Abstract
The motor relearning program can significantly improve various functional disturbance induced by ischemic cerebrovascular diseases. However, its mechanism of action remains poorly understood. In injured brain tissues, glial fibrillary acidic protein and neurofilament protein changes can reflect the condition of injured neurons and astrocytes, while vascular endothelial growth factor and basic fibroblast growth factor changes can indicate angiogenesis. In the present study, we induced ischemic brain injury in the rhesus macaque by electrocoagulation of the M1 segment of the right middle cerebral artery. The motor relearning program was conducted for 60 days from the third day after model establishment. Immunohistochemistry and single-photon emission CT showed that the numbers of glial fibrillary acidic protein-, neurofilament protein-, vascular endothelial growth factor- and basic fibroblast growth factor-positive cells were significantly increased in the infarcted side compared with the contralateral hemisphere following the motor relearning program. Moreover, cerebral blood flow in the infarcted side was significantly improved. The clinical rating scale for stroke was used to assess neurological function changes in the rhesus macaque following the motor relearning program. Results showed that motor function was improved, and problems with consciousness, self-care ability and balance function were significantly ameliorated. These findings indicate that the motor relearning program significantly promoted neuronal regeneration, repair and angiogenesis in the surroundings of the infarcted hemisphere, and improve neurological function in the rhesus macaque following brain ischemia.
Keywords: neural regeneration, brain injury, stroke, motor relearning program, rhesus macaque, brain ischemia, animal model, neurological function, neurotrophic factor, single-photon emission CT, cerebral blood flow, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
We established a brain ischemia model in the rhesus macaque, as they exhibit more similar properties in inheritance and physiology compared with rodents.
-
(2)
We examined the influence of a motor relearning program on neurological function and expression of glial fibrillary acidic protein, neurofilament protein, vascular endothelial growth factor and basic fibroblast growth factor in brain regions surrounding the ischemic region.
-
(3)
The motor relearning program significantly promoted neuronal repair, regeneration and angiogenesis, and improved neurological function of the rhesus macaque after brain ischemia.
INTRODUCTION
The motor relearning program can significantly improve the functions of patients with brain ischemia, and can produce neuroprotective effects[1,2,3,4,5,6,7,8,9,10,11,12,13]. However, the mechanism by which this therapy benefits brain functional repair and reconstruction post central lesion remains unknown. Several studies have established rat, gerbil, pig, dog and cat models of brain ischemia to investigate the neuronal regeneration and angiogenesis in the surroundings of injury site, but there are only a few primate studies[3,4].
Some questions in studies of nervous system repair include the influence of motor relearning program on neurons and astrocyte in injured brain tissues post brain ischemia, the influence on angiogenesis and blood flow in the surroundings of ischemic areas or ischemic penumbra, and whether this program promotes angiogenesis and increases secretion and growth of related factors that benefit angiogenesis in the injured area.
Neurofilament expression can reflect the growth of neurons and is used as a marker of neuronal function. Glial fibrillary acidic protein is a structural protein of intermediate fibers of astrocyte in the brain, and is used as a marker of mature and reactive astrocytes. Thus, neurofilament and glial fibrillary acidic protein changes can reflect the repair of injured neurons and astrocytes[14,15,16]. Vascular endothelial growth factor can promote proliferation and migration of vascular endothelial cells, induce expression of corresponding ligand and receptor, increase levels of intercellular adhesion molecule and vascular cell adhesion molecule, upregulate expression of endothelial cell surface integrin receptor, degrade dense extracellular matrix, and promote angiogenesis. Basic fibroblast growth factor can trigger angiogenesis[17,18]. Changes in expression of these factors are used to indicate angiogenesis in the surroundings of injured brain tissues. Thus, in the present study, we assessed neurofilament and glial fibrillary acidic protein to investigate neural regeneration, and determined vascular endothelial growth factor and basic fibroblast growth factor expression to investigate angiogenesis, in injured brain tissues. In addition, single-photon emission CT (SPECT) was used to detect cerebral blood flow post brain ischemia to analyze the influence of the motor relearning program on blood supply of injured brain tissues. This may provide insight into the mechanism of the motor relearning program.
This study has several important characteristics. First, we established a brain ischemia model in the rhesus macaque, as they have more similar properties in inheritance and physiology compared with rodents. We also investigated the influence of the motor relearning program on neurological function and expression of neurofilament and glial fibrillary acidic protein in the regions around ischemic tissues, and examined whether the motor relearning program could promote nerve growth and development and repair nerve injury. In addition, we examined the influence of the motor relearning program on neurological function and expression of vascular endothelial growth factor and basic fibroblast growth factor in regions surrounding ischemic tissues to explore the mechanism by which the motor relearning program promotes angiogenesis. Finally, we determined the influence of motor relearning program on cerebral blood flow post brain ischemia.
The overall aim of this study was to investigate whether the motor relearning program can promote neural regeneration and angiogenesis, and to examine the relationship between motor relearning program with brain functional reconstruction, neural regeneration and angiogenesis.
RESULTS
Quantitative analysis of experimental animals
Nine rhesus macaques were randomly assigned to three groups (n = 3): training (middle cerebral artery occlusion + motor relearning program training), ischemic injury, and sham-surgery (exposure of middle cerebral artery). The model was successfully established in three animals of the training group. However, all three rhesus macaques died post-surgery in the ischemic injury group, and two died in the sham-surgery group. The three rhesus macaques in the training group presented a similar injury degree, including left central facial and lingual palsy, complete paralysis in the left limbs and unilateral sensory loss. Thus, three rhesus macaques were included in the final analysis.
