Abstract
Objective
To study the association between wheezing in children with cystic fibrosis (CF) and lung function in later life.
Methods
We used data from the Epidemiologic Study of CF, a longitudinal observational study from 1994–2005. Wheezing phenotypes were defined as: no wheezing in the first 6 years of life (NW), transient wheezing (TW; wheezing <3 years old, but no wheezing after 3), late wheezing (LW; wheezing only after age 3 years), and persistent wheezing (PW; wheezing both before and after age 3 years). A linear regression model was developed predicting the best forced expiratory volume in 1 second (FEV1) at age 6 or 7 years (6–<8) for each wheezing phenotype.
Results
1,302 children met the cohort inclusion criteria; 61% of the cohort had wheezing in the first 6 years of life. A history of any wheezing in the first 6 years of life was associated with a significantly lower FEV1 at 6–<8 years compared to children with NW. The FEV1 of children with NW at age 6–<8 years was 104% predicted, whereas the mean FEV1 in TW, LW, and PW groups was 98%, 100%, and 96% predicted respectively (P<0.05 compared to NW).
Conclusions
Childhood wheezing in CF is common and is associated with lower lung function at age 6–<8 years. Our results identify a clinical feature in early childhood CF associated with lower lung function in later life.
Keywords: cystic fibrosis, epidemiology, spirometry, wheeze, infants
Introduction
Wheezing is a common finding in young children with and without cystic fibrosis (CF). Half of all children in the general population have at least one episode of wheezing in the first 6 years of life [1, 2]. The timing of this wheezing is associated with different physiologic findings and long term pulmonary outcomes. Children who wheeze in the first 3 years of life but not at age 6 years seem to have diminished airway function at birth [1]. Their lung function improves by age 6 years, but a deficit persists through adolescence relative to children who do not wheeze in the first 6 years [3]. In contrast, children who wheeze both in the first 3 years of life and at age 6 appear to be born with good lung function which declines by age 6 years, and they too continue to show a deficit through adolescence [3].
The natural history of wheezing in normal children has been studied extensively, but the relationship between early childhood wheezing and pulmonary outcomes is less well understood in CF. Reisman, et al. reported that wheezing before the age of 2 years was associated with lower lung function at 13 years but not at 7 years [4]. Their study was a retrospective single center analysis which had the potential for recall and ascertainment bias. The Epidemiologic Study of Cystic Fibrosis (ESCF) is a large multicenter study with a prospective study design including contemporaneous physician documented wheezing. This data source offers an opportunity to overcome the limitations of previous studies of wheezing in CF.
We used data from ESCF to test the hypothesis that CF patients exhibit a similar relationship between wheezing phenotypes and lung function as seen in the general population. We characterized patterns of early childhood wheezing in CF and related them to lung function at around age 6 years. Furthermore, we assessed differences across the wheezing phenotypes in clinical characteristics associated with lung disease in CF.
Methods
The design and implementation of ESCF have previously been described [5]. ESCF is a prospective, encounter-based, observational study of CF patients in North America from 1994 to 2005. Data collected included pulmonary function, height and weight, clinical signs and symptoms, respiratory microbiology, other medical conditions (including asthma), and use of CF-related therapies. The presence or absence of wheezing on physical examination was recorded at every encounter. Informed consent was obtained based on decisions by a central or a local human subjects review board.
To ensure adequate 6-year follow-up, we limited this analysis to patients born from 1994–1998. Each patient had to have at least one encounter in the first year of life and for six subsequent years (to age 6 or 7 years), with ≥1 spirometry at age 6 or 7 (6–<8) years. Our prespecified wheezing phenotypes were patterned similarly to the Tucson Children’s Respiratory Study (CRS) [1] and were defined as: no wheezing in the first 6 years of life (NW), transient wheezing (TW; wheezing <3 years old, but no wheezing age 3–5 years), late wheezing (LW; wheezing only age 3–5 years), and persistent wheezing (PW; wheezing both age <3 years and age 3–5 years).
A linear regression model was developed predicting the best forced expiratory volume in 1 second (FEV1) percent predicted (%pred) with wheezing phenotype as a fixed effect and adjusted for the following covariates: sex, genotype, history of sinusitis, history of allergic bronchopulmonary aspergillosis, weight-for-age percentile, height-for-age percentile, cough, sputum production, clubbing, crackles, respiratory tract culture positive for Pseudomonas aeruginosa (Pa), respiratory tract culture positive for Staphylococcus aureus, and pancreatic enzyme use. Covariates that vary over time were measured at the visit closest to the best FEV1 %pred. Pa and S.aureus were coded as negative/missing, ever positive before age 3 years, or only positive age 3–<6 years. The same model was applied to forced vital capacity (FVC) %pred, FEV1/FVC %pred, and forced expiratory flow between 25–75 % of FVC (FEF25–75) %pred as measured at the same best FEV1 %pred. %pred values were obtained using the equations of Stanojevic [6].
