Abstract
Objectives
We determined whether any individual cancers are increased or decreased in a cohort of 595 patients with systemic lupus erythematosus (SLE) followed for up to 32 years at the University College London Hospitals Lupus Clinic, looking for any associated clinical or serological factors and the prognosis after cancer diagnosis.
Methods
We undertook a careful retrospective review of the medical records and identified all individuals diagnosed with cancer. For controls, we selected three other patients in the cohort who had not developed cancer, carefully matched for age, sex, ethnicity and disease duration, to determine if any obvious differences emerged in a nested case-control design.
Results
Thirty-three patients developed cancer after being diagnosed with SLE. There was a statistically insignificant small increase in overall cancer risk, standardized incidence ratios (SIRs) 1.05 (95% CI 0.52–1.58) and increased SIRs for cervical, prostate, anal and pancreatic cancers and reduction in breast cancer SIRs.
Haematological and musculoskeletal manifestations, anticardiolipin and antithyroid globulin antibodies were found to be positively associated with cancer risk in multivariate analysis. There was no drug, dose or duration was associated with cancer risk. There was a reduction in survival with a cancer fatality rate of 84.2% (p < 0.0001).
Conclusion
We found a very small but statistically insignificant increased cancer risk with reduction in survival. Whereas some cancers appear to be more common in SLE, notably prostate and cervical cancer, others, particularly breast cancer, are less frequent. Multiple clinical and serological factors are involved in the increased risk of malignancy in SLE. No drug dose or duration effect was identified.
Keywords: Systemic lupus erythematosus (SLE), neoplasm, risk factors, immunosuppressive therapy
Introduction
Over the past 50 years the survival of systemic lupus erythematosus (SLE) patients has improved, with rates improving from less than 50% at five years in 1955 to over 92% at 10 years.1
This improvement is attributed to better and earlier diagnosis, optimized management of disease and prevention of organ damage. Despite these advances in care, the life expectancy of patients with SLE remains lower than the general population.2 This reduction is attributed to the fact that as care improves, more chronic factors (cardiovascular morbidity, malignancy and increased risk of infections) now contribute to the long-term sequelae of the disease and its management.3,4
Links between malignancy and autoimmune rheumatic disease have focused on lymphoma, especially Non-Hodgkin’s lymphoma (NHL).5 However, is there really an overall increased risk of malignancy in SLE? Various clinical series and cohort studies have sought to clarify the issue; some have shown an increased risk6–8 whilst others have not.9,10 In the most comprehensive report so far, the Systematic Lupus International Collaborating Clinics (SLICC) group, in an international multicentre cohort study involving 9547 patients, reported an increased risk of malignancy in SLE, especially NHL.11
In spite of various attempts, the question of whether medication influences this risk is unresolved. Studies in patients with rheumatoid arthritis (RA) and post-organ transplant have confirmed the increased risk of malignancy associated with immunosuppressive drugs, but this still remains inconclusive in patients with SLE.12–14
There are several hypotheses about the pathogenesis of malignancy in SLE, including medication use, interaction with viruses, disordered immunosurveillance, and coexistence of other diseases like Sjögren’s and RA that have a known association with malignancy.15–19
To explore the possible pathogenic risk factors further, we undertook a detailed analysis within the University College London Hospitals (UCLH) lupus cohort of 36 patients who have developed cancer, analysing the disease manifestations, autoantibody profile, associated diseases and in particular, the steroid and immunosuppressive regimens they were treated with. We have taken advantage of the unusually long-term follow-up (up to 32 years) and data recording in this cohort to establish, as accurately as possible, the relative risk of cancer and the specific types of malignancy after the diagnosis of SLE. We were particularly interested to discover whether the increased incidence, if any, is associated with the clinical characteristics of patients or the type of immunosuppressive therapy and if so, what cumulative dose or time interval of exposure is associated with this risk, and to review the prognosis and cause of death in the cohort after developing cancer.
Methods
Approval for this study was obtained from the UCLH Research and Development Committee.
Study population
The UCLH Lupus Clinic has a cohort of 595 SLE patients diagnosed and followed up for up to 32 years, i.e. from 1978 to the end of December 2010. All patients followed up over time are entered into the cohort when they fulfil the American College of Rheumatology (ACR) revised classification criteria for SLE.20
Selection of cases and controls
We identified all 36 individuals who have developed cancer after being diagnosed with SLE and selected as controls three individuals for each of the “cancer cases” who have SLE, but have not developed cancer in the cohort during this period of observation. The controls were carefully matched for age, sex, ethnicity and disease duration. These control patients had disease duration within five years of their matched index case.
No cases of drug-induced, discoid lupus or undifferentiated autoimmune disease were included in this cohort study. However, cases of SLE meeting the ACR criteria, who have met standard criteria for other autoimmune conditions, were included to examine the possible links with concomitant diseases, such as Sjögren’s syndrome, which has been found to increase cancer risk.