Neurological function of rhesus macaques with brain ischemia
The neurological function of animals was assessed using Clinical Rating Scale for stroke[19] at 1 day prior to, and 3, 10 and 60 days following model establishment. Kruskal-Wallis test showed that the motor relearning program training significantly improved the neurological function of three rhesus macaques (P < 0.01; Figure 1), manifested by improved motor function, consciousness, self-care ability and balance function (concordant limbs and facial muscles). However, the muscle strength of paralytic limbs was not significantly ameliorated.
Figure 1.
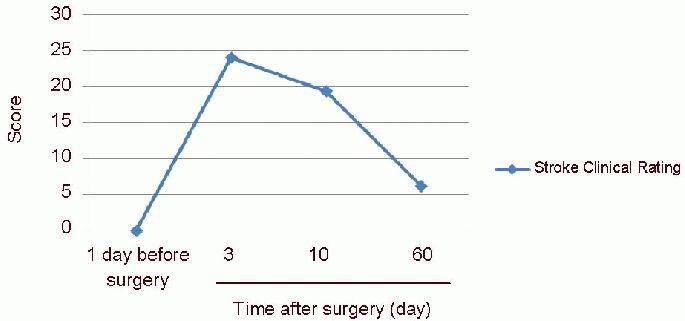
Neurological function changes in rhesus macaques at 1 day prior to and 3, 10 and 60 days following model establishment.
At 1 day prior to model establishment, scores of Clinical Rating Scale for stroke were 0, indicating normal neurological function. At 3 days post-surgery, the scores were 24, indicating peak degree of neurological function injury. Up to 10 days, the scores decreased to 20, demonstrating that the neurological function improved (higher scores indicate more severe injury). At 60 days, the scores further decreased to 6, indicating that neurological function was further improved, but not restored to normal (mean, n = 3, Kruskal-Wallis test). As the scores of Clinical Rating Scale for stroke were not normally distributed, the mean was not expressed as SD or SEM.
The neurologic function changes of three rhesus macaques that succeeded in middle cerebral artery occlusion are shown in Figure 1. Expression of neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor in the precentral motor area of rhesus macaques following motor relearning program training. Following model establishment, the rhesus macaques were subjected to the motor relearning program, and were then sacrificed at 60 days. Immunohistochemistry was used to determine expression of neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor in the precentral motor area. Pathological examination showed that the brain tissues of the ischemic side were liquefied, necrosed, shrunk and formed a stroke capsule (Figure 2).
Figure 2.
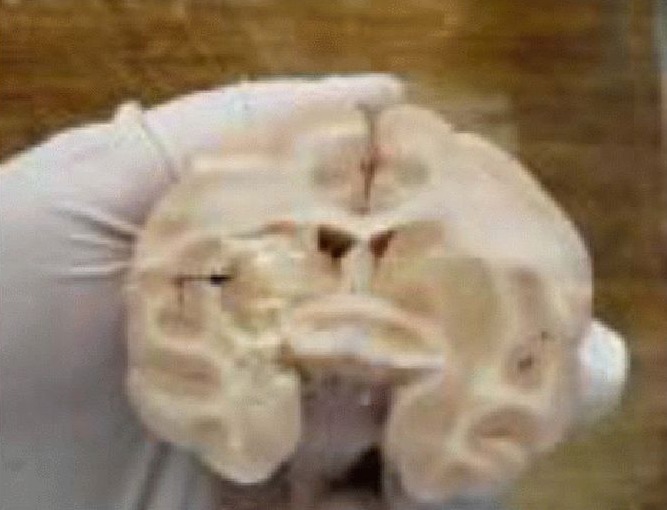
Pathological sections of the rhesus macaques.
The animals were sacrificed at 60 days following the motor relearning program training. The brain tissues were softened (arrow) in the infarcted hemisphere induced by the occlusion of the M1 segment of the right middle cerebral artery using the electrocoagulation method.
High power microscopy showed that the number of neurofilament-, glial fibrillary acidic protein-, vascular endothelial growth factor- and basic fibroblast growth factor-positive cells was significantly increased in the infarcted side compared with the contralateral side, and the positive cells were mainly distributed at the regions surrounding the ischemia (Figure 3).
Figure 3.
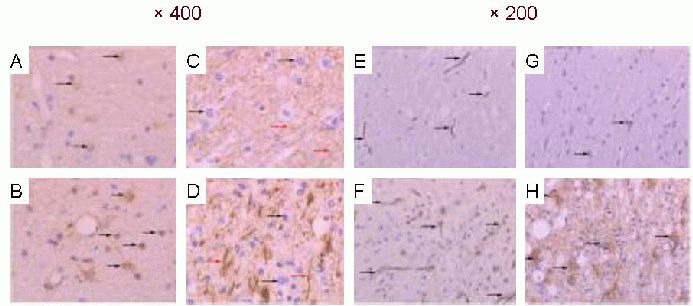
Expression of nerve factors in the precentral motor area of the brain of rhesus macaques (immunohistochemical staining, light microscope; A–D: × 400; E–H: × 200).