These analyses were repeated for the subset of patients with spirometry data available at age 8 (8–<9) years. For each spirometry measure, we summarized the subset at age 6–<8, at age 8–<9, and the change from the earlier to the later age.
Statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). All tests were two-sided and P<0.05 was considered statistically significant. All comparisons among the four wheezing phenotypes were adjusted for multiple comparisons, using the Bonferroni method for demographic and clinical characteristics and the Tukey method for all spirometry outcomes.
Results
We identified 4,480 patients enrolled in ESCF who were born in the period 1994–1998 and had complete enrollment data. Of the 2,204 patients who had ≥1 encounter in their first year of life, 1,364 had ≥1 encounter in each of the six years following the first year of life. After omitting 62 patients who did not have any spirometry data at age 6–<8 years, 1,302 patients met the cohort inclusion criteria. Of these patients, 833 had ≥1 spirometry at age 8–<9 years.
The distribution of wheezing phenotypes and associated clinical features are shown in Table 1. Wheeze before age 3 years was observed in 52% of the patients, about evenly divided between TW and PW. Only 9% of patients had wheeze for the first time at age 3–5 years (LW). Boys were more likely than girls to have wheezing (65.7% vs. 55.7%, P<0.001). In general, children with wheezing were more likely to have daily cough, daily sputum production, and crackles at age 6–<8 years compared to children who never wheezed. A history of sinusitis was more common in the PW group than the NW group.
Table 1.
Demographic and clinical characteristics (N=1,302).
ABPA, allergic bronchopulmonary aspergillosis; NW, No Wheeze; TW, Transient Wheeze; LW, Late Wheeze; PW, Persistent Wheeze.
| Characteristic | Wheezing Phenotype | |||
|---|---|---|---|---|
| NW | TW | LW | PW | |
| All, N (%) | 508 (39.0%) | 363 (27.9%) | 118 (9.1%) | 313 (24.0%) |
| Male*, n (%) | 238 (46.9%) | 213 (58.7%) | 61 (51.7%) | 181 (57.8%) |
| Genotype, n (%) | ||||
| Delta F508 homozygous | 242 (47.6%) | 178 (49.0%) | 62 (52.5%) | 184 (58.8%) |
| Delta F508 heterozygous | 165 (32.5%) | 107 (29.5%) | 37 (31.4%) | 80 (25.6%) |
| Other | 36 (7.1%) | 21 (5.8%) | 7 (5.9%) | 18 (5.8%) |
| Unknown | 65 (12.8%) | 57 (15.7%) | 12 (10.2%) | 31 (9.9%) |
| Diagnosis by clinical symptoms, n (%) | 32 (6.3%) | 29 (8.0%) | 8 (6.8%) | 20 (6.4%) |
| Diagnosis by family history, n (%) | 104 (20.5%) | 61 (16.8%) | 24 (20.3%) | 56 (17.9%) |
