Abstract
There is considerable interest in the structural and functional properties of the angular gyrus (AG). Located in the posterior part of the inferior parietal lobule, the AG has been shown in numerous meta-analysis reviews to be consistently activated in a variety of tasks. This review discusses the involvement of the AG in semantic processing, word reading and comprehension, number processing, default mode network, memory retrieval, attention and spatial cognition, reasoning, and social cognition. This large functional neuroimaging literature depicts a major role for the AG in processing concepts rather than percepts when interfacing perception-to-recognition-to-action. More specifically, the AG emerges as a cross-modal hub where converging multisensory information is combined and integrated to comprehend and give sense to events, manipulate mental representations, solve familiar problems, and reorient attention to relevant information. In addition, this review discusses recent findings that point to the existence of multiple subdivisions in the AG. This spatial parcellation can serve as a framework for reporting AG activations with greater definition. This review also acknowledges that the role of the AG cannot comprehensibly be identified in isolation but needs to be understood in parallel with the influence from other regions. Several interesting questions that warrant further investigations are finally emphasized.
Keywords: inferior parietal lobule, connectivity, cross-modal integration, semantic, default network
There is a rich and fascinating history of discoveries about the angular gyrus (AG). From a devastating effect on word processing when the left AG is damaged (Dejerine 1891) to triggering an out-of-body experience when the right AG is electrically stimulated (Blanke and others 2002), the AG has not yet revealed all its secrets and is still attracting a huge interest in the neuroscience community. A search on PubMed using angular gyrus as a keyword limited to the “title/abstract” retrieved nearly 500 studies. This brief review aims to integrate previous findings about the AG, particularly regarding its potential role(s) and whether it can be subdivided into multiple areas. The studies reviewed here are limited to functional studies of healthy populations irrespective of task or topic of interest. Given the huge number of functional neuroimaging studies that reported interesting effects in the AG, it was not possible to evaluate all their findings thoroughly. Therefore, the focus here is made on consistent results rather than the differences between previous studies. Specifically, effects of interest are limited to the functions and processes that have been shown with a compelling consistency in previous meta-analysis reviews. Because of space limitations, a large proportion of selective citations are made here of reviews with the expectation that each of these reviews would provide a comprehensive list of previous studies that reported activation in the AG in different tasks and contexts.
This review is divided into three sections. A first section provides some useful definitions about the anatomy of the AG. The second section reviews the different roles and functions that have been associated with the AG. The third section succinctly presents some of the emerging evidence of multiple subdivisions within the AG.
The Anatomy of the AG
Localization in the Posterior Inferior Parietal Lobule
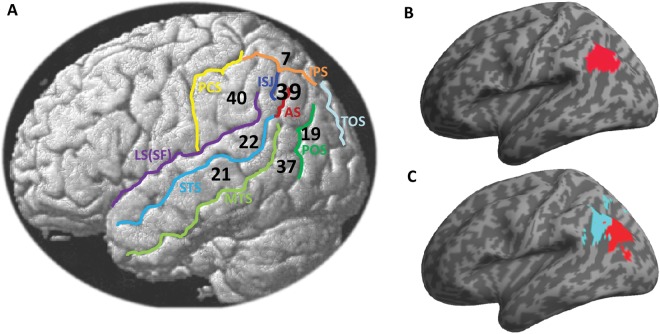
The AG occupies a posterior part of the inferior parietal lobule corresponding to Brodmann area (BA) 39, von Economo and Koskinas area PG, or area 69 in the unified nomenclature of Triarhou (2007). It can be seen as the continuation of the superior/middle temporal gyri into the inferior parietal lobule with a medial boundary defined by the intraparietal sulcus (Rademacher and others 1992). Its anterior boundary with the supramarginal gyrus is marked by the descending portion of the intermediate sulcus of Jensen (Ribas 2010), and its posterior boundary is set by the dorsal part of the anterior occipital sulcus (Rademacher and others 1992). On a sagittal view, it can easily be identified by its horseshoe shape near the dorsal-posterior segment of the superior temporal sulcus that is also called the angular sulcus (Naidich and others 1995) (see Fig. 1). Recent cytoarchitectonic studies (e.g., Caspers and others 2008; Caspers and others 2006) have suggested that the AG extends rostrally to area PGa and caudally to area PGp, as illustrated in Figure 1C. Its cortical volume is estimated at around 13.2 cm3 and 11.7 cm3 in the left and right hemisphere, respectively (Rademacher and others 1992), suggesting a relative left-greater-than-right structural asymmetry (see also Eidelberg and Galaburda 1984). It is worth noting that the AG belongs to a set of parietal regions that are lightly myelinated compared with sensory or modality-specific regions (Box 1). Last but not least, an increasing literature points to an extensive training-induced structural plasticity in bilateral AG in the adult brain, particularly when learning new skills that require spatial coordination and verbal memory (see summary in Box 2).
Figure 1.
(A) Localization of the angular gyrus (Brodmann area [BA] 39) with respect to some anatomical landmarks that are close to the angular gyrus (AG), illustrated on a lateral view of a single subject brain. Numbers correspond to BA 7, 19, 21, 22, 37, 39, and 40. STS = superior temporal sulcus; MTS = middle temporal sulcus; IPS = intraparietal sulcus; LS(SF) = lateral sulcus (sylvian fissure); PCS = postcentral sulcus; POS = parieto-occipital sulcus; TOS = transverse occipital sulcus; AS = angular sulcus; ISJ = intermediate sulcus of Jensen. Projection of the AG from two widely used anatomical toolboxes in region-based analyses in functional neuroimaging, including (B) the AAL atlas (http://www.cyceron.fr/web/aal_anatomical_automatic_labeling.html) and (C) the probabilistic anatomy toolbox (http://www.fz-juelich.de/inm/inm-1/EN/Home/home_node.html) showing two cytoarchitectonic areas PGa (cyan) and PGp (red), as detailed in Caspers and others (2008).
Box 1. AG Maturation.
Myelin content and maturational curves constitute some of the key anatomical properties that shape regional parcellation and function. In the inferior parietal lobule, it has been shown that gyration starts by 27 weeks post–gestational age, and the cortical folding of intraparietal and parieto-occipital sulci that anatomically delineate the AG may progress from 27 to 31 weeks gestational age (Dubois and others 2008). This early morphometric development may explain why the AG has been activated in previous studies in very young subjects; for instance, three-month-old infants showed strong fMRI activations in the left AG for normal mother’s voice speech compared with reversed speech (Dehaene-Lambertz and others 2002). Early studies (as reviewed in Geschwind 1965) suggested very late myelination in bilateral AG (e.g., Flechsig’s myelination map; see Figure 14 in Glasser and Van Essen 2011), being probably the latest in the whole parietal lobe. However, recent studies have observed earlier myelination than initially thought. For instance, across childhood, gray matter maturation in the AG peaks between 8.5 (Gogtay and others 2004) and 13 years (Westlye and others 2010) and then declines with age. The topology of the AG in terms of anatomical parcellation and boundaries is already well established in school-age children (Barnes and others 2011). In adults, it has been shown that the whole inferior parietal cortex, including the AG, is lightly myelinated compared with primary and secondary sensory regions (Glasser and Van Essen 2011).
Box 2. Structural Plasticity.
There is increasing evidence about a training-induced neuroanatomic plasticity even in the adult brain that results in detectable structural changes following learning new skills (for review, see Draganski and May 2008). These structural changes can occur at the macroscopic level and thus can be revealed by morphometry analyses on high-resolution anatomical scans. Interestingly, several studies have reported structural changes in the AG during learning. For instance, when brain scans were compared in subjects before and after training to juggle, increased gray matter density was detected in the left AG and bilateral mid-temporal regions (Draganski and others 2004), probably reflecting an increase in the coordination and storage of complex visual motion. In the language domain, a comparison between bilinguals and monolinguals revealed stronger gray matter density in the bilingual brain in the anterior AG that also correlated significantly with the second-language proficiency (Mechelli and others 2004). In addition, gray matter density in bilateral AG was shown to be significantly higher in subjects who learned to read as adults (late literates) compared with a matched group of adult illiterates (Carreiras and others 2009). Recently, by measuring creative productivity scores across different skills that included visual arts, music, creative writing, scientific discovery, and invention, a whole-brain correlation between cortical thickness and creativity scores was only significant in the AG (Jung and others 2010). This literature points to a phenomenal structural plasticity in the AG when subjects are learning new skills that tap on spatial coordination, verbal storage, and creativity.