Case definition
Cancers were identified through the hospital medical records and confirmed by histological or autopsy reports. Cancer cases had been identified by clinical features suggestive of cancer and incidental findings, and some cases of cervical cancer were identified through a routine screening programme offered to all women in the United Kingdom (UK) in the relevant age group.
The health system in the UK requires patients to have family practitioners, and regular correspondence between the practitioner and the lupus clinic helps to guarantee that any malignancy identified by either “side” will be communicated effectively. As part of the malignancy studies conducted by the SLICC group, we have been in contact with the UK National Cancer Registry to check for any cancer cases our SLE patients reported to them. Thus it is most unlikely that any case of malignancy was missed in our cohort.
Index date
The follow-up period was from the date of diagnosis of SLE till the last review date or death or 31 December 2010. (Disease duration was ascertained as the time of diagnosis of SLE, i.e. when the patients reach their fourth lupus criterion, to the date of their cancer diagnosis.)
We undertook retrospective analysis of the medical records of the patients, collecting data on age, sex, date of diagnosis of SLE and cancer, duration between cancer and SLE diagnosis, type of cancer diagnosed, time to death if relevant, clinical manifestations, autoantibody profile, smoking history, nonsteroidal anti-inflammatory drug (NSAID) use, immunosuppressive drug use- type, cumulative dosage and duration of use and other concomitant conditions.
Patients were generally deemed to have been “on” individual immunosuppressive drugs if they had been taking them for a minimum of three months. Anti-double-stranded DNA (anti-dsDNA) and extractable nuclear antibodies (ENA) were assessed using a Euro Diagnostica enzyme-linked immunosorbent assay (ELISA). Anti-dsDNA antibodies were also tested by the immunofluorescent Crithidia assay. Patients were considered to be anti-dsDNA antibody positive if they were ever Crithidia positive or had an ELISA result more than twice the upper limit of normal on two occasions. Complement component levels were measured by laser nephelometry. We have used the same anti-DNA, anti-ENA and C3 assays for over 20 years and, utilizing stored serum samples for patients who lived (and in some cases died), we have tested samples obtained early in the course of disease with the current methods utilizing stored samples so that we can genuinely say that all of our patients have had their serum tested by the same methodology. We used results obtained around the period they were classified as fulfilling the ACR criteria for SLE for consistency.
Use of drugs and immunosuppressive agents from time of SLE diagnosis up to the time of the diagnosis of cancer (ever or never, cumulative dose over time and duration of use) was calculated for cases with cancer and up to the defined end of follow-up for those without.
To assess the damage accrued, we used the SLICC/ACR damage index. Though not a measure of disease activity, it has been shown to reflect cumulative disease activity over time.21
Statistical analysis
Statistical Packages for Social Sciences version 18.0 (SPSS Inc, Chicago, IL, USA) was used for data entry and analysis. Standardized incidence ratios (SIRs, the quotient of observed to expected cancers) were also calculated for males and females, overall cancer and individual cancers using the Thames Cancer Registry’s five-year annualized age and gender-specific cancer rates per 100,000 population for the southeast of England22 and their 95% confidence intervals (CIs) were attained by using observed cases as a Poisson variable and its associated interval from formulae based on the Poisson distribution.23 The expected number of malignancies (E) was calculated as the sum of all person-years at risk in the age group (i) from the cohort study (ni) multiplied by the age- and sex-specific cancer rates for the South East of England in the age group i (Ri) using the expression E = ∑ (ni) × Ri.
The relation between cancer and the probable risk factors was explored by comparing clinical and serological characteristics of patients who developed malignancies and those who had not developed malignancies in a nested case-control design. Case fatality rate was calculated as a percentage of deaths in the cohort with malignancy over total number of cases in that cohort through their disease course and cancer fatality rate as deaths directly due to cancer over deaths in the cohort with malignancy. With development of cancer (cases) as the dependent variable and demographic characteristics, clinical and serological factors as independent variables, univariate analysis was conducted using the 2 × 2 contingency table for each of the independent variables to determine any association between them and the development of malignancy. Following the univariate analysis, variables that attained a p value < 0.1 were retained for multivariate analysis. However, as few variables met the criteria for inclusion into the multivariable analysis (i.e. p value < 0.1), all variables were included into the model. A backward elimination procedure form of the logistic regression model was used to calculate the odds ratio (ORs) and 95% CIs for the risk of cancer development.
Results
A total of 595 SLE patients were followed up until 31 December 2010. Of these patients, 81 died and a further 58 were no longer being followed up in our clinic. Eight have been followed up in other (notably renal) clinics in our hospital and 50 have moved away, some to be followed up in other UK clinics and others overseas.
Out of the 595 patients, we estimate that we have lost complete contact, long-term, with only 25 patients. In this cohort analysis we did not get complete data for only one patient and could not completely match all the male cancer cases. A total of 37 malignancies were diagnosed in 36 patients. Four of these were diagnosed in patients before SLE onset. One patient was diagnosed with two different malignancies, one before the onset and one afterwards. Those malignancies before the onset of SLE were eliminated from the analysis, which was therefore undertaken on 33 patients.
