Abstract
Objective
Recent advances in lifestyle intervention programs, pharmacotherapy, and bariatric surgery have enabled the development of medical models for the treatment of obesity. Regarding pharmacotherapy, in 2012 the U.S. Food and Drug Administration approved two new effective and safe weight-loss medications, phentermine/topiramate extended release and lorcaserin, which has greatly augmented options for medically assisted weight loss.
Methods
The rationale for advantages of a complications-centric medical model over current body mass index (BMI)-centric indications for therapy is examined.
Results
Currently, the baseline BMI level is the principle determinant of indications for obesity treatment using medication and surgery. However, the BMI-centric approach fails to target therapy to those obese patients who will benefit most from weight loss. In contrast, a complications-centric medical model is proposed that will earmark the modality and intensity of the therapeutic intervention based on the presence and severity of complications that can be ameliorated by weight loss.
Conclusion
The complications-centric approach to “medicalizing” obesity care employs weight loss primarily as a tool to treat obesity-related complications and promotes the optimization of health outcomes, the benefit/risk ratio, and the cost-effectiveness of therapy.
INTRODUCTION
Obesity is a disease with genetic, environmental, and behavioral determinants that confers increased morbidity and mortality (1). Obesity prevalence rates began to sharply increase approximately 3 decades ago, and obesity has emerged as a critical public health crisis worldwide. Currently, in the United States, 35% of the adult population is obese (body mass index [BMI] ≥30 kg/m2), and an additional ~35% of the population is overweight (BMI of 25 to 29.9 kg/m2) (2). Obesity adversely affects mortality, morbidity, and quality of life (3,4) as a consequence of its complications. Foremost among obesity-related complications in terms of the public health burden are those that relate to cardiometabolic disease, which encompasses metabolic syndrome, prediabetes, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD) (5,6).
Therapy for obesity has included lifestyle modification and bariatric surgery, whereas pharmacologic options have been relatively limited (4). Phentermine (and several other sympathomimetic drugs) suppress appetite by increasing neuronal release of norepinephrine and are approved for short-term therapy (<3 months), which does not address treatment of obesity as a life-long disease (7,8). Orlistat is approved for long-term therapy and is an intraluminal gastrointestinal lipase inhibitor that promotes weight loss by inducing fat malabsorption (9). Sibutramine, a serotonin-norepinephrine re-uptake inhibitor, had been available but was withdrawn from the market in 2010 after the Sibutramine Cardiovascular Outcomes Trial (10) showed an increase in composite CVD events in patients with preexisting vascular disease. These medical options are or were modestly effective. In the summer of 2012, however, the U.S. Food and Drug Administration (FDA) approved two new medications for treatment of overweight (BMI ≥27 and <30 kg/m2) patients with comorbidities such as diabetes, hypertension, and dyslipidemia and for treatment of obesity (BMI ≥30 kg/m2) regardless of whether comorbidities are present or not. These medications, phentermine/topiramate extended release (ER) (Qsymia®) and lorcaserin (Belviq®), are indicated as adjuncts to lifestyle modification (11) and are described below in more detail. Currently available weight loss medications are listed in Table 1.
Table 1.
Weight-Loss Medications
| Generic | Proprietary | Mechanism | FDA Approval |
|---|---|---|---|
| Phentermine/Topiramate ER | Qsymia (Vivus) | Sympathomimetic amine/gabaminergic, carbonic anhydrase inhibitor | 2012 |
| Lorcaserin | Belviq (Arena/Esai) | 5HT2c serotonin receptor agonist | 2012 |
| Orlistat | Xenical (Roche) | Intra-intestinal lipase inhibitor | 1999 |
| Phentermine | Adipex-P, (Gate) Suprenza (Akrimax) |
Sympathomimetic amine | 1959 |
| Phendimetrazine | Bontril (Valeant) | Sympathomimetic amine | 1982 |
| Diethylpropion | (generic) | Sympathomimetic amine | 1959 |
| Benzphentamine | Didrex (Pfizer) | Sympathomimetic amine | 1960 |
| Methamphetamine | Desoxyn (Lundbeck) | Sympathomimetic amine | 1943 |
| Naltrexone SR/Buproprion SR | Contrave (Orexigen/Takeda) | Opioid antagonist/ dopamine-norepinephrine re-uptake inhibitor | Phase III |
| Liraglutide (3 mg) | (Novo Nordisk) | GLP-1 agonist | Phase III |
Abbreviations: 5HT2c = 5-hydroxytryptamine 2C; ER = extended release; GLP-1 = glucagon-like peptide 1; SR = sustained release.