Structural Connectivity
With its location at the junction between the occipital, temporal, and parietal lobes, the AG is considered an important interface that conveys and integrates information between different modalities and processing subsystems. For instance, large-scale connectivity analyses have shown that the AG is one of the major connector hubs linking different subsystems (Hagmann and others 2008; Tomasi and Volkow 2011). It is thus essential to identify the set of regions that anatomically connect to the AG as this would shed some light on its role during different cognitive processes. Because of the lack of consensus about the exact homology of the AG between humans and monkeys, it was not possible to discuss here the rich literature of anatomical tracer studies in the nonhuman primate (see Box 3). Thanks to recent advances in diffusion tensor imaging and tractography techniques, noninvasive visualization of white matter tracts that link the AG to other brain structures has been made possible (e.g., Catani and Thiebaut de Schotten 2008; Oishi and others 2008; Wakana and others 2004). Below is a list of some major tracts and bundles from previous diffusion tensor imaging studies that defined the AG as a seed, a target, or an intermediate connecting region in their tractography analyses. These different tracts are mentioned here using the same labels as in the original studies and are schematically illustrated in Figure 2.
Box 3. Homology with the Nonhuman Primate Brain.
An interesting observation from previous comparative studies is the striking differences in location and size of the inferior parietal cortex between humans and animals (Hyvarinen 1982; Orban and others 2006). For instance, previous reports have suggested that there is no clear homologue in monkeys to the human AG (Geschwind 1965; Zilles and Palomero-Gallagher 2001; see illustration in Figure 1 of Culham and Kanwisher 2001). This is largely because expansion of the posterior parietal lobe in humans has taken place largely in the inferior parietal lobule and in particular in the AG (Hyvarinen 1982). A few studies, however, have tentatively proposed a homology between the AG and areas 7a/PG of the macaque brain (McCulloch 1944; Petrides and Pandya 2009). Orban and others (2004) argued that the intraparietal sulcus in monkeys may correspond not only to the intraparietal sulcus in humans but also to a part of the AG (cf. Figure 2 of Orban and others 2004). Furthermore, anatomical tracer studies in the monkey have revealed rich efferent and afferent connections between the different subareas of the posterior parietal cortex with multiple inferior frontal, dorsal prefrontal, parahippocampal, hippocampal, and thalamic, caudate, cingulate, superior temporal, and frontal eye field (e.g., Andersen and others 1990; Cavada and Goldman-Rakic 1989; Pandya and Seltzer 1982; Petrides and Pandya 2009). However, because a homology between the human AG and its counterpart in the nonhuman primate is still a matter of debate, it is unclear to what extent this rich literature of monkey anatomical tracer studies can be compared with connectivity studies of the human brain (for a similar rationale, see Uddin and others 2010).
Figure 2.
Schematic illustration of some white matter tracts mapped in previous studies that used diffusion tensor imaging and tractography analysis. It is, however, important to keep in mind some of the limitations in using such techniques to quantify structural connectivity in vivo (see review in Jones 2010; Jones and Cercignani 2010). For ease of illustration, the tracts are oversimplified (i.e., not shown at their exact extent and localization). The names of the tracts are kept identical to the ones used in the original studies. The exact localization and full extent of each tract can be retrieved from their original studies as explicitly listed below: SLF-II = superior longitudinal fasciculus–second branch (cf. Figure 3 of Makris and others 2005); SLF-III = superior longitudinal fasciculus–third branch (cf. Figure 8 of Frey and others 2008); MLF = middle longitudinal fasciculus (cf. Figures 5–7 of Makris and others 2009, Figure 7 of Frey and others 2008); ILF = the inferior longitudinal fascicle (cf. Figure 5 of Rushworth and others 2006); OFF = occipitofrontal fascicle (cf. Figure 8 of Makris and others 2007); IOFF = inferior occipitofrontal fascicle (cf. Figure 5 of Uddin and others 2010). OFF and IOFF may correspond to the same tract (see discussion in Makris and others 2007), although other studies have suggested the existence of both inferior and superior OFF in humans. BA = Brodmann area; AG = angular gyrus; SOG = superior occipital gyrus; Pcu = precuneus; pSMG = posterior supramarginal gyrus; MTG/ITG = middle temporal gyrus/inferior temporal gyrus; HP/PHG = hippocampus/parahippocampal gyrus; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus.
Left and right AG are interconnected via the dorsal areas of the splenium and isthmus of the corpus callosum (Park and others 2008). The AG connects to the ipsilateral frontal and opercular areas via the superior longitudinal fasciculus (SLF) (Makris and others 2005). Specifically, the second branch of the SLF (SLF-II), located at the central core of the white matter above the insula, connects the AG to the caudal-lateral prefrontal regions (Makris and others 2005), and the third branch (SLF-III) links the AG directly to the inferior frontal gyrus (Broca area) at the level of areas BA 44 (Frey and others 2008) and BA 45 (Kelly and others 2010). It is also connected to the caudal posterior temporal regions via the middle longitudinal fasciculus (Frey and others 2008; Makris and others 2009) and to other temporal regions via the posterior segment of the arcuate fasciculus (Catani and others 2005). The AG also connects to the precuneus (BA 7) and the superior frontal gyrus (BA 8) via the occipitofrontal fasciculus (Makris and others 2007), to the caudate via the inferior occipitofrontal fasciculus (Uddin and others 2010), and to both parahippocampal gyrus (Rushworth and others 2006) and hippocampus (Uddin and others 2010) via the inferior longitudinal fascicle. Regarding its connections within the inferior parietal lobule, the AG connects to a posterior part of the supramarginal gyrus via local arcuate (U-shaped) connections (Lee and others 2007). Last but not least, it is argued that the AG receives little or no direct input from primary sensory areas (see discussion in Binder and others 2009). This rich anatomical connectivity pattern (Figure 2) enables considerable interactivity between the AG and temporofrontal subsystems in addition to other medial regions such as the hippocampus, caudate, and precuneus. As detailed below, connectivity may vary with the AG subregion used in seed-based tractography analyses (see examples in Mars and others 2011; Uddin and others 2010).
Multiple Functions
Functionally, the AG is commonly considered part of the heteromodal parietal association cortex (Rademacher and others 1992). At the system level, it is one of the major connecting hubs, as shown in previous functional connectivity studies (e.g., Tomasi and Volkow 2011). An abundant literature on healthy adult populations revealed that both left and right AG are implicated in numerous tasks and processes. For instance, using the NeuroSythn database (Yarkoni and others 2011), a “reverse inference” at coordinates [x = −48, y = −56, z = 36] revealed that several concepts and keywords previously have been associated with the left AG in numerous fMRI and PET studies, with the top 10 concepts including retrieval, default mode network, memory, semantic, sentence, semantic memory, consciousness, narrative, intentional, and familiar. This section succinctly reviews some of the most common functions associated with the AG on the basis that these functions consistently implicated the AG across numerous studies, as demonstrated from previous meta-analysis reviews. As a selection criterion, the AG should at least be listed in the meta-analysis tables of previous reviews. Each function is briefly introduced in separate paragraphs below. Table 1 provides some of the most consistent coordinates for the AG in different tasks from previous meta-analysis reviews.
Table 1.
Meta-Analysis Studies That Identified Consistent Angular Gyrus (AG) Activation across Numerous Functional Neuroimaging Studies (Listed Alphabetically: First Author + Year)
| Task/Process | Left AG | Right AG | |
|---|---|---|---|
| Arsalidou 2011 | Visuospatial facts retrieval in calculations | — | 30 –58 32a |
| Dehaene 2003 | Verbal retrieval of numbers | −41 −66 36 | — |
| Kim 2010 | Memory retrieval: self-referential processing | −40 −74 24 | — |
| Memory retrieval: control processing | — | 42 −60 26 | |
| Laird 2009 | Default mode network | −42 −66 18 | 46 −66 16 |
| Mar 2011 | Story-based theory-of-mind | −42 −72 34 | 54 −54 26 |
| Non-story-based theory-of-mind | −46 −62 32 | 52 −54 34 | |
| Mazoyer 2001 | Default mode network | −52 −74 18 | 46 –68 26 |
| Nee 2007 | Conflict resolution in go/no-go tasks | — | 42 −64 32 |
| Shulman 1997 | Default mode network | −45 −67 36 | — |
| Spaniol 2009 | Episodic memory: retrieval success | −34 −60 44a | 46 –50 28 |
| Episodic memory: subjective recollection | −40 −72 32 | 52 −68 16 | |
| Sperduti 2011 | External-agency attribution | −50 −56 44 | 58 x02212;56 36 |
| Spreng 2009 | Default mode network | −43 –69 32 | 49 −63 20 |
| Autobiographical memory | −47 −61 26 | 49 −59 27 | |
| Visuospatial navigation | −34 −78 34 | 42 −74 32 | |
| Theory-of-mind | −43 −68 39 | — | |
| Prospection | −49 –64 29 | — | |
| Turkeltaub 2010 | Phoneme discrimination | −38 −60 42a | — |
| Vigneau 2006 | Semantic processing | −45 −68 26 | — |
| Vilberg 2008 | Memory retrieval: successful recollection | −43 −66 38 | 53 −61 25 |
| Wager 2005 | Inhibition in go/no-go tasks | — | 41 −64 35 |
| Wang 2010 | Concrete versus abstract concepts | −34 −76 34 | — |
— = AG not identified.