| Diagnosis by screening, n (%) | 16 (3.2%) | 8 (2.2%) | 5 (4.2%) | 3 (1.0%) |
| History of pancreatic enzyme use, n (%) | 503 (99.0%) | 362 (99.7%) | 116 (98.3%) | 313 (100.0%) |
| History of sinusitis†, n (%) | 200 (39.4%) | 159 (43.8%) | 53 (44.9%) | 164 (52.4%) |
| History of ABPA, n (%) | 4 (0.8%) | 6 (1.7%) | 3 (2.5%) | 10 (3.2%) |
| Weight-for-age percentile‡, Mean ± SD | 37.44 ± 25.11 | 37.50 ± 26.47 | 37.26 ± 25.98 | 36.97 ± 26.05 |
| Height-for-age percentile‡, Mean ± SD | 30.78 ± 24.58 | 28.38 ± 25.18 | 31.01 ± 24.43 | 26.99 ± 23.19 |
| Daily cough‡§, n (%) | 134 (26.4%) | 113 (31.1%) | 40 (33.9%) | 128 (40.9%) |
| Crackles†‡, n (%) | 31 (6.1%) | 33 (9.1%) | 9 (7.6%) | 42 (13.4%) |
| Clubbing‡, n (%) | 201 (39.6%) | 156 (43.0%) | 45 (38.1%) | 150 (47.9%) |
| Daily sputum*‡, n (%) | 39 (7.7%) | 37 (10.2%) | 16 (13.6%) | 56 (17.9%) |
| Number of encounters‖¶, Mean ± SD | 25.74 ± 8.09 | 29.07 ± 10.33 | 28.19 ± 8.88 | 35.32 ± 14.06 |
| Number of cultures*‖, Mean ± SD | 10.60 ± 7.58 | 11.40 ± 8.11 | 12.69 ± 8.90 | 13.39 ± 9.36 |
| First positive P.aeruginosa culture, n (%) | ||||
| Never/unreported P.aeruginosa | 184 (36.2%) | 121 (33.3%) | 30 (25.4%) | 84 (26.8%) |
| Before age <3 years | 237 (46.7%) | 187 (51.5%) | 65 (55.1%) | 175 (55.9%) |
| Age 3–<6 years | 87 (17.1%) | 55 (15.2%) | 23 (19.5%) | 54 (17.3%) |
| First positive S.aureus culture, n (%) | ||||
| Never/unreported S.aureus | 98 (19.3%) | 71 (19.6%) | 10 (8.5%) | 46 (14.7%) |
| Before age <3 years | 304 (59.8%) | 224 (61.7%) | 80 (67.8%) | 193 (61.7%) |
| Age 3–<6 years | 106 (20.9%) | 68 (18.7%) | 28 (23.7%) | 74 (23.6%) |
| Inhaled bronchodilators†, n (%) | 372 (73.2%) | 300 (82.6%) | 99 (83.9%) | 279 (89.1%) |
| Inhaled corticosteroids#, n (%) | 139 (27.4%) | 133 (36.6%) | 51 (43.2%) | 169 (54.0%) |
Differences below represent Bonferroni corrected P<0.05.
PW>NW, TW>NW
PW>NW
Assessed at or around the best FEV1 %pred at ages 6–<8.
PW>NW, PW>TW
Assessed 0–5 years of age.
PW>NW, PW>TW, PW>LW, TW>NW
PW>NW, PW>TW, LW>NW, TW>NW
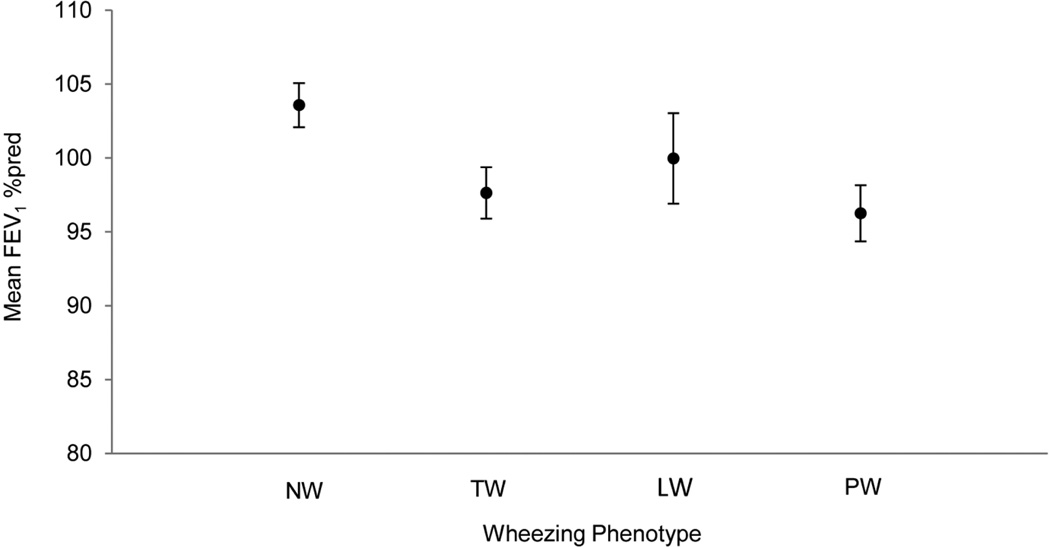
Figure 1 shows FEV1 %pred at age 6–<8 years in each of the wheezing phenotypes adjusted for all covariates. Children with persistent wheeze (PW) had a significantly lower FEV1 %pred compared to those with no history of wheezing (NW). The transient wheeze (TW) group also had significantly lower FEV1 %pred than the no wheeze group (NW). Patients in the LW group are not statistically different from any of the other groups.