The future holds great promise for additional weight-loss drugs. Phase III trials have been completed for two other mediations seeking an obesity indication: naltrexone/buproprion (12) and high-dose liraglutide (13). Furthermore, there have been substantial advances in our understanding of the molecular mechanisms that regulate energy balance since the discovery of leptin in 1994, resulting in the identification of multiple targets for new drugs. Development has included: (1) mimetics or antagonists of peripheral signals that provide input to the arcuate nucleus of the hypothalamus and register systemic fuel storage/availability; these signals can originate from adipose tissue (leptin), the pancreas (amylin, insulin), stomach (gherlin), upper intestine (cholecystokinin), lower intestine (peptide YY), and colon (glucagon-like peptide-1); (2) drugs that act directly in the hypothalamus to either inhibit orexigenic (neuropeptide Y receptor antagonists) or stimulate anorexigenic pathways (serotonin receptor agonists, opioid receptor antagonists); and (3) drugs that act on ascending pathways and higher cortical centers controlling appetite (melanin-concentrating hormone-1R antagonists). Thus, not only have the recently approved drugs greatly enhanced options for pharmacotherapy, but we can anticipate that additional safe and effective medications will become available over time for the treatment of obesity.
These exciting developments in pharmacotherapy have been accompanied by the evolution of effective lifestyle intervention programs for weight loss as well as options for bariatric surgery. Clinical trials have established that diet and exercise can be used to produce and sustain weight loss, leading to prevention and treatment of diabetes and improvements in cardiovascular risk factors (14–16). Principles embodied in these clinical trials have been translated into community-based programs for weight loss (17) and have been incorporated into effective structured treatment programs that can be remote or web-based (18), offered commercially (19,20), or used in multidisciplinary clinic-based programs (21). In addition, bariatric surgical approaches have been developed and refined, together with pre- and postoperative care practices, which have expanded options and enhanced outcomes (22–24). Thus, there have been advancements in all three treatment modalities for obesity.
In summary, the recently approved weight-loss medications have greatly enhanced capabilities for pharmacotherapy, and this has been accompanied by advances in lifestyle therapy and bariatric surgery. These expanded options in all three modalities of obesity treatment can be utilized to produce a broad range of weight loss in patients and have enabled the development of robust medical models for obesity treatment. As described below, the medical model that will achieve good patient outcomes with optimal benefit/risk ratio and cost-effectiveness will involve a complications-centric as opposed to a BMI-centric approach.
RECENTLY APPROVED WEIGHT-LOSS MEDICATIONS
Table 2 documents the relative efficacy of weight-loss medications in published clinical trials. In each instance, all patients were placed on a lifestyle intervention program and randomized to drug versus placebo. In the absence of head-to-head studies, the table highlights values for placebo-subtracted weight loss as the parameter that best reflects differences in drug-assisted weight loss.
Table 2.