The neurofilament (NF)-, glial fibrillary acidic protein (GFAP)-, vascular endothelial growth factor (VEGF)- and basic fibroblast growth factor (bFGF)-positive cells in the precentral motor area are observed as brown yellow or brown particles in the cytoplasm. The number of GFAP-, NF-, VEGF- and bFGF-positive cells was larger in the infarcted side (A, C, E, G) compared with the contralateral side (B, D, F, H; black arrows). In particular, NF fibers were distributed evenly and normally in the non-injured side (red arrows in C), but were disordered and significantly increased in the infarcted side (red arrows in D).
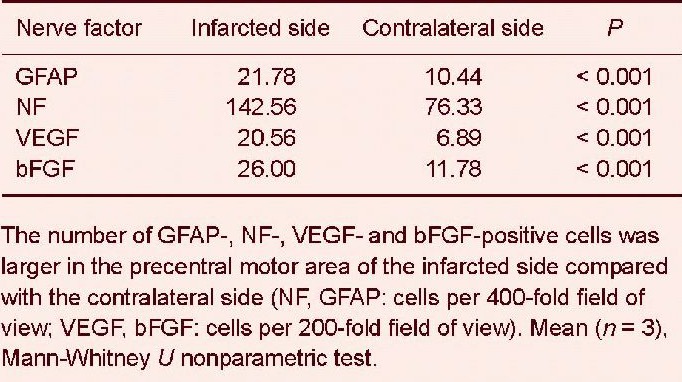
The expression of neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor was significantly increased in the precentral motor area of infarcted side compared with the normal side at 60 days following the motor relearning program training (P < 0.001). This indicates that the motor relearning program training significantly increased content of neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor in the ischemic hemisphere (Table 1).
Table 1.
Number of neurofilament (NF)-, glial fibrillary acidic protein (GFAP)-, vascular endothelial growth factor (VEGF)- and basic fibroblast growth factor (bFGF)-positive cells in the precentral motor area of rhesus macaques with brain ischemia.
Cerebral blood flow in rhesus macaques with brain ischemia following motor relearning program training
Kruskal-Wallis test showed that brain ischemia significantly altered regional cerebral blood flow of the rhesus macaques (P = 0.042; Figure 4).
Figure 4.
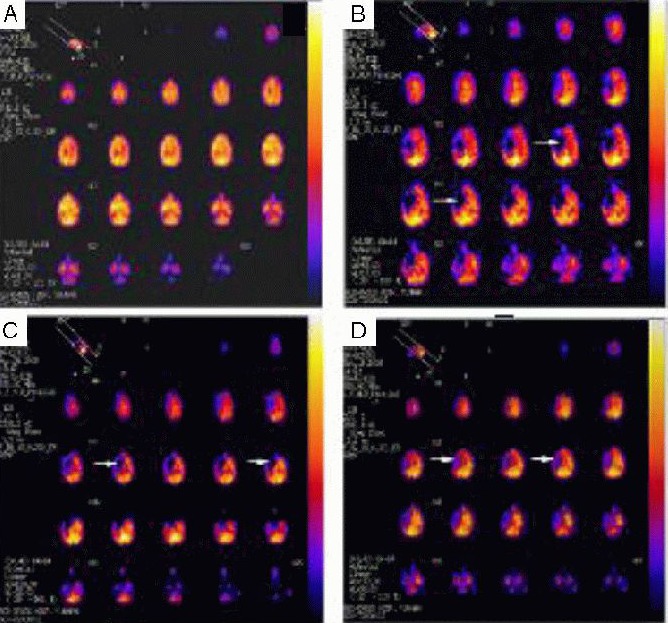
Changes of cerebral blood flow in rhesus macaques with brain ischemia (SPECT of one rhesus macaque as representative).
(A) 1 day prior to surgery; (B) 3 days post-surgery; (C) 10 days post-surgery; (D) 60 days post-surgery. Arrows represent the blood supply of the M1 segment of the right middle cerebral artery. The blue region reflects that the radioactivity of regional brain tissues was significantly reduced or even lost, indicating ischemic focus. The red region indicates normal radioactivity of regional brain tissues, suggesting normal blood flow perfusion. The yellow region (even white) represents higher radioactivity of regional brain tissues than normal brain tissues, indicating increased blood flow. At 3 days post-surgery, ischemia was evident in the injury area. Up to 60 days, the ischemic region was significantly diminished, but not restored to normal.
