Abstract
We report on a stroke patient who showed delayed gait recovery between 8 and 11 months after the onset of intracerebral hemorrhage. This 32-year-old female patient underwent craniotomy and drainage for right intracerebral hemorrhage due to rupture of an arteriovenous malformation. Brain MR images revealed a large leukomalactic lesion in the right fronto-parietal cortex. Diffusion tensor tractography at 8 months after onset revealed that the right corticospinal tract was severely injured. At this time, the patient could not stand or walk despite undergoing rehabilitation from 2 months after onset. It was believed that severe spasticity of the left leg and right ankle was largely responsible, and thus, antispastic drugs, antispastic procedures (alcohol neurolysis of the motor branch of the tibial nerve and an intramuscular alcohol wash of both tibialis posterior muscles) and physical therapy were tried to control the spasticity. These measures relieved the severe spasticity, with the result that the patient was able to stand at 3 months. In addition, the improvements in sensorimotor function, visuospatial function, and cognition also seemed to contribute to gait recovery. As a result, she gained the ability to walk independently on even floor with a left ankle foot orthosis at 11 months after onset. This case illustrates that clinicians should attempt to find the cause of gait inability and to initiate intensive rehabilitation in stroke patients who cannot walk at 3–6 months after onset.
Keywords: neural regeneration, brain injury, stroke, diffusion tensor imaging, sequelae, gait, walk, motor recovery, corticospinal tract, rehabilitation, intracerebral hemorrhage, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
This case report describes a stroke patient who demonstrated delayed gait recovery between 8 and 11 months after the onset of intracerebral hemorrhage.
-
(2)
Severe spasticity of the lower extremity seemed to be largely responsible for gait inability in this patient.
-
(3)
Clinicians should find the cause of gait inability and initiate intensive rehabilitation in stroke patients.
INTRODUCTION
Stroke is a leading cause of major adult disability. In fact, 20–30% of stroke patients do not recover gait ability, and therefore, gait dysfunction is one of the most serious disabling sequelae of stroke[1,2]. Gait is important for mobility and for maintaining general health. In addition, gait ability is closely related to cognition and gait disturbance is closely related to cognitive impairment[3,4]. Therefore, regaining gait ability in stroke is a primary goal of neurorehabilitation, along with the recovery of hand function and cognition[1].
Previous studies have demonstrated the extensive potential for gait recovery even in cases of complete injury of the corticospinal tract, which is the most important motor tract in the human brain[5,6,7]. Furthermore, gait is a less demanding motor function than hand function[6,7,8]. Stroke patients can walk when motor function is recovered in the proximal joint (hip and knee), at least to the degree of being able to oppose gravity. However, hand function requires a larger degree of recovery to distal joints[8]. These phenomena suggest that detailed knowledge of gait could aid more stroke patients in recovering their gait ability. In general, most motor recovery after stroke occurs within 3–6 months after onset, and gait function usually recovers within 3 months of stroke onset[1,8,9,10,11,12,13]. Therefore, clinicians need to look for the cause of gait inability and perform intensive rehabilitation for stroke patients who cannot walk after 3–6 months after insult. We report on a stroke patient who showed delayed gait recovery between 8 and 11 months after the onset of intracerebral hemorrhage.
CASE REPORT
A 32-year-old, right-handed female underwent craniotomy and drainage at the neurosurgery department of a hospital for a right intracerebral hemorrhage caused by rupture of an arteriovenous malformation. Brain MR images taken 8 months after onset, showed a large leukomalactic lesion in the right fronto-parietal cortex including the primary motor cortex, centrum semiovale, and corona radiata (Figure 1A). At intracerebral hemorrhage onset, the patient had presented with complete paralysis of the left arm and leg, and had undergone rehabilitative management at the rehabilitation department of the hospital (for 2 months), a university hospital (for 2 months), and at a local rehabilitation hospital (for 2 months) from 2 to 8 months after onset.
Figure 1.