Demographic characteristics
Thirty females and three males developed cancer after diagnosis of SLE (Table 1). The mean duration of follow-up was 14.7 years. Total person-years of follow-up was 8910.51, and the mean age at diagnosis of SLE and cancer was 33.5 (SD 12.7) years and 50.3 (SD 14.8) years, respectively. The mean time interval between diagnosis of SLE and cancer was 16.4 (SD 9.73) years. The majority of patients were Caucasian (90.9%) and most cancer cases occurred in Caucasians. Nineteen (57.6%) of the 33 cancer patients died during the follow-up period, with a cancer fatality rate of 84.2%; significantly more people died from cancer than noncancer causes (p < 0.0001).
Table 1.
Univariate analysis with odds ratio (OR), 95% confidence intervals (95% CIs) and p values comparing clinical, serological characteristics and medical treatment of SLE cancer cases and controls
| Demographic Characteristics | Cases n = 33 (%) | Controls n = 94 (%) | OR | 95% CIs | p value < 0.05 |
|---|---|---|---|---|---|
| Clinical features | |||||
| Mucocutaneous | 30 (90.9%) | 73 (77.7%) | 2.9 | (0.8, 10.4) | 0.12 |
| Musculoskeletal | 2 (6.1%) | 1 (1.0 %) | 5.9 | (0.5, 67.7) | 0.17 |
| Neuropsychiatric | 7 (21.2%) | 23 (24.5%) | 0.8 | (0.3, 2.2) | 0.81 |
| Cardio respiratory | 7 (21.2%) | 23 (24.5%) | 0.8 | (0.3, 2.2) | 0.81 |
| Gastrointestinal | 0 (0%) | 2 (2.1%) | – | – | NA |
| Ophthalmic | 14 (42.4%) | 53 (56.4%) | 0.6 | (0.3, 1.3) | 0.22 |
| Renal | 15 (45.5%) | 34 (36.2%) | 1.5 | (0.6, 3.3) | 0.41 |
| Haematological | 20 (60.6%) | 83 (88.3%) | 0.2 | (0.1, 0.5) | 0.001 |
| Immunological profile | |||||
| Antinuclear antibodies | 33 (100%) | 90 (97.9%) | 1.4 | (1.2, 1.5) | 0.39 |
| Anti-dsDNA antibodies | 18 (54.5%) | 61 (64.9%) | 0.7 | (0.3, 1.5) | 0.30 |
| Rheumatoid factor | 11 (33.3%) | 28 (29.8%) | 1.2 | (0.5, 2.8) | 0.80 |
| Anti-Sm antibodies | 3 (9.11%) | 12 (12.8%) | 0.4 | (0.1, 2.1) | 0.35 |
| Anti-RO antibodies | 7 (21.2%) | 29 (30.9%) | 0.6 | (0.2, 1.5) | 0.37 |
| Anti-La antibodies | 6 (18.2%) | 9 (9.6%) | 2.1 | (0.7, 6.4) | 0.21 |
| Anti-RNP antibodies | 9 (27.3%) | 23 (24.5%) | 1.2 | (0.5, 2.8) | 0.82 |
| Low C3 | 11 (33.3%) | 44 (46.8%) | 0.6 | (0.3, 1.3) | 0.22 |
| Lupus anticoagulant | 7 (21.2%) | 13 (13.8%) | 2.2 | (0.8, 5.9) | 0.16 |
| Anticardiolipin antibodies | 9 (27.3%) | 33 (35.9%) | 0.4 | (0.2, 1.2) | 0.07 |
| Coombs positivity | 6 (18.2%) | 25 (26.6%) | 0.6 | (0.3, 1.6) | 0.48 |
| Antithyroid globulin antibodies | 2 (6.1%) | 25 (26.6%) | 0.2 | (0.4, 0.8) | 0.01 |
| Smoking (ever) | 15 (45.5%) | 44 (46.8%) | 0.9 | (0.4, 2.1) | 0.92 |
| NSAID use | 6 (19.4%) | 24 (25.8%) | 0.6 | (0.2, 1.7) | 0.48 |
| Aspirin use | 13 (41.9%) | 35 (37.6%) | 1.2 | (0.5, 2.7) | 0.68 |
| Steroid use | 25 (80.6%) | 74 (78.7%) | 1.3 | (0.5, 3.5) | 0.41 |
| Hydroxychloroquine | 24 (77.4%) | 67 (71.3%) | 1.1 | (0.4, 2.6) | 1.00 |
| Azathioprine (AZA) | 15 (46.9%) | 45 (47.9%) | 0.9 | (0.4, 2.1) | 1.00 |
| Methotrexate (MTX) | 2 (6.5%) | 8 (8.5%) | 0.8 | (0.2, 4.1) | 1.00 |
| Cyclophosphamide (CYC) | 8 (25.8%) | 24 (25.5%) | 0.9 | (0.4, 2.5) | 1.00 |
| Cyclosporine | 1 (3.0%) | 3 (3.2%) | 0.9 | (0.1, 16.4) | 1.00 |
| Mycophenolate mofetil (MMF) | 2 (3.0%) | 13 (13.8%) | 0.4 | (0.1, 1.8) | 0.35 |
| Rituximab | 0 (0%) | 19 (20.2%) | – | – | NA |