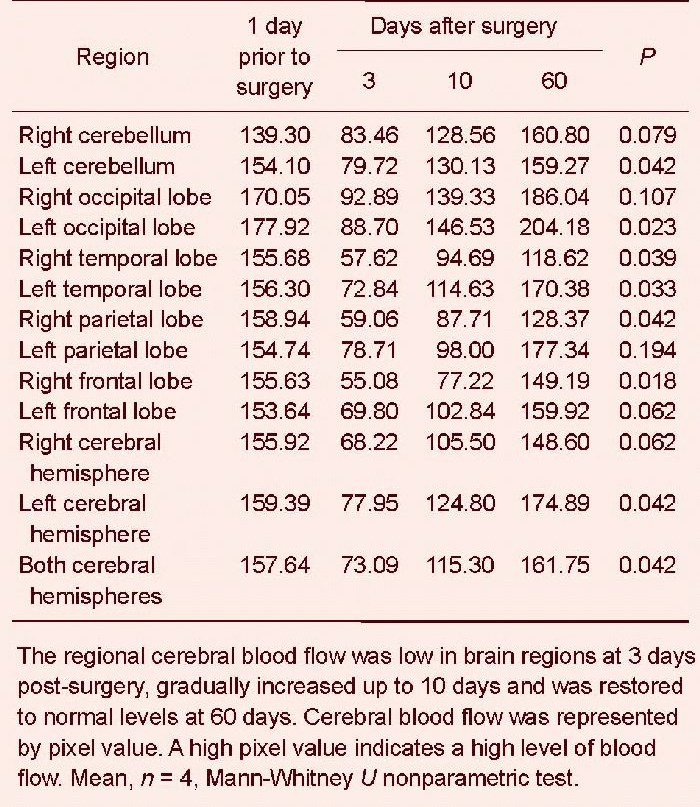
In particular, the regional cerebral blood flow in the left cerebellum (P = 0.042), left occipital lobe (P = 0.023), right temporal lobe (P = 0.039), left temporal lobe (P = 0.033), right parietal lobe (P = 0.042), right frontal lobe (P = 0.018) and left hemisphere (P = 0.042) was significantly reduced. In addition, the regional cerebral blood flow of the above brain regions was reduced at 3 days post-surgery compared with 1 day prior to surgery; the blood flow gradually increased at 10 days post-surgery, and was restored to normal up to 60 days. In particular, the blood flow in some brain regions was even higher than prior to surgery, including the bilateral cerebellum, bilateral occipital lobe, left temporal lobe, left parietal lobe, left frontal lobe, left hemisphere and the entire brain (Table 2). At 3 days post-surgery, the ischemic injury was evident (P < 0.001); at 10 days, the ischemic areas gradually decreased (P < 0.001); up to 60 days, the ischemic areas were further decreased (Figure 4).
Table 2.
Regional cerebral blood flow changes in different brain regions prior to and following model establishment (single-photon emission CT).
DISCUSSION
Stroke, also termed a cerebrovascular accident or cerebral paralysis, is a sudden disease due to disturbance in the blood supply to the brain. Stroke has a high morbidity, mortality, disability rate and recurrence rate. Approximately 2 million new patients with stroke appear yearly in China, and 70–80% of them cannot live independently because of disability[20]. Modern rehabilitation theory and practice demonstrate that effective rehabilitation training can ameliorate functional disability in stroke patients, improve their quality of life, improve rehabilitation, reduce potential nursing costs and decrease social resources[21,22]. A motor relearning program is a rehabilitation training focusing on motor function recovery post central nervous system injury, and is based on theories of biomechanics, sports science, neuroscience and cognitive psychology. This method aims to restore the motor function of patients using scientific motor and learning methods[23,24,25,26,27,28,29,30]. The motor relearning program involves rehabilitation in room, and is: (1) active: the patient should actively participate in the training with the guidance of therapists, and they should analyze factors and causes of their functional disturbance and solve the problems through persistent training (this is the basis of the motor relearning program); (2) scientific: the motor relearning program is a scientific training method as it is designed according to the theories of biomechanics, sports science, neuroscience and cognitive psychology specifically to common motor disturbance in stroke patients; (3) specific: the learning and training content of the program is designed individually according to motor disturbance in different patients; (4) practical: the motor training in the program is closely related with functional activities in daily life; and (5) systematic: in addition to learning in the treatment room, learning transfer and persistence are involved in the program, which requires participation of the patients families. Thus, the motor relearning program is regarded as one of the most advanced rehabilitation approaches.
In the present study, we used the motor relearning program in rhesus macaques with brain ischemia, and the Clinical Rating Scale for stroke showed that the motor relearning program significantly improved neurological function in injured animals. The motor relearning program can effectively promote motor function recovery and significantly improve consciousness, self-care ability and balance function (concordance of limbs and facial muscles) of animals. However, the muscle strength of paralytic limbs was not significantly ameliorated. This may be because the rhesus macaque is a quadruped, and they are good at adjusting balance concordance. In addition, there may be a self-repair mechanism in the rhesus macaque post injury. Nevertheless, our data indicate that the motor relearning program may promote neural regeneration of injured brain tissues.
To further investigate whether the motor relearning program could influence neurons and astrocytes in the ischemic brain tissues, we examined neurofilament and glial fibrillary acidic protein expression to assess the neural regeneration post brain ischemia[31,32,33,34,35,36,37]. By immunohistochemistry, expression of neurofilament protein and the number of neurofilament-positive nerve fibers in the precentral motor area were significantly greater in the infarcted hemisphere compared with the contralateral following the motor relearning program. In addition, the content of glial fibrillary acidic protein in glial cells in the injured area was significantly increased compared with the contralateral side following the motor relearning program. These data suggest that although the brain tissues were markedly damaged in the infarcted hemisphere, neurofilament and glial fibrillary acidic protein expression was altered in the bilateral hemispheres following the motor relearning program. On the one hand, the injured brain structures may exhibit a compensatory increase of neurofilament and glial fibrillary acidic protein expression post ischemia (i.e., natural repair of the brain). On the other hand, the motor relearning program also increased their expression in the brain tissues surrounding the infarction compared with the normal hemisphere, demonstrating a neuroprotective role of the motor relearning program.
Brain ischemia can induce neuronal degeneration and necrosis, resulting in neurological function impairment. Thus, restoration of blood supply in the injured areas is critical for neurological function recovery. Angiogenesis and pro-angiogenic factors play important roles in this process[38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Thus, in the present study, we also determined changes of vascular endothelial growth factor and basic fibroblast growth factor, which can reflect angiogenesis. Results showed that vascular endothelial growth factor and basic fibroblast growth factor expression levels were significantly greater in the brain tissues of infarcted hemisphere compared with the normal side following the motor relearning program. Moreover, the quantity of newly generated blood capillaries was significantly greater in the infarcted hemisphere than in the normal side. This indicates that the motor relearning program may construct new blood supply and promote cerebral blood flow by inducing angiogenesis resulting from increased vascular endothelial growth factor and basic fibroblast growth factor expression.