Figure 1.
Adjusted mean FEV1 % predicted at age 6–<8 years of age by wheezing phenotype (N=1,302). Error bars indicate 95% confidence intervals.
Table 2 shows the effect at age 6–<8 years of wheezing phenotype for all 4 spirometry outcomes from a multivariate analysis after adjusting for all covariates. With the exception of FVC %pred, the percent of predicted lung function in wheezing phenotypes are in the order of NW>LW>TW>PW, but not all group differences are statistically significant. For FVC %pred, PW is between LW and TW. Group differences that are statistically significant after adjustment for multiple comparisons are indicated in the table.
Table 2.
Adjusted* spirometry at age 6–<8 years by wheezing phenotype.
NW, No Wheeze; TW, Transient Wheeze; LW, Late Wheeze; PW, Persistent Wheeze.
| Wheezing Phenotype |
FEV1 %pred | FVC %pred | FEV1/FVC %pred | FEF25–75 %pred | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Total, N | 1,302 | -- | 1,293 | -- | 1,293 | -- | 1,293 | -- | |
| NW | 508 | 103.95 ± 0.77 | 504 | 103.95 ± 0.75 | 504 | 99.98 ± 0.40 | 502 | 102.97 ± 1.39 | |
| TW | 363 | 97.64 ± 0.88 | 362 | 100.09 ± 0.86 | 362 | 97.73 ± 0.46 | 361 | 92.37 ± 1.60 | |
| LW | 118 | 99.98 ± 1.55 | 116 | 101.81 ± 1.53 | 116 | 98.18 ± 0.81 | 118 | 96.99 ± 2.80 | |
| PW | 313 | 95.66 ± 1.00 | 311 | 100.64 ± 0.97 | 311 | 95.24 ± 0.52 | 312 | 86.48 ± 1.81 | |
| P<0.05† | NW>TW NW>PW |
NW>TW NW>PW |
NW>TW NW>PW LW>PW TW>PW |
NW>TW NW>PW LW>PW |
|||||
A linear regression model was developed predicting each spirometry measure at the time of the best FEV1 %pred at age 6–<8 years with wheezing phenotype as a fixed effect and the following covariates: sex, genotype, history of sinusitis, history of allergic bronchopulmonary aspergillosis, weight-for-age percentile, height-for-age percentile, cough, sputum production, clubbing, crackles, number of encounters, number of respiratory tract cultures, culture positive for Pseudomonas aeruginosa, culture positive for Staphylococcus aureus, and pancreatic enzymes use.
P-value calculated from t statistic with Tukey adjustment.
The models were repeated on the subset of patients who also had spirometry at age 8–<9 years (Table 3). These 833 patients had significantly better FEV1 %pred (+2.4), FVC %pred (+2.1), and FEF25–75 (+3.8) %pred at age 6–<8 years than the 469 patients who did not have data at the later time. FEV1/FVC ratio was similar in the two groups. The pattern of change from age 6–<8 years to age 8–<9 years was similar for all wheezing phenotypes and all outcomes.
Table 3.
Adjusted* FEV1 %pred at age 6–<8 years and age 8–<9 years by wheezing phenotype.
NW, No Wheeze; TW, Transient Wheeze; LW, Late Wheeze; PW, Persistent Wheeze.
| Wheezing Phenotype |
All Patients Age 6–<8 Years | Subset of Patients with Data at Age 8–<9 Years | ||||
|---|---|---|---|---|---|---|
| At age 6–<8 years | At age 8–<9 years | Difference | ||||
| n | Mean ± SE | n | Mean ± SE | Mean ± SE | Mean ± SE | |
| Total | 1302 | 833 | ||||
| NW | 508 | 103.95 ± 0.77 | 325 | 103.80 ± 0.93 | 97.91 ± 0.95 | −5.89 ± 0.66 |
| TW | 363 | 97.64 ± 0.88 | 230 | 99.69 ± 1.08 | 92.59 ± 1.10 | −7.10 ± 0.76 |
| LW | 118 | 99.98 ± 1.55 | 63 | 103.17 ± 2.07 | 97.32 ± 2.12 | −5.85 ± 1.46 |
| PW | 313 | 95.66 ± 1.00 | 215 | 96.37 ± 1.17 | 90.32 ± 1.19 | −6.05 ± 0.82 |
| P<0.05† | NW>TW NW>PW |
NW>TW NW>PW LW>PW |
NW>TW NW>PW LW>PW |
NS‡ | ||
A linear regression model was developed predicting FEV1 %pred at the time of the best FEV1 %pred at age 6–<8 years and at the time of the best FEV1 %pred at age 8–<9 years for the subset of patients with data at both age intervals. Wheezing phenotype was included as a fixed effect with the following covariates: sex, genotype, history of sinusitis, history of allergic bronchopulmonary aspergillosis, weight-for-age percentile, height-for-age percentile, cough, sputum production, clubbing, crackles, number of encounters, number of respiratory tract cultures, culture positive for Pseudomonas aeruginosa, culture positive for Staphylococcus aureus, and pancreatic enzymes use.