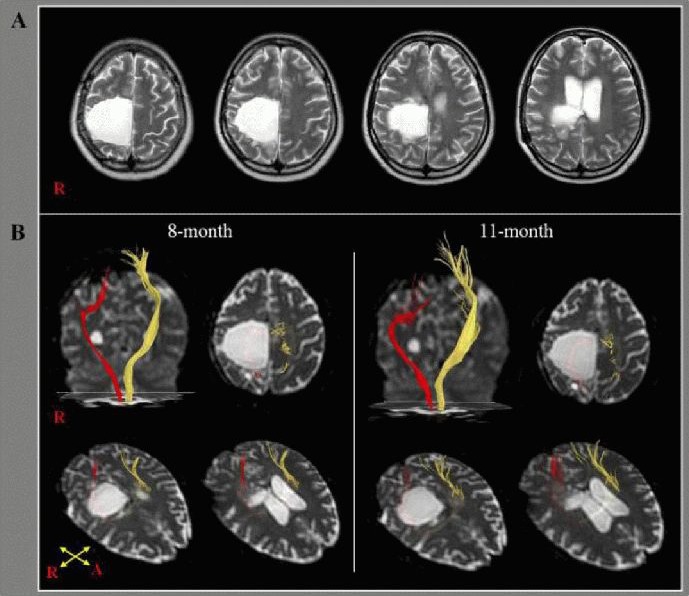
T2-weighted brain MR and diffusion tensor tractography in a 32-year-old, right-handed female with right intracerebral hemorrhage.
(A) T2-weighted brain MR images 8 months after onset showing a large leukomalactic lesion in the right frontoparietal cortex including the primary motor cortex, centrum semiovale, and corona radiate. R: Right.
(B) Diffusion tensor tractography of the corticospinal tract (CST). The CST of the left hemisphere (yellow) originated from the primary sensorimotor cortex and descended through the known CST pathway at 8 and 11 months after stroke. By contrast, the right CST (red) originated from the posterior parietal cortex and descended through the posterior margin of the leukomalactic lesion at 8 months. No significant change was observed at 11 months. R: Right; A: anterior.
At 8 months after onset, the patient was admitted to the rehabilitation department of the Yeungnam University Hospital for further rehabilitation. At the time, Mini-Mental State Exam (MMSE), Motor-Free Visual Perceptual Test (MVPT), and ideomotor apraxia test scores were 20 (cut-off score < 25), 17 (cut-off score < 27), and 40 (cut-off score < 32), respectively (Table 1)[14,15,16]. In terms of motor function, she was able to extend the left hip and knee without gravity. Subscale scores for tactile sensation (full mark: 20) and kinesthetic sensation (full mark: 24) of the Nottingham Sensory Assessment (NSA) were 10 and 3, respectively[17] on the left side.
Table 1.
Clinical data of the patient at 8 and 11 months after intracerebral hemorrhage
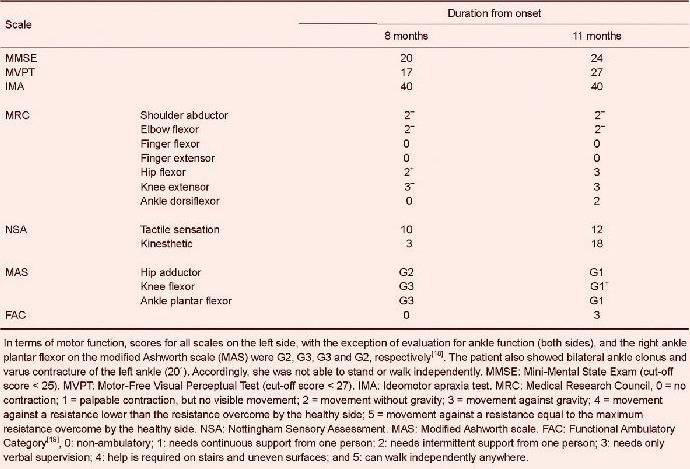
Spasticities of the left hip adductor, knee flexor and ankle plantarflexor, and the right ankle plantarflexor on the modified Ashworth scale (MAS) were G2, G3, G3 and G2, respectively[18]. She also showed bilateral ankle clonus and varus contracture of the left ankle (20°). Accordingly, she was not able to stand or walk independently.