Brain ischemia can induce alteration in regional blood flow. Our SPECT results showed that middle cerebral artery occlusion significantly reduced regional cerebral blood flow of the rhesus macaque, while the motor relearning program significantly improved cerebral blood flow in different brain regions, manifested by restored blood flow in the infarcted hemisphere and increased blood flow in some brain regions compared with prior to model establishment. Thus, the motor relearning program enhances the self-regulation of cerebral blood flow to rebuild cerebral blood circulation, and increases cerebral blood flow through training of muscle strength, balance, coordination, sitting position, standing, walking, upper limbs and head, face and oral cavity functions[52,53,54].
During model establishment, five rhesus macaques died and one was not successful in establishing the ischemic model because of inaccurate operation or localization, such as excessive injury scope, cerebral edema, herniation of brain and infection. Thus, these risk factors should be carefully monitored to minimize the death of animals.
In summary, the motor relearning program effectively improved neurological function of the rhesus macaque with brain ischemia, potentially because of positive effects on neural regeneration, angiogenesis and cerebral blood flow in the regions surrounding the ischemic lesion[55,56,57,58,59]. This study provides theoretical evidence for the clinical application of the motor relearning program for treatment of brain ischemia. However, further studies are required with a large number of samples to further elucidate the mechanisms underlying the actions of the motor relearning program.
MATERIALS AND METHODS
Design
An animal study of neuroimaging.
Time and setting
The experiments were conducted in the Laboratory of Primate Motor, Sensation and Integration, Kunming Institute of Zoology, Chinese Academy of Sciences, China from December 2009 to May 2011.
Materials
Nine healthy male rhesus macaques, aged 9 years, weighing 7–9 kg, were provided by the Laboratory Animal Center of Kunming Institute of Zoology, Chinese Academy of Sciences, China (license No. SYXK (Dian) 2008-0001). The rhesus macaques were separately raised at constant humidity and temperature with a 12-hour day/night cycle. Their food was specifically prepared, and contained fruits and vegetables with abundant cellulose and vitamin. Animals had free access to water. Animal procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[60].
Methods
Establishment of middle cerebral artery occlusion model by electrocoagulation
The brain ischemia model was induced by occlusion of the single great vessel using a previously described method[61], with some modification. This method produced models with a similar degree of functional disability. In brief, food, but not water, was deprived for 24 hours prior to surgery. The rhesus macaques were routinely anesthetized with 10 mg/kg ketamine hydrochloride, 20–30 mg/kg sodium pentobarbital and 0.3 mg/kg atropine, and placed on the operation table. The right skull was sterilized, and a hole was drilled at 2 cm above the center of the right orbit (sagittal sinus of the right skull). The cranium was removed, and an incision was made at the cerebral dura mater of the right frontal lobe to the frontal pole, sagittal sinus and ventral temporal lobe. The frontal pole was raised under an operating microscope, and the middle cerebral artery was found at the juncture of the frontal lobe and the temporal lobe along the olfactory tract. The M1 segment of the right middle cerebral artery, 2 mm medial to the olfactory tract (Figure 5), was electrocoagulated using a bipolar electrocoagulation system (Erbe Elektromedizin TÜbingen, Germany). The animals were returned to the cage and observed until they recovered from anesthesia. Successful injury was demonstrated by animals exhibiting left central facial and lingual palsy, complete paralysis in the left limbs and unilateral sensory loss. Mannitol, furosemide and albumin were used to reduce cranial pressure if necessary.
Figure 5.
Appearance of the M1 segment (arrow) of the normal right middle cerebral artery.
Modified motor relearning program
According to the properties of the rhesus macaque, the motor relearning program was modified and was performed for 1 hour daily for 60 days. In brief, one physiotherapist began to train the animals from the third day post-surgery, including consciousness, self-care ability, limb strength, general balance coordination, sitting, standing, walking, upper limb function and motor skills of hands (and fingers). During 1 to 30 days, the training mainly targeted awakening consciousness using food induction[62], including tactile stimulation (perception, alertness, stare, movement or aggressive acts and defensive reaction in response to approaching of the physiotherapist). In addition, the self-care ability was enhanced by training including swallowing water feeding into the mouth, chewing the food placed into the mouth and taking the food placing in the hands of the physiotherapist or from the case. In the training of limb strength and coordination ability, the animals were coaxed using food to lift up their healthy upper limbs, attempted to catch the cage or other supports using the healthy limbs and to shake or pull the cage to maintain standing. The animals were also trained to sit straight and stably with or without supports using food induction, to stand in the cage, to sit and stand, to stand and sit and to walk in the cage. During 30 to 60 days, goal-oriented tasks were conducted using food induction to enhance their body posture, such as moving around the cage, sitting out of the cage, static and dynamic equilibrium of standing and walking out of the cage. The training of limb strength and coordination ability included lifting up the hemiplegic upper limbs, attempting to catch the cage using the hemiplegic upper limbs, catching the cage or other supports using the hemiplegic upper limbs, shaking or pulling the cage using the hemiplegic upper limbs to maintain standing and maintaining coordination of bilateral upper limbs and four limbs (Figure 6).
