200223551–559 [DOI] [PubMed] [Google Scholar]
- 2.Langenbach F, Naujoks C, Laser A, et al. Improvement of the cell-loading efficiency of biomaterials by inoculation with stem cell-based microspheres, in osteogenesis J Biomater Appl 201226549–564 [DOI] [PubMed] [Google Scholar]
- 3.Dawes G, Fratila-Apachitei LE, Necula BS, et al. Effects of dexamethasone-loaded PLGA microspheres on human fetal osteoblasts J Biomater Appl 201227477–483 [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Lee GS, Shin US, et al. Self-hardening microspheres of calcium phosphate cement with collagen for drug delivery and tissue engineering in bone repair J Am Ceram Soc 201194351–354 [Google Scholar]
- 5.Mulia K, Witkamp G-J, Dawes GJS, et al. Drug release from PLGA microspheres attached to solids using supercritical CO2 J Biomater Appl 201125401–412 [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Hong SJ, Kim HY, et al. Evacuated calcium phosphate spherical microcarriers for bone regeneration Tissue Eng A 2010161681–1691 [DOI] [PubMed] [Google Scholar]
- 7.Brunner TJ, Grass RN, Bohner M, et al. Comparison of amorphous TCP nanoparticles to micron-sized α-TCP as starting materials for calcium phosphate cements J Mater Chem 2007174072–4078 [DOI] [PubMed] [Google Scholar]
- 8.Brown WE, Chow LC.A new calcium phosphate setting cement J Dent Res 198362672–672 [Google Scholar]
- 9.Oh SA, Noh KT, Lee KS, et al. Osteoclastic cell behaviors affected by the alfa-tricalcium phosphate based bone cements J Mater Sci Mater Med 2010213019–3027 [DOI] [PubMed] [Google Scholar]
- 10.Lee GS, Park JH, Won JE, et al. Alginate combined calcium phosphate cements: mechanical properties and in vitro rat bone marrow stromal cell responses J Mater Sci Mater Med 2011221257–1268 [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa K, Miyamoto Y, Kon M, et al. Non-decay type fast-setting calcium phosphate cement: composite of FSCPC with sodium alginate Biomaterials 199516527–532 [DOI] [PubMed] [Google Scholar]
- 12.Lee GS, Park JH, Shin US, et al. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering Acta Biomater 201173178–3186 [DOI] [PubMed] [Google Scholar]
- 13.Augst AD, Kong HJ, Mooney DJ.Alginate hydrogels as biomaterials Macromol Biosci 20066623–633 [DOI] [PubMed] [Google Scholar]
- 14.Kokubo T, Takadama H.How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006272907–2915 [DOI] [PubMed] [Google Scholar]
- 15. Nam SY, Won JE, Kim CH, et al. Odontogenic differentiation of human dental pulp stem cells stimulated by the addition of calcium phosphate porous granules. J Tissue Eng 2011; 2011: 812547. [DOI] [PMC free article] [PubMed]
- 16. Oh SA, Kim SH, Won JE, et al. Effects on growth and osteogenic differentiation of mesenchymal stem cells by the zinc-added sol-gel bioactive glass granules. J Tissue Eng 2011; 2010: 475260. [DOI] [PMC free article] [PubMed]
- 17.Haugh MG, Murphy CM, O’Brien FJ.Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes Tissue Eng Part C 201016887–894 [DOI] [PubMed] [Google Scholar]
- 18.Chung TW, Yang J, Akaike T, et al. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment Biomaterials 2002232827–2834 [DOI] [PubMed] [Google Scholar]
- 19.Annabi N, Nichol JW, Zhong X, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering Tissue Eng Part B 2010163711–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TK, Yoon JJ, Lee DS, et al. Gas foamed open porous biodegradable polymeric microspheres Biomaterials 200627152–159 [DOI] [PubMed] [Google Scholar]
- 21.Shin US, Park JH, Hong SJ, et al. Porous biomedical composite microspheres developed for cell delivering scaffold in bone regeneration Mater Lett 2010642261–2264 [Google Scholar]