The coordinates of these dorsal-medial clusters may extend to other parietal subareas of the intraparietal sulcus (see Caspers and others 2008).
But first it is important to keep in mind some conceptual issues and limitations on the kind of inferences that someone can make from previous literature (see Box 4 for more details). For instance, it is worth noting the discrepancy in previous studies and reviews when it comes to labeling their AG foci using sometimes different names for the same localization. The nomenclature of region thus tends to vary depending on the cognitive domain under study. For example, activation in the AG is more often described as (or part of) the temporoparietal junction in social cognition. In the language and reading literature, the labels posterior middle temporal gyrus and temporo-parieto-occipital cortex are sometimes used. Other studies, particularly in the domain of attention, default mode, number processing, and memory, have used unspecific labels such as inferior parietal lobule, posterior parietal cortex, or ventral parietal cortex to designate their AG activation, as these labels mix AG with other neighbor regions such as the supramarginal gyrus and the lateral bank of the intraparietal sulcus. In particular, several distinct areas may exist along the banks of the intraparietal sulcus (e.g., Culham and Kanwisher 2001; Grefkes and Fink 2005; Konen and Kastner 2008), and thus it is important to not conflate them with the AG. When these labels were used, the stereotaxic coordinates (if provided) were compared with typical localization of the AG (as defined in previous atlases of Fig. 1B,C) to decide whether to include such activations in the review.
Box 4. Methodological Issues.
Identifying the exact role of a given brain structure is not straightforward in the context of the many-to-many relationship between structure and function. In this context, the exact role of the AG critically depends on the set of regions it is interacting with during a given task/process. This implies that the role of the AG cannot comprehensibly be identified in isolation but ideally needs to be understood in parallel with the influence from other regions—for instance, by combining the AG with other interacting regions to enable meta-analyses at the system level. This kind of systemic meta-analysis may help, for instance, to identify brain regions that are consistently coactivated with the AG and how they interact with task demands, modality, and stimulus domain.
Existing differences in functional properties and lateralization between the left and right AG have not always been taken into account in previous studies and reviews, although strong bilateral AG activations are not uncommon. Indeed, some interesting differences between the left and right AG may get “blurred” and thus were omitted in previous meta-analysis reviews; therefore, differences between the left and right AG are not strongly emphasized in this review.
It is obvious to note the diversity in tasks that were previously used to activate the AG, varying in terms of stimulus material, domain, modality, baseline condition, response type, paradigm design, and sequence. In the same way, reporting activation in the AG during a given function/domain with fMRI or PET (i.e., typically by contrasting different conditions/tasks) does not necessarily mean that the AG was essential for that function/domain. Here, emphasis was particularly put on consistent findings, and thus such inevitable methodological differences were ignored.
There is a sort of “borrowing” of several concepts across different domains for the interpretation of activations in the AG, which inevitably resulted in the same concept being associated with different processes in completely different tasks. For instance, the use of “magnitude/size” in spatial attention and number-processing studies, “referential” in social cognition and the default network studies, or “semantic” in language and episodic memory studies may reflect different processes despite being described under the same concept.
Previous studies and reviews have used different terms to describe the responses that are common (or independent) to several modalities, including multimodal, heteromodal, trimodal, amodal, intermodal, cross-modal, polymodal, transmodal, and modality independent. Although subtle differences exist between these concepts, the term cross-modal is used here to stress the significant activation in the AG across several modalities.
Semantic Processing
Semantic processing is the most consistent function that activates the AG, particularly in the left hemisphere. Early functional imaging studies demonstrated left AG activation during semantic tasks on auditory (Demonet and others 1992) and visual (Vandenberghe and others 1996) stimuli, and these findings have been replicated with high consistency and reliability across numerous studies (see meta-analysis reviews in Binder and others 2009; Vigneau and others 2006). For instance, Binder and others (2009) found that the most consistent semantic activation across 120 functional neuroimaging studies was located within the left AG (Binder and others 2009), with a less strong but consistent effect in the right AG as well. Moreover, the AG plays a major role in processing concrete relative to abstract concepts (Wang and others 2010). More specifically, the left AG seems to be engaged in all aspects of semantic processing that require concept retrieval and conceptual integration (Binder and others 2009), and it provides semantic constraints during language comprehension (Price CJ 2010; Seghier and others 2010). As detailed below, semantics are inherently present in multiple tasks that activate the AG.
Reading and Comprehension
The involvement of the AG in reading comprehension was first suggested by Dejerine (1891), who documented a loss in the capacity to read and write words following damage to the left AG. This finding was then popularized by the seminal work of Geschwind (1965). In his model, Geschwind (1965) defined the AG as a visual memory center for words that turns written language into spoken language and vice versa. Activation in the left AG during reading was found to be highly consistent in children as well (see meta-analysis in Houde and others 2010); however, the left AG was not sensitive to visual word forms because it was not activated during reading aloud single words relative to fixation (see discussion in Price CJ 2000). This nonselectivity to word forms is also supported by several meta-analysis reviews showing that the AG was not consistently activated in single word reading in skilled adult readers (Fiez and Petersen 1998; Purcell and others 2011; Turkeltaub and others 2002). As discussed below in the next paragraph, this absence of activity for words relative to fixation might be explained by the strong task-independent deactivation that is typically seen in bilateral AG. This deactivation relative to fixation and rest has been attributed to greater semantic associations during free thought than during reading (Binder and others 1999). A semantic account of the AG during reading was also supported by the strong positive correlation between activation in bilateral AG and both word frequency and imageability (Binder and others 2005; Graves and others 2010). However, Graves and others (2010) argued that the AG is not involved in mapping from semantics to phonology, a role attributed to middle and inferior temporal gyri, but it is likely to support semantic feature knowledge (Graves and others 2010). Thus, what emerges from this large literature is that the AG engages in reading when semantic associations are made (Price CJ and Mechelli 2005), an involvement that is particularly enhanced during sentence reading and more generally in comprehension of speech and written language (Obleser and Kotz 2010; Xu and others 2005). In addition to this dominant semantic account in comprehension, it is worth noting the potential role of the AG in phoneme discrimination in sublexical speech perception (see meta-analysis in Turkeltaub and Coslett 2010).
The Default Network
The “default network” or the “default mode network” (Greicius and others 2003; Raichle and others 2001) designates a set of brain regions that are strongly deactivated during goal-directed tasks as compared with rest or passive baselines. It is thought to participate in internal mentation that becomes prominent when people are not engaged in external interactions (Buckner and others 2008), and it forms one of the most consistent resting-state networks (Smith and others 2009). These task-independent deactivations include specifically the bilateral inferior parietal, medial frontal, and posterior cingulate cortex. The deactivation in the inferior parietal cortex that includes the bilateral AG is remarkably reliable (Shehzad and others 2009) and consistent across different tasks, paradigms, subjects, and studies (see recent meta-analysis reviews in Buckner and others 2008; Laird and others 2009; Spreng and others 2009). Several hypotheses have been put forward to explain such consistent deactivations in the AG. One influential hypothesis suggests that the AG is involved in task-free semantic and conceptual processes that result from the manipulation of acquired knowledge about the world during rest that is interrupted during effortful tasks (Binder and others 1999; see also Seghier and others 2010; Wirth and others 2011). Others have suggested that the bilateral AG act as dynamic self-referential regions during rest that are associated with significant behavioral profiles in interoception and somesthesis (Laird and others 2009). Alternatively, the AG might be engaged in constructing mental scenes based on memory during rest or when subjects envision themselves in the future (Andrews-Hanna and others 2010). To summarize this paragraph, the common denominator between these different hypotheses is the engagement of the AG in the manipulation of conceptual knowledge and mental representations when the mind wanders during “rest.”
Number Processing
Early neuroimaging studies have shown strong AG activation during digit subtraction (Roland and Friberg 1985) that has been replicated with high consistency across functional studies with varieties of tasks that manipulated different numerical operations and presentations (for review, see Dehaene and others 1998; Dehaene and others 2003). For instance, the AG has been shown to mediate spatial representations of numbers (Gobel and others 2001) and might be specific to Arabic digit perception even under passive tasks (Price GR and Ansari 2011); however, its specificity for numbers is still debatable. For example, bilateral AG were activated during a conceptual decision on numbers, but this activation was similar to conceptual decisions on object names in the left AG (Cappelletti and others 2010), which argues against a selective role of the left AG for number processing (e.g., Cappelletti and others 2007). In this context, Dehaene and others (2003) argued that the left AG is mainly involved in the verbal coding of numbers because it was strongly activated during small problems of addition and multiplication that require the retrieval of arithmetic facts stored in the verbal memory. For example, by comparing problem solving of small versus large problems over different arithmetic operations, a significant difference was found in the left AG (Grabner and others 2009), which supports its role in arithmetic fact retrieval. Interestingly, the left AG seems also to play a major role during the transfer of facts between arithmetic operations (Ischebeck and others 2009). Although the left AG has dominated the number-processing field, activations in the right AG have not been infrequent. For instance, in a recent meta-analysis, the right AG has been shown to be a highly consistent cluster that is most likely to be involved in visual-spatial attention when calculation problems are being solved (Arsalidou and Taylor 2011).