| Hypothyroid | 2 (6.5%) | 12 (12.8%) | 0.4 | (0.1, 2.1) | 0.35 |
| Hyperthyroid | 0 (0%) | 4 (4.3%) | – | – | NA |
| Rheumatoid arthritis | 1 (3.0%) | 2 (2.1%) | 1.4 | (0.1, 16.4) | 1.00 |
| Undifferentiated autoimmune rheumatic disease | 0 (0%) | 0 (0%) | – | – | – |
| Sjögren’s | 3 (9.1) | 9 (9.6) | 0.9 | ||
| Diabetes | 2 (6.5%) | 5 (5.3%) | 1.1 | – | – |
| Sarcoidosis | 0 (0.0%) | 1 (1.1%) | – | – | – |
| Myositis | 0 (0.0%) | 2 (2.1%) | – | – | NA |
| Liver cirrhosis | 0 (0%) | 2 (2.1%) | – | – | NA |
| Antiphospholipid syndrome | 1 (3.0%) | 9 (9.6%) | 0.3 | (0.0, 2.4) | 0.45 |
| Myasthenia gravis | 0 (0.0%) | 0 (0.0%) | – | – | NA |
| Scleroderma | 1 (3.0%) | 1 (1.1%) | 2.9 | (0.8, 47.8) | 0.45 |
| Other autoimmune disease | 15(45.5%) | 40 (42.6%) | 1.1 | (0.5, 2.5) | 0.84 |
| SLICC damage | 26 (47.3%) | 29 (52.7%) | 0.12 | (0.0,0.36) | 0.0001 |
Abbreviations: n: number; NA; not analysed; SLE: systemic lupus erythematosus; NSAID: nonsteroidal anti-inflammatory drug; anti-dsDNA: anti-double-stranded DNA; SLICC scores: Systemic Lupus Erythematosus International Collaborating Clinic scores.
There was a small but statistically insignificant increase in overall cancer risk, SIR 1.05 (95% CI 0.52–1.58).The most frequently occurring cancers following the diagnosis of SLE were breast and lung cancers, with five cases each (15.2%).
Using the South East England age standardized cancer rates, there were statistically increased SIRs for cervical, prostate, anal and pancreatic cancers. The age standardized incidence rates for the other cancers were not available for comparison (Table 2).
Table 2.
Cancers observed and expected in SLE cancer patients with standard incidence ratios (SIR) and 95% confidence intervals (95% CIs) compared to the general population. Risks for other malignancies were not calculated as there were no comparable data for the general population
| Characteristics | Observed | Expected | SIR | 95% CIs |
|---|---|---|---|---|
| All cancers | 33 | 31.5 | 1.05 | 0.52–1.58 |
| Female | 30 | 29.0 | 1.03 | 0.66–1.40 |
| Male | 3 | 3.0 | 1.00 | 0.29–1.06 |
| Lung cancer | 5 | 2.9 | 1.72 | 0.97–2.47 |
| Breast cancer | 5 | 10.5 | 0.48 | 0.35–0.64 |
| Cervical cancer | 2 | 0.5 | 4.00 | 3.50–4.50 |
| Anal cancer | 2 | 1.1 | 1.80 | 1.48–2.12 |
| Prostate cancer | 3 | 0.7 | 4.29 | 1.09–10.24 |
| Non-Hodgkin’s lymphoma (NHL) | 1 | 1.1 | 0.91 | 0.87–0.95 |
| Pancreatic cancer | 1 | 0.7 | 1.43 | 1.32–1.54 |
| Hodgkin’s disease | 2 | NR | NR | NR |
| Vulval cancer | 1 | NR | NR | NR |
| Cholangiocarcinoma | 1 | NR | NR | NR |
| Appendiceal cancer | 1 | NR | NR | NR |
| Large granular lymphocytic leukaemia | 1 | NR | NR | NR |
| Cancer of the cheek | 1 | NR | NR | NR |
| Gall bladder cancer | 1 | NR | NR | NR |
| Cancer of the tongue | 1 | NR | NR | NR |
| Skin cancer | 2 | NR | NR | NR |
| Bowel cancer | 2 | NR | NR | NR |
| Hepato-biliary cancer | 1 | NR | NR | NR |
SLE: systemic lupus erythematosus; NR: not recorded; p value < 0.05.
The majority of cases, nine (27.3%), were in the 30 - to 39-year age group, and 69.7% were > 40 years. Malignancy was diagnosed in two patients less than a year after SLE diagnosis and both cases were breast cancers in patients less than 50 years of age (Table 3).
Table 3.