P-value calculated from t statistic with Tukey adjustment.
No differences significant at P<0.05.
Discussion
Wheezing occurs commonly in infants and young children, but its clinical significance in CF is unclear. Using data from the ESCF, we found a high prevalence of wheezing in young children with CF. In a birth cohort study of normal full term infants, Martinez, et al. reported that 33.6% wheezed in the first 3 years of life and 48.6% in the first 6 years; we found 51.9% and 61.0%, respectively [1]. Some of the higher prevalence of wheeze in CF may be attributable to the greater frequency of routine visits associated with CF care [7]. Similar to what others have found with normal children, we found a history of wheezing any time in the first 6 years of life was associated with lower lung function at age 6 and age 8 in children with CF.
Although there have been several studies of the prevalence of asthma in CF [8], there have been few studies in CF of wheezing itself, especially in early life, and its relationship to pulmonary outcomes. A retrospective single center study of patients born 1965–1979 found 25% of CF patients had wheezing during the first two years of life [4]. That study found no effect of wheezing on FEV1 at age 7 years, but significantly lower FEV1 at age 13 years (69 % pred for wheezing vs. 78 % pred for no wheezing, p=0.02). This difference of 9 % pred between groups is similar to the 7.6 % pred difference we found at age 6 between the non-wheezing and persistent wheezing groups.
Wheezing in infancy and childhood in the non-CF population has been the focus of many studies [1, 2, 9, 10]. The Tucson CRS found a lower prevalence of wheezing in the first 6 years of life than we found in ESCF, but the pulmonary outcomes had some similarities [1]. In the Tucson CRS, the transient and persistent wheezing groups had lung function lower than non-wheezing children at age 6 years. The late wheezing group showed no significant difference from non-wheezing children at age 6 years [3]. We found similar relationships among the wheezing phenotypes in ESCF patients at age 6 years: persistent and transient wheezing groups were substantially lower than non-wheezers, with late wheezing children in between and not significantly different from any other groups.
The mean FEV1 decline in the NW group from age 6–<8 to age 8–<9 was 4.33 % predicted/year, which is slightly higher than the overall mean FEV1 decline in children born in the years of our analysis. A comparison with other patients in ESCF who had an encounter in their birth year (one of our inclusion criteria) revealed a very similar decline (4.32 % predicted/year). However, a lower rate of decline (2.66 % predicted/year) was observed among another group of patients diagnosed near birth but without an encounter in their birth year, presumably because they were born late in the year or were not enrolled immediately in ESCF. This difference in rate of decline may reflect the fact that patients seen in the first year of life have more severe disease. Children for whom PFT data were available at both ages 6–8 and 8–9 years had slightly higher mean FEV1 than children who only had data for 6–8 years. This may be because they were followed more closely. These observations do not affect our primary conclusion that wheezing in early childhood is associated with lower lung function at age 6.
The most common cause of wheezing in children age less than 3 years is lower respiratory viral infection, usually due to human rhinovirus and respiratory syncytial virus [11, 12]. Wheezing-associated respiratory illness induced by viral infection is associated with acute airway injury and inflammation, as well as respiratory sequelae for many years post infection [11, 13]. Indeed, a history of cough and sputum at age 6 years were more likely in the transient and persistent wheezing children and may be an indication of chronic CF lung disease rather than asthma.
Transient wheezing may be a reflection of reduced airway conductance in early life, which in non-CF children improves by age 6 years but remains lower than for non-wheezers [1, 14]. In non-CF children with persistent wheezing, it may be that chronic airways inflammation results in dysynaptic lung growth and progressive loss in maximal forced expiratory flow. Children with CF presumably bear the usual risks of non-CF wheeze with the addition of airway inflammation that is exacerbated by chronic bacterial infection., such as the increased early Pa acquisition we found in the wheezing groups. We would argue that it is important to understand those mechanisms given that wheezing is highly prevalent in the CF population and may represent a marker for progression of lung disease in early life.