Attention and Spatial Cognition
Several functional neuroimaging studies have suggested strong AG involvement in attention mechanisms (see review in Corbetta and Shulman 1998; Singh-Curry and Husain 2009). In particular, the AG might be involved in the reorienting or shifting of attention—for instance, when shifting the attentional system toward particular stimuli that have high salience in terms of motion, emotion, value, or meaning (Gottlieb 2007). For example, it has been shown that attentional reorienting originates in the right AG thanks to its causal role in using task history to update attentional selection (Taylor and others 2011). The right inferior parietal lobule including the AG may also play an important role in maintaining attention as well as encoding salient events in the environment (Singh-Curry and Husain 2009). Furthermore, in their recent meta-analysis about the attention and memory systems, Ciaramelli and others (2008) argued that the inferior parietal cortex, including the supramarginal gyrus and the AG, is part of a “bottom-up” attentional subsystem that mediates the automatic allocation of attention to task-relevant information (Ciaramelli and others 2008), particularly in attending to retrieved memories (Cabeza and others 2008).
It is interesting to note that the bilateral AG are involved in a wide range of tasks in spatial cognition, which reflects our ability to process and integrate all spatial aspects of our environment, including the spatial analysis of external sensory information and internal mental representations (for review, see Sack 2009). One common example of such spatial cognition processes is the ability to discriminate left from right. This left-right discrimination consists first of a perceptual or spatial encoding process and then the ability to associate each side with the word left or right. As shown recently, the left AG is the site where spatial information is integrated with the meaning of the words left and right (Hirnstein and others 2011). Interestingly, the important supporting role of the AG in spatial cognition, particularly in the right hemisphere, explains why the AG is critical for perceptual learning (see discussion in Rosenthal and others 2009). This again highlights the major role of the AG in integrating spatial information with conceptual knowledge.
Memory Retrieval
The AG is associated with verbal working memory, particularly during the retrieval of verbal material (Jonides and others 1998). Recent meta-analysis reviews have demonstrated a strong involvement of the AG during episodic memory retrieval, particularly during successful (Ciaramelli and others 2008; Vilberg and Rugg 2008) and subjective recollection (Spaniol and others 2009). In addition, bilateral AG, as part of the inferior parietal lobule mediating the automatic “bottom-up” attentional resources (Cabeza and others 2008), play a critical role in monitoring the retrieval output continuously, with an activation level that increases when memory performance is high and when recognizing items with high compared with low confidence (Cabeza 2008; Ciaramelli and others 2008). However, others have argued that attention and memory can take distinct forms in the posterior parietal cortex, with the contribution of attention to memory varying between the left and right AG, and may differ between episodic encoding and retrieval (Hutchinson and others 2009). Moreover, AG activity has also been shown to act as a marker of violations in memory expectations, where a violation reflects a lack of correspondence between retrieval outcomes and expectations (O’Connor and others 2010). Others have alternatively suggested that the AG, particularly in the left hemisphere, serves as a memory buffer for intention maintenance that sustains episodic information until the execution of an action (e.g., Kalpouzos and others 2010). Another set of evidence is provided by studies that compared AG involvement in episodic memory with the default network (e.g., see meta-analysis in Kim 2010). For instance, Sestieri and others (2011) compared the activation profile of the default network during episodic memory retrieval and found that the strongest memory search–related activations were observed in the bilateral AG (Sestieri and others 2011). Last but not least, the AG is also part of the autobiographical memory system (Spreng and others 2009; Svoboda and others 2006). It is worth noting that the important role of the AG in memory is facilitated by its strong connectivity with the hippocampal system as mentioned above (i.e., Fig. 2).
Conflict Resolution
This concerns a broad range of tasks where participants need to select or execute an appropriate response in the context of conflict or interference from other conditions or stimuli. This conflict can be semantic, spatial, or emotional in nature (see examples in Fan and others 2003). One particular class of conflict tasks that strongly activated the AG are go/no-go tasks that present participants with two types of stimuli, one requiring a response and the other requiring the withholding of a response. Specifically, the right AG was found to be strongly involved during the inhibition of the inappropriate response across a variety of go/no-go tasks (see meta-analysis reviews in Nee and others 2007; Wager and others 2005). Regarding the left AG, previous meta-analysis reviews failed to identify consistent activations across neuroimaging studies that used different conflict tasks. One possible hypothesis is the need for a strong contextual/semantic conflict to activate the left AG. For instance, when comparing between three different conflict tasks, the right AG was strongly activated in all conflict tasks, whereas the left AG was only involved in a sentence comprehension task that included a conflict between plausible and implausible sentential representations (Ye and Zhou 2009). In the same way, when manipulating semantic and referential anomalies during sentence comprehension, the right and left AG were strongly involved in solving referential ambiguity (Nieuwland and others 2007).
Theory-of-Mind and Social Cognition
Theory-of-mind or mentalizing is a framework used in social cognition to infer the mental states of others (i.e., the attribution of mental states) at the level of their beliefs, emotions, goals, and motivations. It is an essential capacity that helps the human brain to reason about other people to effectively communicate and navigate in the social world. Theory-of-mind tasks can be verbal or nonverbal in nature and typically involve false belief stories, attribution of mental state to one or more characters of a story, cartoon stories, or animations of rigid geometric shapes that depict social interactions (e.g., Gallagher and others 2000). In this large literature of social cognition, a consistently activated region extended over posterior temporal to posterior parietal cortices (Decety and Lamm 2007), including the AG (Mar 2011). For instance, numerous functional neuroimaging studies have shown strong involvement of bilateral AG in theory-of-mind or mentalizing tasks (see meta-analysis reviews in Buckner and others 2008; Mar 2011; Spreng and others 2009). As shown recently, bilateral AG showed strong involvement in both (verbal) story-based and (nonverbal) non-story-based theory-of-mind tasks, which may suggest its involvement in some aspects of sequencing and scene construction (see discussion in Mar 2011). Alternatively, other studies have suggested a role of bilateral AG in theory-of-mind in accessing story content and episodic memories (Calarge and others 2003), in external-agency attribution (Sperduti and others 2011), or when inferences about human intention are made during discourse processing (Mason and Just 2011). In sum, the AG in social cognition seems to support access to mental representations and judgment making on contextual associations.
AG as a Cross-Modal Integrative Hub
It is striking to see the high similarity in task-free deactivation in the AG as in the default network with multiple networks that, in addition to the ones cited above, also included other high-order (meta-cognitive) systems such as envisioning the future, moral decision making, and prospection (Buckner and others 2008; Spreng and others 2009). Given this high similarity, it might be more informative to think of the AG beyond the boundaries of each domain. This short paragraph thus aims to provide a unified picture of AG function that transcends specific domains or tasks. For instance, as argued by Binder and others (2009), the AG may play a particular role in all tasks requiring fluent conceptual combination, such as sentence comprehension, discourse, problem solving, and planning (Binder and others 2009). In another model, the AG was proposed as a module of an analyzing block responsible for accessing subparts of stored items (Shalom and Poeppel 2008). But before discussing specifically the fundamental features that may define AG function, it is useful to see how the AG was portrayed in some previous seminal studies.
By thoroughly reviewing earlier neuropsychological and comparative studies, Geschwind (1965) argued that intermodal associations become powerful in humans thanks in part to the emergence of the AG, which acts as a “visual memory centre for words” and has contributed tremendously to the development of language and speech. Thirty years ago, Joseph (1982) defined the AG with Broca and Wernicke areas as components of a language axis that, with its complex interactions with the thalamic system, enables the formulation of speech and thought. Specifically, he proposed that the AG is “involved in the assimilation of diverse information variables, their integration, the calling-up of relevant associations, and functions as a necessary intermediary for all conscious functioning, particularly in the development and comprehension of language and thought. . . . [The AG] increases the capacity for the organization, categorization, and labeling of sensory-motor events” (Joseph 1982, p. 22). He then defined the AG as a processing center “where cross-modal associations such as visual, somasthetic and other sensory-motor concommitants are aroused, integrated, organized, assimilated, and finally comprehended” (Joseph 1982, p. 24). In addition, in his generalized model of sensation-to-cognition, Mesulam (1998) suggested that transmodal areas that include the posterior parietal cortex “provide critical gateways for transforming perception into recognition, word-forms into meaning, scenes and events into experiences, and spatial locations into targets for exploration.” In particular, thanks to its role in multimodal associations, the posterior parietal cortex (including the AG) also supports spatial awareness and working memory–executive function (Mesulam 1998).