Figure 6.

Motor relearning program training for the rhesus macaque.
(A) Tests in cage: the left forelimbs of animals were weak during induction to walk, stand, sit and balance function training at the 7th day post-surgery. (B) Tests out of cage: the left forelimbs of animals could load weight during walking training out of the cage at the 48th day post-surgery.
The Clinical Rating Scale for stroke[19] was used to evaluate the behaviors of rhesus macaques with brain injury at 1 day prior to and 3, 10 and 60 days following model establishment. Higher scores represent more severe neurological impairment.
Immunohistochemical staining for expression of neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor in the precentral motor area of rhesus macaques
The animals were anesthetized and sacrificed at 60 days post-surgery. The brain was fixed in 4% paraformaldehyde, and brain tissues in the precentral motor area of blood supply region of bilateral middle cerebral arteries were harvested, dehydrated, cleared, immersed in paraffin, embedded and sectioned (3 μm thick). The sections were dewaxed, hydrated, retrieved with antigen, incubated with peroxidase blocking agent at room temperature for 10 minutes and washed with PBS for 3 × 3 minutes. The PBS was removed, and the sections were incubated with rat anti-human neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor monoclonal antibodies (ready-to-use; Maixin Biotechnique, Fuzhou, China) at room temperature for 60 minutes or overnight at 4°C, washed with PBS for 3 × 3 minutes, followed by rabbit anti-rat IgG at room temperature for 15 minutes. After washes with PBS for 3 × 3 minutes each, the sections were mixed with freshly prepared 3,3’-diaminobenzidine (Maixin Biotechnique), washed with tap water to terminate the reaction, counterstained with hematoxylin, differentiated, returned to blue, dehydrated with gradient alcohol, cleared with xylene and mounted with neutral gum. The sections were observed and photographed using a light microscope (Olympus, Tokyo, Japan). The control group was treated with PBS, and the results were negative.
Image acquisition and analysis
The stained sections were magnified under a BX51 light microscope (Olympus; neurofilament and glial fibrillary acidic protein at 400 ×; vascular endothelial growth factor and basic fibroblast growth factor at 200 ×). Five regions were randomly selected from the infarction region. Brain tissue sections from one site of the infarction region contained one hematoxylin-eosin stained section, one neurofilament, glial fibrillary acidic protein, vascular endothelial growth factor and basic fibroblast growth factor antibody, for five sections in total. The number of positive cells in one region was quantified and the results were represented by the mean value.
SPECT for cerebral blood flow of injured brain regions
99Tcm-ethyl cysteinate dimer SPECT perfusion imaging technique was used to semiquantitatively detect cerebral blood flow in related brain regions[63] using a dual-detector GCA7200A/UA SPECT system (Toshiba, Tokyo, Japan) equipped a with low energy high-resolution parallel hole collimator, matrix 128×128, and magnification 2.0. 99Tcm-ethyl cysteinate dimer was used as the developing agent. 99Tcm was provided by Beijing Atom HighTech Co., Ltd., Beijing, China, and ethyl cysteinate dimer was provided by the College of Chemistry, Beijing Normal University, China. The SPECT detection was performed at 1 day prior to and 3, 10 and 60 days following model establishment. In brief, the rhesus macaques were deprived of food and water for 4–6 hours prior to detection. Animals were fed with tomato injected with 20 mg/mL potassium perchlorate solution (0.5 mL/kg) to block the choroid plexus. After 30 minutes, atropine (0.05 mg/kg) was intramuscularly injected to prevent vagus reflex and vomiting; after 5 minutes, 5.0 mg/kg ketamine and 2.0 mg/kg sodium pentobarbital were injected into the muscle group of both lower limbs to anesthetize the animals. After 1 hour, black eyeshades and cotton were used to block the eyes and ears. After 15 minutes, 99Tcm ECD at a dose of 2.5 mCi/kg was injected via femoral vein. After another 15 minutes, the animals were placed in supine position on the examining table, and the head and body were fixed using adhesive tape. The camera was rotated around the head for 360 degrees using 60 frame images, 1 frame/6°, 15 seconds/frame and a net counting rate of 40–80 k/frame. The raw data were filtered using a Butterworth low-pass filter (Toshiba), and images were reconstructed with Ramp function filtered back projection. The original transverse section images were corrected with the OM line (the line that connects the lateral canthus and the external auditory meatus), and transverse, sagittal and coronal images were reconstructed. The reconstructed transverse section images were 20–22 slices and 2.0 mm thick.
The images were semiquantitatively analyzed by two nuclear medicine physicians blinded to the experiment. The symmetric region of interest of the frontal lobe, parietal lobe and occipital lobe were outlined at the 13th to 15th slices (three continuous slices) above the OM line, and the symmetric region of interest of the temporal lobe and cerebellum outlined at the 18th to 20th slices (three continuous slices). One side of the region of interest of the brain lobes was outlined clearly at the transverse section images, and then mirrored to the contralateral brain lobe to ensure equal region of interest size and appearance. For brain lobes with reduced radioactive distribution, the region of interest mirror of the normal brain lobe was copied in this brain region. The mean radioactive counting of the bilateral region of interest of each brain region was calculated according to the formula (sum of mean region of interest in each slice of one brain region/slices of this brain region). The degree of ischemia was evaluated by statistical analyses. Cerebral blood flow was represented by pixel value.