SLE cases with cancer and their observed cancer frequencies for various age ranges and duration between SLE and cancer diagnosis
| Age range in years for SLE cases with cancer | Observed cancer frequency |
|---|---|
| 0–20 | 0 |
| 20–29 | 1 |
| 30–39 | 9 |
| 40–49 | 7 |
| 50–59 | 7 |
| 60–79 | 7 |
| > 80 | 2 |
| Duration between SLE diagnosis and cancer in years | Observed cancer frequency |
| < 1 | 2 |
| 1–5 | 2 |
| 6–10 | 8 |
| 11–15 | 5 |
| 16–20 | 4 |
| > 20 | 12 |
SLE: systemic lupus erythematosus.
Univariate analysis found a statistically significant association between cancer and antithyroid globulin antibodies, haematological manifestations and SLICC damage scores (Table 1).
As few of the variables met a p value cut-off point of 0.1 for inclusion into the model, all variables were entered into the multivariate analysis model. There was a statistically significant positive association found with musculoskeletal 7.94 (95% CI 1.68–37.82) and haematological manifestations 4.64 (95% CI 2.05–10.06), anticardiolipin 9.17 (95% CI 2.73–30.78), and antithyroid globulin antibodies 2.91 (95% CI 1.0–12.64).
None of the medications used showed an association with cancer risk, and further analysis showed no critical dose or duration effect for cyclophosphamide use and no association with cancer.
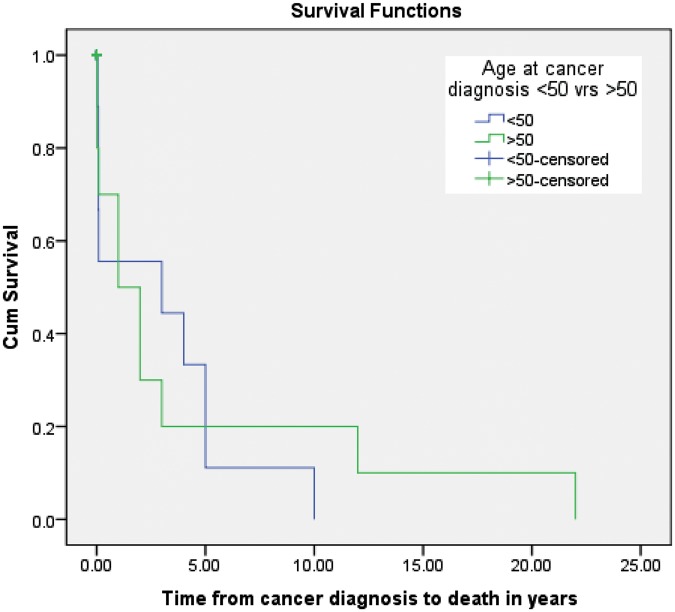
There was no clear association between duration of disease and susceptibility to either a solid or haematological malignancy. Seventy-five per cent of cases that occurred less than ten years after SLE diagnosis were solid tumours and 25% were haematological, namely Hodgkin’s lymphoma and one large granular lymphocytic (LGL) leukaemia. Two lung cancers occurred less than ten years after SLE onset, and for the haematological malignancies, 50% of patients were younger than 50 years at diagnosis and the other half older than 50 years. All cancers occurring after 20 years of disease duration were solid tumours. Cumulative survival fell in the first five years after a cancer diagnosis with a very sharp drop in the first year; survival at five years was less than 20%; at 20 years less than 10%; and almost 0% at 22 years. Only three of the controls died, two from ischemic heart disease and one committed suicide. Those < 50 years when they developed cancer after SLE diagnosis tended to die faster than the 50-plus year groups (Figure 1).
Figure 1.
Kaplan Meier survival function curve: survival curve for cancer cases comparing ages < 50 and >50years.
Discussion
Determining accurate cancer risk in a disease like SLE, which nowadays may affect patients for several decades, is very challenging. The SLICC study11 captured cancers reported in almost 10,000 patients. While the denominator is most impressive (and our group was a contributor to this effort), given that over 23 centres were involved it is inevitable that variation in the quality and completeness of data must have occurred. Furthermore, the mean duration of follow-up was only eight years, limiting the ability of the study to link long-term immunosuppressive use to cancer development. The SLICC study was also, for understandable reasons, unable to comment on any cancer association with lupus serology, disease activity or damage.
The present study has attempted to address some of these problems. We have followed up almost 600 SLE patients for up to 32 years as assiduously as possible, losing only 25 patients to any kind of long-term follow-up. Thus mean duration of our patient follow-up is almost twice that of the SLICC study. We have reported on links to serological abnormalities (using the same assays throughout the study) damage and indirectly (albeit incompletely) disease activity. The nested case-control study design in our study is advantageous in that the estimates derived in this way should be virtually identical to a full cohort analysis, and if associations are found this could be explored further in cohort studies.
The main findings in this study are a small but statistically insignificant overall increased risk of cancer in SLE in contrast to previously reported studies of increased cancer,6–8,11,24–28 with a particular increase in prostate, anal, pancreatic and cervical cancers but smaller than expected numbers of breast cancer.