Furthermore, Damasio (1989) introduced the concept of a convergence zone to describe the function of the posterior parietal cortex (including the AG) and other regions at the system level. Convergence zones are assumed to be amodal, and they sustain integration in a multimodal system (Damasio 1989). They are purposely considered “a critical gateway for accessing, binding and integrating information related to the conceptual representation and exploration of the extrapersonal space. . . . They register combinations of components in terms of coincidence or sequence, in space and time” (Damasio 1989). This framework has recently been adapted by Binder and Desai (2011), who proposed that the AG belongs to the convergence zones that store increasingly abstract representations of entity and event knowledge. They pointed out that the level of activation in the AG reflects the amount of semantic information that can be successfully retrieved from a given input, which suggests that the AG may play a unique role in the representation of event concepts (Binder and Desai 2011).
Fortunately, these models seem to agree on some fundamental features that shape AG function. These features include cross-modal associations (or, in Damasio’s model, trimodal combinations), integration, meaning, and event representations. Given also the consistent involvement of the AG in the default network, memory retrieval, and spatial and social cognition, it is important to reckon other extra features that include the sense of agency and action awareness (Farrer and others 2008; Farrer and Frith 2002; Kim 2010; Sperduti and others 2011). These extra features of agency and action awareness complete the set of key features that embody the multiple roles of the AG because both seem necessary to accurately compass the dynamic nature of semantics (i.e., as events and experiences) where persons, concepts, objects, and actions bind in time and space (Zhuge 2010).
To conclude this paragraph, it becomes clear that the AG resembles a “core facility” used by different subsystems to access concepts when interfacing perception-to-recognition-to-action. More specifically, given its rich connectivity and its location where multisensory information converges, the AG resembles a cross-modal integrative hub that gives sense and meaning to an event within a contextualized environment, based on prior expectations and knowledge, and toward an intended action. Although integration and amodality have been associated with more anterior temporal regions (see reviews in Jung-Beeman 2005; Patterson and others 2007; Stowe and others 2005), it is plausible that the AG supports initial (or first-order) integration that provides direct access to conceptual representations. This is supported by recent evidence that showed, for instance, the involvement of the AG in audiovisual speech integration (Bernstein and others 2008) and face-voice integration during person recognition (Joassin and others 2011). However, this does not preclude strong interactivity between the AG and other integrative hubs (Patterson and others 2007) that may increase with task demands (e.g., Obleser and others 2007).
A Unified Account of AG Multiple Functions
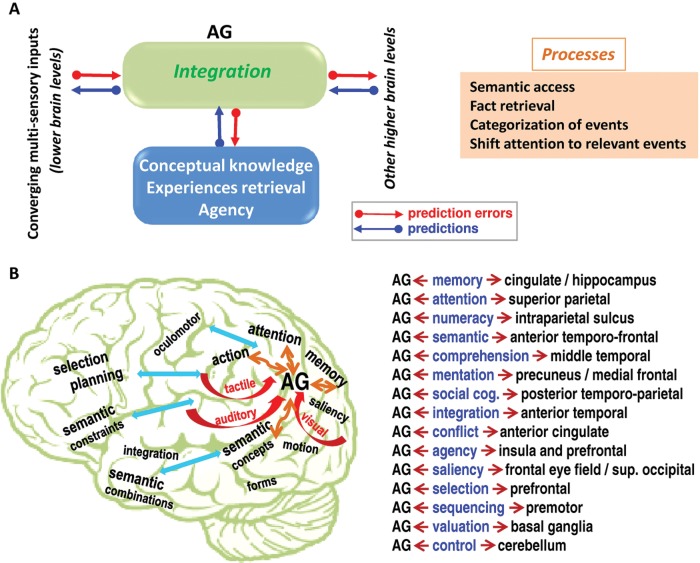
Figure 3 schematically illustrates a unified framework that could account for the different processes/domains that activate the AG as detailed above. This framework is borrowed from the popular predictive coding framework (as reviewed in Friston 2010) that models the brain as a hierarchical inference engine that is trying to optimize probabilistic representations of what caused its sensory input. Specifically, Figure 3A assumes the AG as an interface between the converging bottom-up multisensory inputs and the top-down predictions. Top-down predictions are conveyed by backward connections and are compared with the representations being generated at the AG, with the difference between the two reflecting the prediction error. This prediction error is then forwarded to higher levels to adjust and optimize the predictions. The recurrent exchange of bottom-up prediction errors and top-down predictions proceeds until prediction error is minimized at all levels of the system. Thus, the cross-modal integration in the AG can conceptually be seen as the sum of such recurrent exchange that happens at the level of the AG. The top-down predictions are based on previous knowledge of the external world, similar learned experiences that can be retrieved, and the awareness of own action (sense of agency). They may also come from other subsystems that maintain the intention (i.e., the planned action/decision to be made) and the saliency and the priority given to particular events of interest. The core processes that result from such integration within the AG translate into the categorization of events, access to semantics, fact retrieval, and shifting attention toward relevant information.
Figure 3.
(A) Provides a unified framework that could account for the multiple functions of the angular gyrus (AG). Converging multisensory inputs are integrated in the AG (green box) in a context-dependent fashion. Top-down predictions (blue box) shape the integration of the converging inputs, and these predictions are generated on the basis of prior knowledge about the external world, similar learned experiences that can be retrieved, and the attribution of own action (i.e., the sense of agency). Other top-down predictions may come from other subsystems that code intention, saliency, and priority given to specific targets or events of interest. The integration in the AG proceeds via the recurrent exchange of bottom-up prediction errors (red arrows) and top-down predictions (blue arrows) until prediction error is minimized in the sense of the predictive coding framework (Friston 2010). This integration ultimately contributes in comprehending and reasoning about external events or internal mental representations and results in a set of core processes (orange box) that include events categorization, semantic access, fact retrieval, and shifting attention to relevant information. (B) Schematically illustrates the complex interplay between the AG and other distributed subsystems. It shows the convergence of different multimodel inputs to the AG (red arrows) and the interactions with different subsystems (orange arrows) that include memory, attention, action, and semantics. Interactions with other potential subsystems are indicated with blue arrows. The definition of semantic combinations and constraints is based on Price’s (2010) review, and the role of anterior temporal regions in semantic integration is based on Jung-Beeman’s (2005) review. Candidate regions that may strongly interact with the AG are listed with the most likely function/domain of interest. This is an oversimplified illustration because each domain/system contains several regions that can differently interact with the AG.
This framework can explain the multiple functions that implicate the AG. For instance, access to semantics is a key process in language comprehension and sentence reading. Likewise, fact retrieval reflects the retrieval of learned rules and facts that are important in number processing and in print-to-sound conversion during reading. Categorization of events and shifting attention to relevant information are important in social cognition, memory, and spatial cognition. In the case of the default network, the manipulation of conceptual knowledge, the sense of agency, and the retrieval of previous experiences (as predictions in Figure 3A) can modulate AG activity even in the absence of external sensory inputs. These processes are likely to have a hemispheric bias favoring more the left than the right hemisphere or vice versa; for instance, attention might lateralize toward the right AG and semantic access might lateralize toward the left AG. Critically, it is important to keep in mind that the exact role of the AG depends on the set of regions it is interacting with during a given task/process, as illustrated in Figure 3B. All these issues warrant further investigations.
Multiple Subdivisions
In the section above, the AG was assumed so far as one single region with homogeneous anatomical and functional properties, thus ignoring the large variability across studies in the localization of AG activations. For instance, in a previous meta-analysis of semantic processing, Binder and others (2009) showed a wide distribution of activated peaks across 120 functional studies in bilateral AG (see Figure 2 in Binder and others 2009). Consistent AG peaks across multiple reviews have been considerably variable, as illustrated here in Table 1. This large variability in the AG may potentially reflect the existence of multiple subdivisions within the AG. Indeed, an increasing literature has defined the AG as an aggregate of multiple subdivisions where each subdivision can be characterized by specific functional and connectivity patterns (e.g., Andrews-Hanna and others 2010; Bahnemann and others 2010; Brownsett and Wise 2010; Caspers and others 2008; Eidelberg and Galaburda 1984; Kim 2010; Mars and others 2011; Naidich and others 1995; Nelson and others 2010; Nickel and Seitz 2005; Price GR and Ansari 2011; Rushworth and others 2006; Seghier and others 2010; Seghier and Price 2009; Sharp and others 2010; Uddin and others 2010; Vandenberghe and Gillebert 2009; Yeo and others 2011). This literature has provided an interesting framework for reporting and interpreting AG activations with greater definition, where each subdivision is allowed to have distinct contributions in a given task. This literature is briefly presented below with emphasis on anatomical, connectivity, or functional parcellation.
Anatomy-Based Parcellation
Previous neuroimaging studies have suggested a possible anatomical segregation of the AG according to the anatomical variability in the sulci and gyri around the posterior inferior parietal region. For instance, following a detailed anatomical analysis of the variability in the horseshoe shape of the angular gyrus, it was suggested that an additional accessory pre-angular (pre-AG) area can be reliably defined at the anterior part of the angular sulcus (Naidich and others 1995). In addition, Eidelberg and Galaburda (1984) suggested three possible subareas in the AG using cytoarchitectonic parcellation on 8 postmortem brains (Eidelberg and Galaburda 1984). Recently, using an observer-independent cytoarchitectonic analysis on 10 postmortem brains, two cytoarchitectonic subdivisions were traced and labeled as areas PGa and PGp with high consistency across subjects (Caspers and others 2008; Caspers and others 2006) (see Fig. 1C). This recent anatomical parcellation into PGa and PGp is now widely used in both connectivity and functional region-based analysis.