Statistical analysis
Measurement data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). All data were not normally distributed. The results of neurological function and regional cerebral blood flow prior to and following model establishment were analyzed using Kruskal-Wallis nonparameter test; the results of number of nerve factor-positive cells between infarcted and normal hemispheres were compared with using Mann-Whitney U-test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Yucheng Xie and Tao Sun, Department of Pathology, Fourth Hospital of Kunming Medical University, China for technical support. We also thank Zhengbo Wang and Yingzhou Hu, Kunming Institute of Zoology, Chinese Academy of Sciences, China for their help in model establishment.
Footnotes
Funding: This study was supported by the Combined Specific Foundation of Department of Science and Technology of Yunnan Province and Kunming Medical University, No. 2008CD037.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of the Second People's Hospital of Yunnan Province, China.
(Reviewed by Dean J, Raye Z, Wang F, Tian HL, Zhang QG)
(Edited by Wang J, Su LL, Li CH, Song LP)
REFERENCES
- [1].Carr JH, Shepherd R. Beijing: Peking University Press; 1999. A Motor Relearning Program for Stroke. [Google Scholar]
- [2].Carr JH, Shepherd R. Beijing: Peking University Press; 2007. Stroke Rehabilitation-Guidelines for Exercise and Training to Optimize Motor Skill. [Google Scholar]
- [3].Alexander W, Kiarash JG, Kristian PD, et al. A new model of cortical stroke in the rhesus macaque. J Cereb blood flow Metab. 2009;29(6):1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang Q, Zhang T, Zhan CY, et al. Nonhuman primate chronic stroke model with middle cerebral artery endovascular embolish. Zhongguo Kangfu Lilun yu Shijian. 2012;18(5):401–405. [Google Scholar]
- [5].Wang Q, Zhang T. Advance in research establishment and neurological behavioral assessment of non-human primate local cerebral ischemic model (review) Zhongguo Kangfu Lilun yu Shijian. 2008;14(6):539–541. [Google Scholar]
- [6].Chan DY, Chan CC, Au DK. Motor relearning programme for stroke patients: a randomized controlled trial. Clin Rehabil. 2006;20(3):191–200. doi: 10.1191/0269215506cr930oa. [DOI] [PubMed] [Google Scholar]
- [7].Pandian S, Arya KN, Davidson EW. Comparison of brunnstrom movement therapy and motor relearning program in rehabilitation of post-stroke hemiparetic hand: a randomized trial. J Bodyw Mov Ther. 2012;16(3):330–337. doi: 10.1016/j.jbmt.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [8].Langhammer B, Stanghelle JK. Can physiotherapy after stroke based on the Bobath concept result in improved quality of movement compared to the motor relearning programme. Physiother Res Int. 2011;16(2):69–80. doi: 10.1002/pri.474. [DOI] [PubMed] [Google Scholar]
- [9].Guo WL, Zhang HJ, Feng YQ, et al. Effect of motor relearning program on recovery of motor ability with stroke patients. Zhongguo Shiyong Shenjing Jibing Zazhi. 2009;12(6):91–92. [Google Scholar]
- [10].Yu Q, Li XL, Tan B, et al. Effect of motor relearning program on recovery of motor ability and P300 potential of the cerebral infarction patients. Zhongguo Zuzhong Zazhi. 2009;4(10):824–827. [Google Scholar]
- [11].Qing DW, Guo TL. Effect of motor relearning programme on lower limbs function of stroke patients. Zhongguo Kangfu Lilun yu Shijian. 2010;16(4):372–373. [Google Scholar]
- [12].Wurm F, Keiner S, Kunze A, et al. Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke. 2007;38(10):2833–2840. doi: 10.1161/STROKEAHA.107.485524. [DOI] [PubMed] [Google Scholar]
- [13].Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59(5):735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- [14].Shen Y, Yu HM. Hangzhou: Zhejiang University; 2009. Protective Effect of Erythropoietin on White Matter Damage in Neonatal Rat Brain after Intrauterine infection. [Google Scholar]
- [15].Uchidal A, Brown A. Arrival, reversal, and departure of neurofilament at the tips of growing axons. Mol Biol Cell. 2004;15(9):4215–4225. doi: 10.1091/mbc.E04-05-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomes FC, Paulin D, Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res. 1999;32(5):619–631. doi: 10.1590/s0100-879x1999000500016. [DOI] [PubMed] [Google Scholar]
- [17].Barid A, Waliche P. Fibroblast growth factors. Br Med Bull. 1989;45(2):438–452. doi: 10.1093/oxfordjournals.bmb.a072333. [DOI] [PubMed] [Google Scholar]
- [18].Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004;113(1):14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ben R, Naimath K, Eray T, et al. Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging, and anatomical study. Neurol Res. 2003;25(1):68–78. doi: 10.1179/016164103101200950. [DOI] [PubMed] [Google Scholar]
- [20].Zhao D. Epidemiological studies of stroke in Chinese population. Zhonghua Liuxingbingxue Zazhi. 2003;24(3):236–239. [Google Scholar]
- [21].Thorsén AM, Holmqvist LW, de Pedro-Cuesta J, et al. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: five-year follow-up of patient outcome. Stroke. 2005;36(2):297–303. doi: 10.1161/01.STR.0000152288.42701.a6. [DOI] [PubMed] [Google Scholar]
- [22].Ostwald SK, Davis S, Hersch G, et al. Evidence-based educational guidelines for stroke survivors after discharge home. J Neurosci Nurs. 2008;40(3):173–191. doi: 10.1097/01376517-200806000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carr JH, Sepherd RB, Nordholm L, et al. A motor assessment scale for stroke. Phys Ther. 1985;65:175–180. doi: 10.1093/ptj/65.2.175. [DOI] [PubMed] [Google Scholar]