In the SLICC cohort, a reduced incidence of endometrial and probably breast and ovarian cancers were found. Though breast cancer cases were the highest number of cancer cases recorded together with lung cancer in our cohort, there was no increased risk compared to the general population. A recent study exploring whether risk alleles of the single-nucleotide polymorphisms (SNPs) influencing the risk of acquiring SLE may be offering some protection against breast cancer found only two out of the 17 SLE SNPs studied to be negatively linked with breast cancer in the genome-wide association studies (GWAS) data. The significance of this is, however, not clear and is being explored further, but may help to explain the lowered risk of breast cancer in SLE patients.29
Some previous studies have reported an increased risk of lung cancer with histological distribution comparable to the general population.30 We found a similar distribution, but a much higher incidence of adenocarcinomas (60%, as compared to 30%–40%), including one case of bronchoalveolar adenocarcinoma, small cell carcinoma (20%) and one non-small cell carcinoma (20%).Though there was an increase in lung cancer cases compared to the general population, it was not statistically significant. All our cases of lung cancer were smokers (100%); smokers in the cases and controls were similar, 45.5% and 46.8%, respectively, a higher overall estimate than previous studies of smoking in lupus patients of 17%–21%. Three out of five patients had been exposed to immunosuppressive therapy, two to azathioprine and one to methotrexate. All cases died, with four out of the five cases doing so less than a year and the other case after one year. Five-year lung cancer survival in the general population is estimated at 7%–9%, with 50% dying within four months of diagnosis.31 One of our cases had fibrosing alveolitis, which has also been linked to lung cancer.32–34 Some studies show a decreased risk of prostate cancer in SLE, with low hypoadrenergic states in men with SLE and genetic factors as possible risks;35 in contrast, we found an increased risk of prostate cancer.
We did not find an increase in haematological malignancies, notably the lymphomas, as other studies have reported. An interesting postulation is whether the use of rituximab, which is a treatment for B cell lymphoma, may be causing a reduction in lymphoma incidence as reported in a study in Sjögren’s syndrome where it reduced risk of mucosa-associated lymphoid tissue (MALT) lymphomas.36 It would be interesting to examine data from other cohorts to see if the frequencies of diagnosed lymphomas have reduced in recent times.
Multivariate analysis suggested a positive association between clinical and serological manifestations, notably musculoskeletal, haematological manifestations, anticardiolipin and antithyroid globulin antibodies and cancer risk. The strength of positive association factors was relatively weak with wide CIs, probably due to the small sample size, and larger cohorts studies are needed to confirm these findings.
It would have been optimal to relate the disease activity over time to cancer risk, using tools such as the British Isles Lupus Assessments Groups (BILAG) disease activity score. However, we did not have sufficient data on some patients, notably those followed up for greater than 20 years and therefore could not measure these scores retrospectively.
We obtained our calculation for expected cancers based on person-years of risk calculations, meaning we considered their contribution to development of cancer only up to the time of development of the cancer and not after the disease had developed; we were limited in calculating observed rates for each year that they developed cancer though that would have been a more accurate measure. Serological tests and complement levels were also calculated at a point in time rather than over a period.
Previous studies have linked damage accrual in SLE patients, clearly associated with prior activity with cancer risk.11,37,38 In our study, SLICC damage scores were found to be associated with cancer risk in univariate but not multivariate analysis. However, positive anti-dsDNA antibody status and low C3 levels, markers that are generally regarded as useful measures of activity, were not found to be statistically significantly associated with cancer risk in this study. The association with damage scores though supports the notion that disease activity (and hence eventual damage accrual) may be one of the major determinants of cancer risk development as has been found in RA rather than the use of immunosuppressive drugs.39 Low C3 (hazard ratio (HR) = 6.18, 95% CI 1.57–24.22) was a significant predictor of lymphoproliferative disease and premature death in patients with primary Sjögren’s.40,41
Haematological aberrations, usually manifesting as cytopaenias, is seen in both SLE and some malignancies, typically lymphoproliferative. These cytopaenias form part of the ACR classification criteria, but on occasion may be paraneoplastic phenomena. However, only two of our SLE patients developed cancers in the first two years following SLE diagnosis. Thus in these patients cytopaenias could have been related to the development of malignancy rather than SLE. We did not examine the individual contributions of the various cytopaenias.
There have been reports of excess autoantibodies in the sera of patients with cancer compared to controls, especially lymphomas, including anti-dsDNA, antiribonucleoprotein (anti-RNP) and anti-Smith (anti-Sm) antibodies. These data suggest these antibodies may be related to cancer development or possibly aging. In our cohort these diagnostic tests were detected prior to the patients’ cancer diagnosis and thus are unlikely to be the result of their cancer.