Connectivity-Based Parcellation
Using diffusion tensor imaging, different structural connectivity patterns were observed at different parts of the AG. For instance, tractography-based parcellation of the inferior parietal lobule identified five clusters that included two subdivisions within the AG (Mars and others 2011): a first cluster in the posterior-ventral part of the AG and a second cluster in the anterior-dorsal part of the AG. These two clusters resembled, respectively, the cytoarchitectonic subdivisions PGp and PGa shown in Figure 1C. Using resting-state functional connectivity analysis, it has been suggested that the posterior-ventral subdivision of the AG connects to the parahippocampal gyrus, whereas the anterior-dorsal subdivision of the AG connects more strongly with the anterior prefrontal cortex (Mars and others 2011). Similarly, a direct comparison between the resting-state functional connectivity patterns of seed regions PGa and PGp revealed greater connectivity for PGa with the caudate, bilateral frontal poles, and posterior and anterior cingulate, as well as greater connectivity for PGp with the hippocampus, parahippocampal gyrus, medial prefrontal cortex, and precuneus (Uddin and others 2010). Although tractography from both subdivisions PGp and PGa showed strong connectivity with the caudate, there was stronger structural connectivity from PGp than PGa with the hippocampus and the parahippocampal gyrus (Uddin and others 2010).
Function-Based Parcellation
Some recent functional neuroimaging studies have reported different contributions of distinct parts of the AG over variable tasks and processes. This was, in particular, shown in the left AG. For instance, Nelson and others (2010) used a combined analysis on the profile of memory retrieval–related activity and the membership to large-scale brain networks to dissociate six subregions in the lateral parietal cortex. Two subdivisions were located near the AG: a ventral region where activity correlated with that in the medial prefrontal cortex and a dorsal region where activity strongly correlated with that in the superior frontal gyrus (Nelson and others 2010). Using passive perception of letters and Arabic digits, the left AG was also segregated into a dorsal subdivision that was activated by both familiar letters and digits and a ventral subdivision that was strongly activated for digits compared with letters (Price GR and Ansari 2011).
During speech comprehension, two AG clusters were segregated by manipulating the semantic and acoustic difficulty in a task that required making decisions based on the semantic relatedness between heard nouns (Sharp and others 2010). A first ventral posterior AG cluster showed stronger activation for high semantic versus high acoustic difficult conditions, whereas a dorsal anterior AG cluster showed stronger activation for high semantic versus control speech (Sharp and others 2010). Using similar semantic decision tasks but on written words and pictures of familiar objects, Seghier and others (2010) showed a reliable intersection between the semantic network and the default network at a precise AG location that served as a functional landmark to dissociate three subdivisions in the left AG (Seghier and others 2010). A first subdivision, located at the site of the overlap between the two networks, is involved in semantic associations regardless of the presence or absence of a stimulus; dorsal to the overlap is a second subdivision involved in searching for semantics in all visual stimuli; and ventral to the overlap is a third subdivision involved in the conceptual identification of visual inputs (Seghier and others 2010). On the other hand, evidence for similar subdivisions in the right AG is scarce, although one study of number processing dissociated two ventral and dorsal clusters in the right AG that varied in activation level with number selectivity, response time-related effects, and stimulus-independent task effects (Cappelletti and others 2010).
Figure 4 illustrates the projection of the coordinates of the different left AG subdivisions identified in the four functional neuroimaging studies cited above (cf. Nelson and others 2010; Price GR and Ansari 2011; Seghier and others 2010; Sharp and others 2010), as reported in the standard Montreal Neurological Institute (MNI) space. By grouping these MNI coordinates and looking for consistent localizations, two AG subdivisions can clearly be identified (Fig. 4): 1) a first subdivision that is located dorsally in the AG with mean coordinates at [x = −35, y = −64, z = 45] and 2) a second subdivision located ventrally in the AG with mean coordinates at [x = −42, y = −69, z = 31]. More specifically, dorsal AG subdivision is likely to participate in “bottom-up” processes during semantic search (Price CJ 2010; Seghier and others 2010), fact retrieval (e.g., Ischebeck and others 2009), and the (automatic) allocation of attention to memory (Cabeza and others 2008; Ciaramelli and others 2008), whereas ventral AG subdivision may exert a “top-down” influence in self-referential processing (Kim 2010), providing semantic constraints (Price CJ 2010; Seghier and others 2010), and making judgments on mental representations (e.g., Bahnemann and others 2010; Mar 2011). The dorsal subdivision is bounded by the lateral bank of the intraparietal sulcus, and the ventral subdivision most likely overlaps with the deactivated regions of the default network. It is worth noting that a third subdivision, located more ventrally at MNI–z coordinates of z = +20 mm, was also identified by Seghier and others (2010), as illustrated in magenta in Figure 4. This third ventral AG subdivision showed stronger semantic responses when stimuli were pictures than written words, suggesting its role in direct access to concepts from visual inputs (Seghier and others 2010). Similarly, during social cognition tasks, Bahnemann and others (2010) observed a strong overlap between theory-of-mind tasks and moral judgment tasks at this more ventral AG subdivision that was distinct from other dorsal AG responses.
Figure 4.
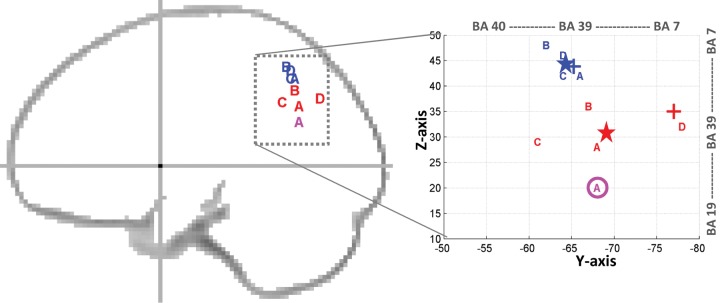
Projection of Montreal Neurological Institute (MNI) coordinates of previous angular gyrus (AG) subdivisions on a schematic sagittal outline (left, at x = −30 mm) and a zoom centered at the AG (right). A = Seghier and others (2010), B = Nelson and others (2010), C = Price GR and Ansari (2011), and D = Sharp and others (2010). Dorsal coordinates are shown in blue and ventral coordinates are shown in red. A third more ventral subdivision at z = +20 mm is shown in magenta (according to Seghier and others 2010). Average locations are illustrated with a star (blue for dorsal subdivision at [x = −35, y = −64, z = 45], red for ventral subdivision at [x = −42, y = −69, z = 31]). The average locations here are remarkably similar to the two subdivisions used in a recent seed-based resting-state functional connectivity study over a large sample of 1000 subjects (noted as PGpd and PGpv in Table 4 and Figure 30 of Yeo and others 2011). Approximate location of the center of gravity of cytoarchitectonic areas PGa and PGp (Caspers and others 2008) is indicated by “+” in blue and red, respectively. BA = Brodmann area.
To conclude this section, both anatomical and functional evidence supports the existence of a high-definition map in the AG. This is particularly visible along the ventral-to-dorsal axis in the left AG, as illustrated in Figure 4. Future studies can use this spatial parcellation of the AG as a roadmap to report their activations.
Future Work
The studies reviewed here have provided valuable insights to our understanding of the exact contribution of the AG in cognition. Several other issues warrant further investigations, as briefly listed below:
Identify the set of core regions that interact with the AG in different processes and how these interactions are modulated by task demands (as illustrated in Fig. 3B)
This important issue would benefit from the popularization of effective connectivity techniques that allow the direction and strength of interregional coupling to be estimated. For instance, Carreiras and others (2009) demonstrated a “top-down” role of the AG on posterior occipital areas during reading aloud relative to object naming (Carreiras and others 2009). Similar studies are thus needed to depict a mechanistic account for AG role(s). A particularly interesting question is the nature of interactions that the AG carries with the rest of the semantic network. The semantic system is composed of a large set of nodes (Binder and others 2009) that may play different roles in semantic processes, including the pars orbitalis, the middle temporal gyrus, and the temporal pole (for more details, see Price CJ 2010). Moreover, there is a lack of literature regarding the potential interactions between the AG and cerebellar regions, particularly when considering the contribution of the cerebellum to different cognitive processes (Schmahmann 2010). For instance, a recent resting-state connectivity analysis has revealed strong functional connectivity between the posterior parietal cortex (including the AG) and a supramodale zone of the cerebellum (see O’Reilly and others 2010).