The patient underwent a comprehensive rehabilitative management program, including neurotrophic drugs (pramipexole, bromocriptine, amantadine, levodopa)[20], antispastic drugs (tizanidine 8 mg, baclofen 60 mg, diazepam 4 mg), movement therapy, and neuromuscular electrical stimulation therapy of left knee extensors and ankle dorsiflexors. Movement therapy was conducted primarily to improve postural control and motor function of the left leg, and was performed 5 days (2.5 hours per day) per week. Alcohol neurolysis for the motor branch of the tibial nerve in both legs, an intramuscular alcohol wash of both tibialis posterior muscles, and physical therapy, including stretching exercise, were performed to control spasticity[21,22].
After 3 months of rehabilitation (11 months after onset), improvements were observed in cognition, visuospatial function, motor-sensory function, and spasticity as follows, MMSE: 20 to 24, MVPT: 17 to 27, Medical Research Council: left hip flexor 2+ to 3, left knee extensor 3− to 3, left ankle plantarflexor 0 to 2, right ankle plantarflexor 4 to 5. NSA: Kinaesthetic 3 to 18, MAS: knee flexor G3 to G1+, left ankle plantarflexor G3 to G1, and right ankle plantarflexor G2 to G1. As a result, she gained the ability to walk independently on even floor with a left ankle foot orthosis at 11 months after onset.
Diffusion tensor imaging was performed at 8 and 11 months after intracerebral hemorrhage using a sensitivity-encoding head coil on a 1.5-T Philips Gyroscan Intera unit (Hoffman-LaRoche Ltd., Best, the Netherlands) using single-shot echo-planar imaging. For each of the 32 non-collinear diffusion-sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line. The imaging parameters used were as follows: matrix = 128 × 128, field of view = 221 × 221 mm2, repetition time/echo time = 10 726/76 ms, sensitivity encoding factor = 2, echo planar imaging factor = 67, b = 1 000 s/mm2, number of excitations = 1, and thickness = 2.3 mm. Fiber tracking was performed using the fiber assignment continuous tracking algorithm implemented within the diffusion tensor imaging task card software (Philips Extended MR WorkSpace 2.6.3)[23]. For corticospinal tract analysis, a seed region of interest was placed on the corticospinal tract at the anterior pontomedullary junction on an axial slice and a target region of interest was drawn in the corticospinal tract area of the anterior medulla[24]. The termination criteria were fractional anisotropy < 0.2 and an angle change of > 45 degrees[25].
The corticospinal tract of the left hemisphere originated from the primary sensori-motor cortex and descended through the known corticospinal tract pathway on both 8- and 11-month diffusion tensor tractography. By contrast, the right corticospinal tract originated from the posterior parietal cortex and descended through the posterior margin of the leukomalactic lesion on the 8-month diffusion tensor tractography. This observation was unchanged on the 11-month diffusion tensor tractography (Figure 1B).
DISCUSSION
We report on a patient that regained the ability to walk independently at 11 months after 3 months of rehabilitation that was started at 8 months after intracerebral hemorrhage onset. This seems to be attributted to functional recovery rather than motor recovery because no significant change of the affected corticospinal tract was observed between 8 and 11 months on diffusion tensor tractography which has the advantage of being able to visualize the architecture and integrity of the corticospinal tract at the subcortical level in three dimensions[23,25,26]. Gait requires the coordination of many brain functions related to motorsensory, execution, attention, and visuospatial functions to achieve stepping movements, adequate leg and trunk muscle power and tone, maintenance of equilibrium, and interlimb coordination[8,27,28,29,30].