The mechanism by which antithyroid globulin antibodies may be linked to cancer risk is unknown. Some studies indicate a significant association between breast cancer and autoimmune and non-autoimmune thyroid disorders and some report an increased incidence of breast cancer in patients with Hashimoto’s thyroiditis.42 An association of antiphospholipid antibodies (aPL) and cancer has been sought. Recent findings imply an increased prevalence of certain cancers in aPL-positive patients; thus, a thorough investigation for an occult malignancy is encouraged in these patients. In addition, several studies reported on elevated levels of aPL antibodies in various malignancies.43
Relatively few patients were taking prescribed NSAIDs. This could have been because their use is not really encouraged in SLE patients. NSAID use has been in found in several studies to be associated with reduced cancer risk, especially colonic cancers and breast cancers and in patients with RA, contrary to the findings in this study.44–46
There appears to be a reduction in survival in SLE patients compared to those without cancer and earlier mortality if diagnosed at a younger age. This is a cause for concern, especially with the higher risk found of cancers associated with high mortality like lung cancer, as it diminishes the good results achieved in improving the morbidity and mortality in SLE.
Mortality from cancer has been reported to be higher in lupus patients than in the general population; previous studies showing increased mortality from lung cancers and NHL support the findings from this study.3,47
Conclusion
Our results show a statistically insignificant, small overall increased risk of cancer in SLE patients, but an interesting dichotomy that some cancers appear to be more common, especially prostate and cervical cancers, but with reduction in breast cancer rates. Increased disease activity may be implicated in this risk. The clinical and serological profile of patients needs to be explored in more detail in future studies to determine the possible pathological pathways in which it predisposes some patients to increased cancer risk. The role of drugs, especially cyclophosphamide, is a subject of concern, but its role in cancer risk in SLE is not definite.
Clinical implications
Multi-factorial causes are involved in the increased risk of malignancy in systemic lupus erythematosus (SLE).
There is increased mortality from cancers and lung cancer in particular.
Lung, prostate and cervical cancers are increased; others, notably breast cancers, are less frequent.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Dr Dey’s fellowship training was partly supported by a training bursary from the European League Against Rheumatism (EULAR).
References
- 1. Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 2003; 82: 299–308 [DOI] [PubMed] [Google Scholar]
- 2. Ward M, Pyun E, Studenski S. Causes of death in systemic lupus erythematosus. Long-term followup of an inception cohort. Arthritis Rheum 1995; 38: 1492–1499 [DOI] [PubMed] [Google Scholar]
- 3. Moss K, Ioannou Y, Sultan SM, Haq I, Isenberg DA. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis 2002; 61: 409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abu-Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol 1995; 24: 1061–1065 [PubMed] [Google Scholar]
- 5. Dias C, Isenberg DA. Susceptibility of patients with rheumatic diseases to B-cell non-Hodgkin lymphoma. Nat Rev Rheumatol 2011; 7: 360–368 [DOI] [PubMed] [Google Scholar]
- 6. Pettersson T, Pukkala E, Teppo L, Friman C. Increased risk of cancer in patients with systemic lupus erythematosus. Ann Rheum Dis 1992; 51: 437–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramsey-Goldman R, Mattai SA, Schilling E, et al. Increased risk of malignancy in patients with systemic lupus erythematosus. J Invest Med 1998; 46: 217–222 [PubMed] [Google Scholar]
- 8. Mellemkjaer L, Andersen V, Linet MS, Gridley G, Hoover R, Jorgen HO. Non-Hodgkin’s lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthritis Rheum 1997; 40: 761–768 [DOI] [PubMed] [Google Scholar]
- 9. Sweeney DM, Manzi S, Janosky J, et al. Risk of malignancy in women with systemic lupus erythematosus. J Rheumatol 1995; 22: 1478–1482 [PubMed] [Google Scholar]
- 10. Abu-Shakra M, Gladman DD, Urowitz MB. Malignancy in systemic lupus erythematosus. Arthritis Rheum 1996; 39: 1050–1054 [DOI] [PubMed] [Google Scholar]
- 11. Bernatsky S, Boivin JF, Joseph L, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum 2005; 52: 1481–1490 [DOI] [PubMed] [Google Scholar]
- 12. Matteson EL, Hickey AR, Maguire L, Tilson HH, Urowitz MB. Occurrence of neoplasia in patients with rheumatoid arthritis enrolled in a DMARD registry. The Rheumatoid Arthritis Azathioprine Registry Steering Committee. J Rheumatol 1991; 18: 809–814 [PubMed] [Google Scholar]
- 13. Silman AJ, Petrie J, Hazleman B, Evans SJ. Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: A 20 year follow up study. Ann Rheum Dis 1988; 47: 988–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. London NJ, Farmery SM, Will EJ, Davison AM, Lodge JP. Risk of neoplasia in renal transplant patients. Lancet 1995; 346: 403–406 [DOI] [PubMed] [Google Scholar]