- [24].Carr JH, Sepherd RB, Nordholm L, et al. Investigation of a new motor assessment scale for stroke patients. Phys Ther. 1985;65(2):175–180. doi: 10.1093/ptj/65.2.175. [DOI] [PubMed] [Google Scholar]
- [25].Carr JH. London: Heinemann Physiotherapy; 1987. A motor relearning programme for stroke. [Google Scholar]
- [26].Carr JH. MD: Aspen Publishers, USA; 1987. Movement Science: Foundations for Physical Therapy in Rehabilitation. [Google Scholar]
- [27].Carr JH, Sepherd RB. A motor learning model for stroke rehabilitation. Physiotherapy. 1989;75(7):372–380. [Google Scholar]
- [28].Carr JH, Mungovan SF, Shepherd RB, et al. Physiotherapy in stroke rehabilitation: bases for Australian physiotherapists’ choice of treatment. Physiother Theory Pract. 1997;10(4):201–209. [Google Scholar]
- [29].Carr JH, Sepherd RB. London, UK: Butterworth-Heinemann; 2003. Stroke rehabilitation: guidelines for exercise and training to optimize motor skill. [Google Scholar]
- [30].Carr JH, Sepherd RB. Neurological rehabilitation: optimizing motor performance. Elsevier India. 2010 [Google Scholar]
- [31].Rosengren LE, Karlsson JE, Sjögren M, et al. Neurofilament protein levels in CSF are increased in dementia. Neurology. 1999;52(5):1090–1090. doi: 10.1212/wnl.52.5.1090. [DOI] [PubMed] [Google Scholar]
- [32].Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- [33].Grant P, Pant HC. Neurofilament protein synthesis and phosphorylation. J Neurocytol. 2000;29(11-12):843–872. doi: 10.1023/a:1010999509251. [DOI] [PubMed] [Google Scholar]
- [34].Sjögren M, Blomberg M, Jonsson M, et al. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res. 2001;66(3):510–516. doi: 10.1002/jnr.1242. [DOI] [PubMed] [Google Scholar]
- [35].Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- [36].Foerch C, Curdt I, Yan B, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77(2):181–184. doi: 10.1136/jnnp.2005.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lumpkins KM, Bochicchio GV, Keledjian K, et al. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65(4):778–784. doi: 10.1097/TA.0b013e318185db2d. [DOI] [PubMed] [Google Scholar]
- [38].Hayashi T, Abe K, Suzuki H, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28(10):2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- [39].Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18(8):887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- [40].Cobbs CS, Chen J, Greenberg DA, et al. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249(2):79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- [41].Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56(3):794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- [42].Issa R, Krupinski J, Bujny T, et al. Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Lab Invest. 1999;79(4):417–425. [PubMed] [Google Scholar]
- [43].Slevin M, Krupinski J, Slowik A, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-β1 in serum of patients with acute ischemic stroke. Stroke. 2000;31(8):1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- [44].Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97(18):10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- [47].Zhang R, Wang L, Zhang L, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- [48].Mu D, Jiang X, Sheldon R, et al. Regulation of hypoxia-inducible factor 1α and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14(3):524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- [49].Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Henry TD, Annex BH, McKendall GR, et al. The VIVA trial vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- [51].Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endoc Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- [52].Ling F. Peking: People's Medical Publishing House; 2007. Cerebral Vascular Disease Therory and Practice. [Google Scholar]
- [53].Purvis T, Cadilhac D, Donnan G, et al. Systematic review of process indicators: including early rehabilitation interventions used to measure quality of acute stroke care. Int J Stroke. 2009;4(2):72–80. doi: 10.1111/j.1747-4949.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- [54].Yin Y, Gu Z, Yang B, et al. Effect of motor relearning program on regional Blood flow (rCBF) of rhesus monkeys with cerebral ischemic stroke. Zhongguo Kangfu. 2012;27(2):83–85. [Google Scholar]
- [55].Del-Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267(2):156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- [57].Kononen M, Kuikka JT, Husso-Saastamoinen M, et al. Increased perfusion in motor areas after constraint, induced movement therapy in chronic stroke: a single-photon emission computerized tomography study. J Cereb Blood Flow. 2005;25(12):1668–1674. doi: 10.1038/sj.jcbfm.9600158. [DOI] [PubMed] [Google Scholar]
- [58].Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99(10):1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- [59].Iwai M, Cao G, Yin W, et al. Erythrupoietin promotes rlellronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- [60].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 [Google Scholar]
- [61].Marshall JW, Ridley RM. Assessment of functional impairment following permanent middle cerebral artery occlusion in a non-human primate species. Neurodegeneration. 1996;5(3):275–286. doi: 10.1006/neur.1996.0036. [DOI] [PubMed] [Google Scholar]
- [62].Mack WJ, King RG, Hoh DJ, et al. An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol Res. 2003;25(3):280–284. doi: 10.1179/016164103101201346. [DOI] [PubMed] [Google Scholar]
- [63].Tanaka F, Vines D, Tsuchida T, et al. Normal patterns on 99mTc-ECD brain SPECT scans in adults. J Nucl Med. 2000;41(9):1456–1464. [PubMed] [Google Scholar]