At the start of our rehabilitation program at 8 months after onset, many problems were found to limit gait function, namely, severe spasticity of both legs, contracture of the affected ankle, somatosensory deficit, visuospatial dysfunction, weakness of the affected leg, and poor postural control. However, after 3 months of rehabilitation therapy (from 8 to 11 months after onset), the patient was able to walk independently on an even floor. Initially, we considered that control of severe spasticity in both legs was the most important factor, and we tried to achieve this using antispastic medication, alcohol neurolysis of the motor branch of the tibial nerve, intramuscular alcohol wash of both tibialis posterior muscles, and physical therapy, including stretching exercises[19,31,32,33]. The severe leg spasticity was relieved and the patient was able to stand with improved postural control. Furthermore, sensorimotor function, visuospatial function, and cognitive improvements also appeared to contribute to the gait recovery. By contrast, the rapid recovery of the left ankle dorsiflexor (from 0 to 2 on the Medical Research Council scale from 8 to 11 months after-onset) suggests the presence of some type of limb kinetic apraxia[34].
Little is known about delayed gait recovery in stroke[34]. In 2012, Kwon and Jang described a patient with intracerebral hemorrhage, who showed unusually delayed leg motor recovery due to the resolution of limb kinetic apraxia, which started at 6 months after intracerebral hemorrhage onset and resulted in the patient being able to walk independently at 9 months after onset. However, functional recovery, mainly attributed to the control of severe spasticity, appears to have been responsible for the regained ability to walk in our patient, not motor recovery. Our results suggest that clinicians should bear in mind the potential for gait recovery, and attempt to determine the causes of gait inability. This investigation will then in turn allow the initiation of intensive rehabilitation aimed at recovering ambulation in stroke patients that cannot walk at 3–6 months after insult.
Footnotes
Funding: This work was supported by the 2012 Yeungnam University Research Grant.
Conflicts of interest: None declared.
(Reviewed by Apricò K, Maxwell R, Rabadi MH, Kisid-Sajewicz K)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Jorgensen HS, Nakayama H, Raaschou HO, et al. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- [2].Jorgensen HS, Nakayama H, Pedersen PM, et al. Epidemiology of stroke-related disability. Clin Geriatr Med. 1999;15:785–799. [PubMed] [Google Scholar]
- [3].Scherder E, Eggermont L, Swaab D, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31:485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [4].Eggermont LH, Gavett BE, Volkers KM, et al. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer's disease. Arch Phys Med Rehabil. 2010;91:584–588. doi: 10.1016/j.apmr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].York DH. Review of descending motor pathways involved with transcranial stimulation. Neurosurgery. 1987;20:70–73. doi: 10.1097/00006123-198701000-00021. [DOI] [PubMed] [Google Scholar]
- [6].Ahn YH, Ahn SH, Kim H, et al. Can stroke patients walk after complete lateral corticospinal tract injury of the affected hemisphere? Neuroreport. 2006;17:987–990. doi: 10.1097/01.wnr.0000220128.01597.e0. [DOI] [PubMed] [Google Scholar]
- [7].Jo HM, Choi BY, Chang CH, et al. The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. Neurorehabilitaiton. 2012;31:207–213. doi: 10.3233/NRE-2012-0790. [DOI] [PubMed] [Google Scholar]
- [8].Jang SH. The recovery of walking in stroke patients: a review. Int J Rehabil Res. 2010;33:285–289. doi: 10.1097/MRR.0b013e32833f0500. [DOI] [PubMed] [Google Scholar]
- [9].Jorgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–412. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- [10].Wade DT, Wood VA, Hewer RL. Recovery after stroke-the first 3 months. J Neurol Neurosurg Psychiatry. 1985;48:7–13. doi: 10.1136/jnnp.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- [12].Kim CH, Kim SJ. Motor recovery after stroke. J of Korean Acad of Rehab Med. 1995;19:55–61. [Google Scholar]