Our analysis was limited to the data collected in ESCF, and therefore did not include data on virology, atopy, family history, or environmental factors such as maternal smoking, that could affect wheezing and lung function [15]. Our model did not account for some covariates that vary over time, such as weight for length or infectious status. While the model may have been even more robust with these variables, their exclusion is unlikely to have affected our ultimate results. Although all patients in this study were diagnosed before age 1 year, we cannot rule out the possibility that some had wheezing prior to enrollment in ESCF, which could result in some misclassification of wheezing phenotype. One of the strengths of our study compared to other studies of wheezing in CF was the prospective collection of physician determined wheezing status at each encounter, which limits recall bias.
The observation that wheezing in early life is associated with lower lung function in later childhood raises the question of whether children with CF who wheeze should be treated more aggressively. In preschool aged non-CF children with an increased risk of asthma, early treatment with inhaled corticosteroids reduced wheezing and improved lung function, but once steroid therapy was withdrawn, symptoms and lung dysfunction returned [14]. A multivariate analysis of risk factors for lung function decline in ESCF found that wheezing among patients age 9–17 years predicted lower rates of decline, which was counter-intuitive[16]. This protective effect of wheeze might be due to increased clinical attention and intervention . In this study, we have shown that wheeze is associated with lower lung function at age 6 but found no statistically significant effect on lung function decline from age 6 to 8 years, which is consistent with the results for this age group in the previous ESCF study.
In summary, our analysis of a cohort of young children followed in a longitudinal, multicenter observational study of CF found a higher prevalence of wheezing compared to non-CF children. We also found that wheezing in the first 6 years of life is associated with lower lung function at age 6 and 8 years. Future studies should focus on elucidating the mechanism of wheeze-associated loss of lung function in CF and on evaluating interventions that can prevent or ameliorate the impact of this association.
Acknowledgements
The authors gratefully acknowledge the more than 400 site investigators and coordinators in ESCF. This study is sponsored by Genentech, Inc.
Footnotes
Disclosure of Conflict of Interest
Clement Ren, Michael Konstan, Margaret Rosenfeld, and Wayne Morgan have received honoraria from Genentech for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF). Michael Konstan and Wayne Morgan have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. David Pasta and Stefanie Millar are employees of ICON Late Phase & Outcomes Research, which was paid by Genentech for providing analytical services for this study.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Young S, Arnott J, O'Keeffe PT, Le Souef PN, Landau LI. The association between early life lung function and wheezing during the first 2 yrs of life. Eur Respir J. 2000;15:151–157. doi: 10.1183/09031936.00.15115100. [DOI] [PubMed] [Google Scholar]
- 3.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisman J, Corey M, Canny G, Levison H, Kerem E, Bentur L. Wheezing in Infants with Cystic Fibrosis: Clinical Course, Pulmonary Function, and Survival Analysis. Pediatrics. 1992;90:703–706. [PubMed] [Google Scholar]
- 5.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. PediatrPulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Stanojevic S, Wade A, Cole TJ, Lum S, Custovic A, Silverman M, et al. Spirometry centile charts for young Caucasian children: the Asthma UK Collaborative Initiative. Am J Respir Crit Care Med. 2009;180:547–552. doi: 10.1164/rccm.200903-0323OC. [DOI] [PubMed] [Google Scholar]
- 7.McColley SA, Ren CL, Schechter MS, Regelmann WE, Pasta DJ, Konstan MW. Risk factors for onset of persistent respiratory symptoms in children with cystic fibrosis. Pediatr Pulmonol. 2012;47:966–972. doi: 10.1002/ppul.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balfour-Lynn IM, Elborn JS. “CF asthma”: what is it and what do we do about it? Thorax. 2002;57:742–748. doi: 10.1136/thorax.57.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–237. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 10.Spahn JD, Covar R. Clinical assessment of asthma progression in children and adults. J Allergy Clin Immunol. 2008;121:548–557. doi: 10.1016/j.jaci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB. Respiratory syncytial virus and parainfluenza virus. NEnglJ Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 12.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 14.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H, Jensen SM, Bonnelykke K. Interaction between Asthma and Lung Function Growth in Early Life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 16.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk Factors For Rate of Decline in Forced Expiratory Volume in One Second in Children and Adolescents with Cystic Fibrosis. J Pediatr. 2007;151:134–139. e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]