Visualize the dynamics of AG activation using high-temporal resolution techniques
In this brief review, it was not possible to do justice to previous electroencephalogram, transcranial magnetic stimulation, or magnetoencephalogram studies because this literature requires its own review. For example, these techniques can help to reveal whether AG activation happens at earlier or later latency than frontal and temporal regions and whether this latency changes with task demands and modality.
Characterize lateralization in the AG and how it is modulated by task and modality
This issue relates to the possible differences in functional properties in the left and right AG over varieties of tasks (Jung-Beeman 2005; Lindell 2006). For instance, as shown above, the involvement of the AG for semantics, spatial cognition, or number processing may vary between left and right hemispheres. A systematic analysis of lateralization effects in the AG will provide important clues for future models of AG function. In the same way, the different AG subdivisions shown above are mainly identified in the left hemisphere. Future studies are needed to investigate whether the same functional subdivisions exist in the right AG.
Characterize specific deficits associated with AG damage
It has been shown that damage to the AG has consequences on a range of skills, including speech comprehension, finger agnosia, spatial disorientation, acalculia, agraphia, and dementia (e.g., Ardila and others 2000; Corbett and others 2009). The wide range of deficits speaks volume to the multiple tasks and processes that depend on the integrity of the AG. Future work can report AG damage at high definition, as reviewed here, to identify whether these variable deficits may reflect damage to specific subparts of the AG.
Explore interindividual variability in AG function
There is an increasing interest in characterizing variability in function between subjects because it can reveal the different cognitive strategies used by subjects when performing the same task (Miller and others 2012; Seghier and Price 2009). Developmental factors may also contribute to such variability, and thus studies are needed to test whether AG structure and function vary over the life span. For instance, it has been shown that the AG is one of the few brain regions where structural asymmetry decreases with age (Kovalev and others 2003). In the same context, the impact of other demographic (gender and handedness) and genetic variables on the anatomy and function of the AG warrants further studies.
Compare the size, location, and connectivity of the AG across different species
For instance, it has been shown that a major temporal lobe projection of the arcuate fasciculus in humans is smaller or even absent in chimpanzees and macaques (Rilling and others 2008). This topic warrants systematic investigation because it can provide new clues to explain the disproportionate expansion of the multimodal associative regions in humans and their significant contribution to our cognitive and linguistic abilities (see discussion in Rosa and Tweedale 2005; Sherwood and others 2008).
Conclusion
This brief review aimed to bring together previous findings to construct a unified picture of the AG during all processes, from perception to action. It highlights the integrative role of the AG in comprehension and reasoning—for instance, when manipulating conceptual knowledge, reorienting the attentional system toward relevant information, retrieving facts for problem solving, and giving meaning to external events based on stored memories and prior experiences. This review also discussed the spatial fractation of the AG into multiple subdivisions that may contribute in “bottom-up” and “top-down” mechanisms across numerous tasks. Future studies have to clarify how the AG communicates with other subsystems to continuously give meaning and sense to the external world. Finally, this highly selective review recognizes that cracking the code that uniquely defines AG function is still an ongoing endeavor.
Acknowledgments
The author thanks Cathy Price for her valuable suggestions on the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for research and/or authorship of this article: This work was supported by the Wellcome Trust.
References
- Andersen RA, Asanuma C, Essick G, Siegel RM. 1990. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296(1):65–113 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain’s default network. Neuron 65(4):550–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, Concha M, Rosselli M. 2000. Angular gyrus syndrome revisited: acalculia, finger agnosia, right-left disorientation and semantic aphasia. Aphasiology 14(7):743–54 [Google Scholar]
- Arsalidou M, Taylor MJ. 2011. Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54(3):2382–93 [DOI] [PubMed] [Google Scholar]
- Bahnemann M, Dziobek I, Prehn K, Wolf I, Heekeren HR. 2010. Sociotopy in the temporoparietal cortex: common versus distinct processes. Soc Cogn Affect Neurosci 5(1):48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Nelson SM, Cohen AL, Power JD, Coalson RS, Miezin FM, and others. 2011. Parcellation in left lateral parietal cortex is similar in adults and children. Cereb Cortex. August 1 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Auer ETJ, Wagner M, Ponton CW. 2008. Spatiotemporal dynamics of audiovisual speech processing. Neuroimage 39(1):423–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. 2011. The neurobiology of semantic memory. Trends Cogn Sci 15(11):527–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19(12): 2767–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. 1999. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11(1):80–95 [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. 2005. Some neurophysiological constraints on models of word naming. Neuroimage 27(3):677–93 [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. 2002. Stimulating illusory own-body perceptions. Nature 419(6904):269–70 [DOI] [PubMed] [Google Scholar]
- Brownsett SLE, Wise RJS. 2010. The contribution of the parietal lobes to speaking and writing. Cereb Cortex 20: 517–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2008. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia 46(7):1813–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. 2008. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci 9(8):613–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O’Leary DS. 2003. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry 160(11):1954–64 [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Barth H, Fregni F, Spelke ES, Pascual-Leone A. 2007. rTMS over the intraparietal sulcus disrupts numerosity processing. Exp Brain Res 179(4):631–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Lee HL, Freeman ED, Price CJ. 2010. The role of right and left parietal lobes in the conceptual processing of numbers. J Cogn Neurosci 22(2):331–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, and others. 2009. An anatomical signature for literacy. Nature 461(7266):983–6 [DOI] [PubMed] [Google Scholar]
- Caspers S, Eichkhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, and others. 2008. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212(6): 481–95 [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. 2006. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33(2):430–48 [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. 2005. Perisylvian language networks of the human brain. Ann Neurol 57(1):8–16 [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de, Schotten M. 2008. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44(8):1105–32 [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. 1989. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287(4):422–45 [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. 2008. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46(7):1828–51 [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. 2009. Different impairments of semantic cognition in semantic dementia and semantic aphasia: evidence from the non-verbal domain. Brain 132:2593–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 1998. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci 353:1353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. 2001. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11(2):157–63 [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1989. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition 33(1–2):25–62 [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. 2007. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13(6):580–93 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. 1998. Abstract representations of numbers in the animal and human brain. Trends Neurosci 21(8):355–61 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. 2003. Three parietal circuits for number processing. Cogn Neuropsychol 20(3):487–506 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. 2002. Functional neuroimaging of speech perception in infants. Science 298:2013–5 [DOI] [PubMed] [Google Scholar]
- Dejerine J. 1891. Sur un cas de cecite verbale avec agraphie, suivi d’autopsie. C.R. Societe de Biologie 43:197–201 [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, and others. 1992. The anatomy of phonological and semantic processing in normal subjects. Brain 115(1, Pt 6): 1753–68 [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. 2004. Neuroplasticity: changes in grey matter induced by training. Nature 427(6972):311–2 [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. 2008. Training-induced structural changes in the adult human brain. Behav Brain Res 192(1): 137–42 [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, and others. 2008. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18(6):1444–54 [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Galaburda AM. 1984. Inferior parietal lobule: divergent architectonic asymmetries in the human brain. Arch Neurol 41(8):843–52 [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. 2003. Cognitive and brain consequences of conflict. Neuroimage 18(1):42–57 [DOI] [PubMed] [Google Scholar]
- Farrer C, Frey SH, Van Horn JD, Tunik E, Turk D, Inati S, and others. 2008. The angular gyrus computes action awareness representations. Cereb Cortex 18(2):254–61 [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. 2002. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 15(3):596–603 [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. 1998. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A 95:914–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. 2008. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28(45):11435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. 2010. The free-energy principle: a unified brain theory? Nat Rev Neurosci 11(2):127–38 [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. 2000. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38(1):11–21 [DOI] [PubMed] [Google Scholar]
- Geschwind N. 1965. Disconnexion syndromes in animals and man: I. Brain 88(2):237–94 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. 2011. Mapping human cortical areas in vivo based on myelin content as revealed by t1- and t2-weighted MRI. J Neurosci 31(32):11597–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel S, Walsh V, Rushworth MF. 2001. The mental number line and the human angular gyrus. Neuroimage 14(6):1278–89 [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, and others. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101(21):8174–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J. 2007. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron 53(1):9–16 [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. 2009. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia 47(2):604–8 [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. 2010. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex 20(8):1799–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. 2005. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat 207(1):3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, and others. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol 6(7):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnstein M, Bayer U, Ellison A, Hausmann M. 2011. TMS over the left angular gyrus impairs the ability to discriminate left from right. Neuropsychologia 49(1):29–33 [DOI] [PubMed] [Google Scholar]
- Houde O, Rossi S, Lubin A, Joliot M. 2010. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci 13(6):876–85 [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. 2009. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem 16(6):343–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J. 1982. Posterior parietal lobe of the primate brain. Physiol Rev 62(3):1060–129 [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Schocke M, Delazer M. 2009. Flexible transfer of knowledge in mental arithmetic—an fMRI study. Neuroimage 44(3):1103–12 [DOI] [PubMed] [Google Scholar]
- Joassin F, Pesenti M, Maurage P, Verreckt E, Bruyer R, Campanella S. 2011. Cross-modal interactions between human faces and voices involved in person recognition. Cortex 47(3):367–76 [DOI] [PubMed] [Google Scholar]
- Jones DK. 2010. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med 2(3):341–55 [Google Scholar]