- [13].Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke. 1990;21:247–251. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- [14].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [15].De Renzi E, Motti F, Nichelli P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol. 1980;37:6–10. doi: 10.1001/archneur.1980.00500500036003. [DOI] [PubMed] [Google Scholar]
- [16].Brown T, Bourne R, Sutton E, et al. The reliability of three visual perception tests used to assess adults. Percept Mot Skills. 2010;111:45–59. doi: 10.2466/03.24.27.PMS.111.4.45-59. [DOI] [PubMed] [Google Scholar]
- [17].Lincoln NB, Jackson JM, Adams SA. Reliability and revision of the Nottingham Sensory Assessment for stroke patients. Physiotherapy. 1998;84:358–365. [Google Scholar]
- [18].Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- [19].Doğan-Aslan M, Nakipoğlu-Yüzer GF, Doğan A, et al. The effect of electromyographic biofeedback treatment in improving upper extremity functioning of patients with hemiplegic stroke. J Stroke Cerebrovasc Dis. 2012;21:187–192. doi: 10.1016/j.jstrokecerebrovasdis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- [20].Scheidtmann K, Fries W, Muller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. 2001;358:787–790. doi: 10.1016/S0140-6736(01)05966-9. [DOI] [PubMed] [Google Scholar]
- [21].Carpenter EB, Seitz DG. Intramuscular alcohol as an aid in management of spastic cerebral palsy. Dev Med Child Neurol. 1980;22:497–501. doi: 10.1111/j.1469-8749.1980.tb04354.x. [DOI] [PubMed] [Google Scholar]
- [22].Jang SH, Ahn SH, Park SM, et al. Alcohol neurolysis of tibial nerve motor branches to the gastrocnemius muscle to treat ankle spasticity in patients with hemiplegic stroke. Arch Phys Med Rehabil. 2004;85:506–508. doi: 10.1016/s0003-9993(03)00468-4. [DOI] [PubMed] [Google Scholar]
- [23].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [24].Jang SH. Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J. 2011;52:553–557. doi: 10.3349/ymj.2011.52.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 2004;3:11–17. doi: 10.2463/mrms.3.11. [DOI] [PubMed] [Google Scholar]
- [26].Jang SH. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation. 2011;28:345–352. doi: 10.3233/NRE-2011-0662. [DOI] [PubMed] [Google Scholar]
- [27].Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56:465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- [28].Keenan MA, Perry J, Jordan C. Factors affecting balance and ambulation following stroke. Clin Orthop Relat Res. 1984;182:165–171. [PubMed] [Google Scholar]
- [29].Friedman PJ. Gait recovery after hemiplegic stroke. Int Disabil Stud. 1990;12:119–122. doi: 10.3109/03790799009166265. [DOI] [PubMed] [Google Scholar]
- [30].Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88:115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- [31].Kakuda W, Abo M, Kobayashi K, et al. Anti-spastic effect of low-frequency rTMS applied with occupational therapy in post-stroke patients with upper limb hemiparesis. Brain Inj. 2011;25:496–502. doi: 10.3109/02699052.2011.559610. [DOI] [PubMed] [Google Scholar]
- [32].Kakuda W, Abo M, Momosaki R, et al. Combined therapeutic application of botulinum toxin type A, low- frequency rTMS, and intensive occupational therapy for post-stroke spastic upper limb hemiparesis. Eur J Phys Rehabil Med. 2012;48:47–55. [PubMed] [Google Scholar]
- [33].Jo HM, Song JC, Jang SH. Improvements in spasticity and motor function using a stretching device in chronic hemiparetic stroke patients. NeuroRehabilitation. 2013;32:369–375. doi: 10.3233/NRE-130857. [DOI] [PubMed] [Google Scholar]
- [34].Kwon H, Jang SH. Delayed recovery of gait function in a patient with intracerebral haemorrhage. J Rehabil Med. 2012;44:378–380. doi: 10.2340/16501977-0962. [DOI] [PubMed] [Google Scholar]