Comparative Efficacy of Weight-Loss Drugsa
| % Weight Loss (ITT-LOCF) | Categorical Weight Loss (ITT-LOCF) | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Drug | Study | Duration | Placebo | Drug | Placebo-Subtracted | Placebo | Drug | Placebo | Drug | |
|
|
||||||||||
| >5% | >10% | |||||||||
|
| ||||||||||
| Phentermine/Topiramate ER | EQUIP | 1 year | 1.6% | 10.9% | 9.3% | 17.3% | 66.7% | 7.4% | 47.2% | 27 |
| CONQUER | 1 year | 1.2% | 9.8% | 8.6% | 21.0% | 70.0% | 7.0% | 48.0% | 28 | |
| SEQUEL | 2 years | 1.8% | 10.5% | 8.7% | 30.0% | 79.3% | 11.5% | 53.9% | 29 | |
|
| ||||||||||
| Lorcaserin | BLOOM | 1 year | 2.2% | 5.8% | 3.6% | 20.3% | 47.5% | 7.7% | 22.6% | 35 |
| BLOOM | 2 years | 2.4% | 5.5% | 3.1% | NR | NR | NR | NR | 35 | |
| BLOSSOM | 1 year | 2.8% | 5.8% | 3.0% | 25.0% | 47.2% | 9.7% | 22.6% | 34 | |
| BLOOM-DM | 1 year | 1.5% | 4.5% | 3.0% | 16.1% | 37.5% | 4.4% | 16.3% | 36 | |
|
| ||||||||||
| Orlistat | XENDOS | 1 year | 5.6% | 9.6% | 4.0% | 45.1% | 72.8% | 20.8% | 41.0% | 9 |
| XENDOS | 4 years | 2.7% | 5.3% | 2.6% | ITTNR | ITTNR | ITTNR | ITTNR | ||
|
| ||||||||||
| Phentermine | Munro et al | 36 weeks | 5.2%2 | 13.0%2 | 7.8%2 | NR | NR | NR | NR | 8 |
|
| ||||||||||
| Naltrexone SR/Buproprion SR | COR-I | 1 year | 1.3% | 6.1% | 4.8% | 16% | 48% | 7% | 25% | 12 |
|
| ||||||||||
| Liraglutide (3 mg) | Astrup et al | 1 year | 2.1% | 8.0% | 5.9% | 28% | 73% | 10% | 37% | 13 |
Abbreviations: ER = extended release; GLP-1 = glucagon-like peptide 1; ITT-LOCF = intention to treat-last observation carried forward; NR = not reported; Ref = reference; SR = sustained release.
Weight loss is expressed as mean % decrease from baseline using intention to treat data unless otherwise indicated. The data in patients randomized to drug reflects weight loss on the maximal treatment dose.
Completers analysis.
Phentermine/Topiramate ER
Phentermine/topiramate ER is an oral combination of immediate-release phentermine hydrochloride and ER topiramate (25). Phentermine is a sympathomimetic amine anorectic agent approved in 1959 for the short-term treatment of obesity at a dose of 30 mg (free base) by mouth once a day. Topiramate is a sulfamate-substituted monosaccharide approved in 1996 as an anti-epileptic agent and for the prophylaxis of migraine headaches at doses up to 400 mg by mouth once a day. Topiramate promotes satiety and has been associated with modest weight reduction. Although the drug augments gabaminergic transmission, inhibits α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor/kainite excitatory glutamate receptors and has carbonic anhydrase activity, the mechanism by which topiramate causes weight loss is unknown. Phentermine/topiramate ER combines lower doses of phentermine and an ER form of topiramate in a once-daily pill to achieve synergism with respect to weight loss at lower adverse event rates when compared with higher doses of these individual medications (25).
Phentermine/topiramate ER was approved by the FDA in July 2012 for treatment of obesity (BMI ≥30 kg/m2) and overweight with complications (BMI of 27 to 29.9 kg/m2). Treatment begins with an initiation dose of phentermine/topiramate ER of 3.75 mg/23 mg for 2 weeks, followed by escalation to the treatment dose of phentermine/topiramate ER of 7.5 mg/46 mg. If weight loss has not reached 3% after 12 weeks, phentermine/topiramate ER should be discontinued or escalated to the top dose of 15 mg/92 mg. In clinical trials, both the treatment dose and top dose produced significantly more weight loss than the top dose of either phentermine (15 mg) or topiramate (92 mg) given alone (26).