- Jones DK, Cercignani M. 2010. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23(7):803–20 [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, and others. 1998. The role of parietal cortex in verbal working memory. J Neurosci 18(13):5026–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. 1982. The neuropsychology of development hemispheric laterality, limbic language, and the origin of thought. J Clin Psychol 38(1):4–33 [DOI] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, and others. 2010. Neuroanatomy of creativity. Hum Brain Mapp 31(3):398–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. 2005. Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9(11):512–8 [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Eriksson J, Sjölie D, Molin J, Nyberg L. 2010. Neurocognitive systems related to real-world prospective memory. PLoS One 5(10):e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, Margulies DS, Castellanos FX, Milham MP, and others. 2010. Broca’s region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. Eur J Neurol 32(3):383–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. 2010. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage 50(4):1648–57 [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. 2008. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci 28(33):8361–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. 2003. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage 19(3):895–905 [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. 2009. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci 29(46):14496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, and others. 2007. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci 27(5):1184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell AK. 2006. In your right mind: right hemisphere contributions to language processing and production. Neuropsychol Rev 16:131–48 [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VSJ, and others. 2005. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15(6): 854–69 [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. 2009. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 19(4):777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN. 2007. The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. Neuroimage 37(4): 1100–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA. 2011. The neural bases of social cognition and story comprehension. Annu Rev Neurosci 62:103–34 [DOI] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, and others. 2011. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci 31(11):4087–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. 2011. Differentiable cortical networks for inferences concerning people’s intentions versus physical causality. Hum Brain Mapp 32(2):313–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, and others. 2001. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54(3):287–98 [DOI] [PubMed] [Google Scholar]
- McCulloch WS. 1944. The functional organization of the cerebral cortex. Physiol Rev 24:390–407 [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, and others. 2004. Neurolinguistics: structural plasticity in the bilingual brain. Nature 431(7010):757. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. 1998. From sensation to cognition. Brain 121:1013–52 [DOI] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Bennett CM, Aminoff EM, Mayer RE. 2012. Individual differences in cognitive style and strategy predict similarities in the patterns of brain activity between individuals. Neuroimage 59(1):83–93 [DOI] [PubMed] [Google Scholar]
- Naidich TP, Valvanis AG, Kubik S. 1995. Anatomic relationships along the low-middle convexity: Part I—normal specimens and magnetic resonance imaging. Neurosurgery 36(3):517–32 [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. 2007. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7(1):1–17 [DOI] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, and others. 2010. A parcellation scheme for human left lateral parietal cortex. Neuron 67(1):156–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J, Seitz RJ. 2005. Functional clusters in the human parietal cortex as revealed by an observer-independent meta-analysis of functional activation studies. Anat Embryol (Berl) 210(5–6):463–72 [DOI] [PubMed] [Google Scholar]
- Nieuwland MS, Petersson KM, Van Berkum JJ. 2007. On sense and reference: examining the functional neuroanatomy of referential processing. Neuroimage 37(3):993–1004 [DOI] [PubMed] [Google Scholar]
- Obleser J, Kotz SA. 2010. Expectancy constraints in degraded speech modulate the language comprehension network. Cereb Cortex 20(3):633–40 [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Alex Dresner M, Scott SK. 2007. Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci 27(9):2283–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. 2010. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci 30(8):2924–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, and others. 2008. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43(3):447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, and others. 2006. Mapping the parietal cortex of human and non-human primates. Neuropsychologia 44(13):2647–67 [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. 2004. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci 8(7):315–24 [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. 2010. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20(4):953–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. 1982. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol 204(2):196–210 [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, and others. 2008. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp 29(5):503–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8(12):976–87 [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2009. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biol 7(8):e1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. 2000. The anatomy of language: contributions from functional neuroimaging. J Anat 197(Pt 3):335–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. 2010. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191(1): 62–88 [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. 2005. Reading and reading disturbance. Curr Opin Neurobiol 15(2):231–8 [DOI] [PubMed] [Google Scholar]
- Price GR, Ansari D. 2011. Symbol processing in the left angular gyrus: evidence from passive perception of digits. Neuroimage 57(3):1205–11 [DOI] [PubMed] [Google Scholar]
- Purcell J, Turkeltaub PE, Eden GF, Rapp B. 2011. Examining the central and peripheral processes of written word production through meta-analysis. Front Psychology 2:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VSJ. 1992. Human cerebral cortex: localization, parcellation, and morphometry with magnetic resonance imaging. J Cogn Neurosci 4(4):352–74 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas GC. 2010. The cerebral sulci and gyri. Neurosurg Focus 28(2):E2. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, and others. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11(4):426–8 [DOI] [PubMed] [Google Scholar]
- Roland PE, Friberg L. 1985. Localization of cortical areas activated by thinking. J Neurophysiol 52(5):1219–43 [DOI] [PubMed] [Google Scholar]
- Rosa MG, Tweedale R. 2005. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philos Trans R Soc Lond B Biol Sci 360(1456):665–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal CR, Roche-Kelly EE, Husain M, Kennard C. 2009. Response-dependent contributions of human primary motor cortex and angular gyrus to manual and perceptual sequence learning. J Neurosci 29(48):15115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. 2006. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex 16(10):1418–30 [DOI] [PubMed] [Google Scholar]
- Sack AT. 2009. Parietal cortex and spatial cognition. Behav Brain Res 202(2):153–61 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. 2010. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 20(3):236–60 [DOI] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. 2010. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci 30(50):16809–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. 2009. Dissociating functional brain networks by decoding the between-subject variability. Neuroimage 45(2):349–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. 2011. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci 31(12):4407–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom DB, Poeppel D. 2008. Functional anatomic models of language: assembling the pieces. Neuroscientist 14(1):119–27 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Awad M, Warren JE, Wise RJS, Vigliocco G, Scott SK. 2010. The neural response to changing semantic and perceptual complexity during language processing. Hum Brain Mapp 31(3):365–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, and others. 2009. The resting brain: unconstrained yet reliable. Cereb Cortex 19(10):2209–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, and others. 1997. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648–63 [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Subiaul F, Zawidzki TW. 2008. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat 212(4):426–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. 2009. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47(6):1434–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, and others. 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106(31):13040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47(8–9):1765–79 [DOI] [PubMed] [Google Scholar]
- Sperduti M, Delaveau P, Fossati P, Nadel J. 2011. Different brain structures related to self-and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct 216(2):151–7 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21(3):489–510 [DOI] [PubMed] [Google Scholar]
- Stowe LA, Haverkort M, Zwarts F. 2005. Rethinking the neurological basis of language. Lingua 115(7):997–1042 [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. 2006. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44(12):2189–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Muggleton NG, Kalla R, Walsh V, Eimer M. 2011. TMS of the right angular gyrus modulates priming of pop-out in visual search: combined TMS-ERP evidence. J Neurophysiol 106(6):3001–9 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2011. Association between functional connectivity hubs and brain networks. Cereb Cortex 21(9):2003–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triarhou LC. 2007. A proposed number system for the 107 cortical areas of Economo and Koskinas, and Brodmann area correlations. Stereotact Funct Neurosurg 85(5):204–15 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB. 2010. Localization of sublexical speech perception components. Brain Lang 114(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16(3, Pt 1): 765–80 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, and others. 2010. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex 20(11):2636–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. 2009. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav Brain Res 199(2):171–82 [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. 1996. Functional anatomy of a common semantic system for words and pictures. Nature 383:254–6 [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, and others. 2006. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30(4):1414–32 [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. 2008. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia 46(7):1787–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. 2005. Common and unique components of response inhibition revealed by fMRI. Neuroimage 27(2):323–40 [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. 2004. Fiber tract-based atlas of human white matter anatomy. Radiology 230(1):77–87 [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. 2010. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum Brain Mapp 31(10): 1459–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjrnerud A, Due-Tnnessen P, Engvig A, and others. 2010. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage 52(1):172–85 [DOI] [PubMed] [Google Scholar]
- Wirth M, Jann K, Dierks T, Federspiel A, Wiest R, Horn H. 2011. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage 54(4):3057–66 [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. 2005. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage 25(3):1002–15 [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8(8):665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhou X. 2009. Conflict control during sentence comprehension: fMRI evidence. Neuroimage 48(1):280–90 [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, and others. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106(3):1125–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuge H. 2010. Interactive semantics. Artificial Intell 174:190–204 [Google Scholar]
- Zilles K, Palomero-Gallagher N. 2001. Cyto-, myelo-, and receptor architectonics of the human parietal cortex. Neuroimage 14(1, Pt 2):S8–S20 [DOI] [PubMed] [Google Scholar]