- 15. Mariette X. Lymphomas in patients with Sjögren’s syndrome: Review of the literature and physiopathologic hypothesis. Leuk Lymphoma 1999; 33: 93–99 [DOI] [PubMed] [Google Scholar]
- 16. Oertel SH, Riess H. Immunosurveillance, immunodeficiency and lymphoproliferation. Recent Results Cancer Res 2002; 159: 1–8 [DOI] [PubMed] [Google Scholar]
- 17. Boubenider S, Hiesse C, Groupy C, Kriaa F, Marchand S, Charpentier B. Incidence and consequences of post-transplantation lymphoproliferative disorders. J Nephrol 1997; 10: 136–145 [PubMed] [Google Scholar]
- 18. Bernatsky S, Boivin JF, Joseph L, et al. Prevalence of factors influencing cancer risk in women with lupus: Social habits, reproductive issues, and obesity. J Rheumatol 2002; 29: 2551–2554 [PubMed] [Google Scholar]
- 19. Ehrenfeld M, Abu-Shakra M, Buskila D, Shoenfeld Y. The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis 2001; 27: 750–756 [DOI] [PubMed] [Google Scholar]
- 20. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725–1725 [DOI] [PubMed] [Google Scholar]
- 21. Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index in patients with systemic lupus erythematosus. Arthritis Rheum 1997; 40: 809–813 [DOI] [PubMed] [Google Scholar]
- 22. Cancer in South East England. Cancer incidence, prevalence, survival and treatment for residents of South East London in 2008, London: Thames Cancer Registry, 2010 [Google Scholar]
- 23. Breslow NE, Day NE. Statistical methods in cancer research. Volume II – The design and analysis of cohort studies. IARC. Sci Publ 1987; 82: 1–406 [PubMed] [Google Scholar]
- 24. Björnådal L, Löfström B, Yin L, Lundberg IE, Ekbom A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand J Rheumatol 2002; 31: 66–71 [DOI] [PubMed] [Google Scholar]
- 25. Cibere J, Sibley J, Haga M. Systemic lupus erythematosus and the risk of malignancy. Lupus 2001; 10: 394–400 [DOI] [PubMed] [Google Scholar]
- 26. Parikh-Patel A, White R, Allen M, Cress R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control 2008; 19: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen YJ, Chang YT, Wang CB, Wu CY. Malignancy in systemic lupus erythematosus: A nationwide cohort study in Taiwan. Am J Med 2010; 123: 1150.e1–1150.e6 [DOI] [PubMed] [Google Scholar]
- 28. Lewis R, Castor CW, Knisley RE, Bole GG. Frequency of neoplasia in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 1976; 19: 1256–1260 [DOI] [PubMed] [Google Scholar]
- 29. Bernatsky S, Easton D, Dunning AM, et al. Decreased breast cancer risk in SLE: Can a genetic basis be determined? Arthritis Rheum 2011; 63: 223–224 [Google Scholar]
- 30. Bin J, Bernatsky S, Gordon C, et al. Lung cancer in systemic lupus erythematosus. Lung Cancer 2007; 56: 303–306 [DOI] [PubMed] [Google Scholar]
- 31. Cancer Research UK. Cancer Statistics key facts, http://www.cancerresearchuk.org/cancerstats (2010)
- 32. Turner-Warwick M, Lebowitz M, Burrows B, Johnson B. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980; 35: 496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris JM, Johnston IDA, Rudd R, Taylor AJ, Cullinan P. Cryptogenic fibrosing alveolitis and lung cancer: The BTS study. Thorax 2010; 65: 70–76 [DOI] [PubMed] [Google Scholar]
- 34. Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000; 161: 5–8 [DOI] [PubMed] [Google Scholar]
- 35. Bernatsky S, Ramsey-Goldman R, Gordon C, Clarke AE. Prostate cancer in systemic lupus erythematosus. Int J Cancer 2011; 129: 2966–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjögren’s syndrome: An open-label phase II study. Arthritis Rheum 2005; 52: 2740–2750 [DOI] [PubMed] [Google Scholar]
- 37. Stoll T, Sutcliffe N, Mach J, Klaghofer R, Isenberg DA. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus – a 5-yr prospective study. Rheumatology (Oxford) 2004; 43: 1039–1044 [DOI] [PubMed] [Google Scholar]
- 38. Chambers S, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 2009; 48: 673–675 [DOI] [PubMed] [Google Scholar]
- 39. Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54: 692–701 [DOI] [PubMed] [Google Scholar]
- 40. Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjögren’s syndrome: A prospective cohort study. Arthritis Rheum 2004; 50: 1262–1269 [DOI] [PubMed] [Google Scholar]
- 41. Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren’s syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006; 65: 796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turken O, Narin Y, Demirbas S, et al. Breast cancer association with thyroid disorders. Breast Cancer Res 2003; 5: 110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reinstein E, Shoenfeld Y. Antiphospholipid syndrome and cancer. Clin Rev Allergy Immunol 2007; 32: 184–187 [DOI] [PubMed] [Google Scholar]
- 44. DuBois RN, Giardiello FM, Smalley WE. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol Clin North Am 1996; 25: 773–791 [DOI] [PubMed] [Google Scholar]
- 45. Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncology Rep 2005; 13: 559–583 [PubMed] [Google Scholar]
- 46. Gridley G, McLaughlin JK, Ekbom A, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst 1993; 85: 307–311 [DOI] [PubMed] [Google Scholar]
- 47. Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006; 54: 2550–2557 [DOI] [PubMed] [Google Scholar]