The efficacy and safety of phentermine/topiramate ER was demonstrated in three large, phase III trials: EQUIP (27), CONQUER (28), and the CONQUER extension study known as SEQUEL (29). In the EQUIP trial, obese patients (BMI ≥35 kg/m2; mean BMI of 42 kg/m2) were all placed on a lifestyle intervention program and randomized to phentermine/topiramate ER 3.75 mg/23 mg, 15 mg/92 mg, or placebo. After 1 year, patients in the placebo group had lost an average of 1.6% (1.9 kg) of their baseline body weight compared to 5.1% (6.0 kg) in the 3.75 mg/23 mg group and 10.9% (12.6 kg) in patients randomized to the 15 mg/92 mg group (27). The CONQUER study enrolled overweight and obese patients (BMI of 27 to 45 kg/m2; mean BMI of 36.6 kg/m2) who had at least two other comorbidities (hypertension, dyslipidemia, prediabetes, or T2DM treated with diet and/or single-agent metformin). Patients were placed on a lifestyle intervention program and actively managed to standard targets for glycemia, blood pressure, and lipids and randomized to phentermine/topiramate ER 7.5 mg/46 mg, 15 mg/92 mg, or placebo. After 1 year, patients in the placebo group lost 1.2% (1.4 kg) of baseline body weight, and those in the lower- and higher-dose phentermine/topiramate groups lost 7.8% (8.1 kg) and 9.8% (10.2 kg), respectively (28). In the SEQUEL trial, patients who had been enrolled in the CONQUER study continued their blinded study regimen for an additional 52 weeks. The weight loss was sustained over the 2-year period; at study end, weight had decreased 9.3% (9.6 kg) and 10.5% (10.9 kg) from baseline in the phentermine/topiramate ER 7.5 mg/46 mg and 15 mg/92 mg groups, respectively, compared with a loss of 1.8% (2.1 kg) in the placebo group (29).
When compared with the lifestyle intervention plus placebo control group, the amount of weight loss produced by phentermine/topiramate ER in these trials was associated with improvements in insulin sensitivity (lower fasting glucose and insulin levels), a profound effect to prevent progression to diabetes in patients with metabolic syndrome or prediabetes at baseline, lower blood pressure and reduce the need for antihypertensive agents in patients with hypertension, lower hemoglobin A1c (HbA1c) values with reduced need for diabetes medications in actively managed patients with T2DM, improvements in dyslipidemia (lower triglycerides and higher high-density-lipoprotein cholesterol [HDL-c]), a dramatic reduction in the apnea-hypopnea index in patients with obstructive sleep apnea, and improvements in cardiovascular risk biomarkers (C-reactive protein [CRP], fibrinogen, adiponectin) (27–33). In a phase II study involving actively managed patients with chronic T2DM, weight loss associated with phentermine/topiramate ER lowered HbA1c from a baseline of 8.8 to 7.2%, concomitant with net reductions in the doses and number of diabetes medications, compared with a decrease from 8.6 to 7.4% in the placebo group, which required net increments in diabetes medications (33).
Lorcaserin
Lorcaserin is a selective serotonin 5-hydroxytryptamine receptor 2C receptor agonist that acts centrally to promote weight loss by reducing food intake and promoting satiety. Lorcaserin was approved for treatment of obesity (BMI ≥30 kg/m2) and overweight with complications (BMI of 27 to 29.9 kg/m2) in June 2012 and is administered at a dose of 10 mg orally twice a day (BID) as an adjunct to lifestyle intervention therapy. After 12 weeks on treatment, if the patient has not lost ≥5% of baseline body weight, lorcaserin should be discontinued. The efficacy and safety of lorcaserin were evaluated in three separate randomized, double-blind, placebo-controlled phase III trials. In the 1-year Behavior Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) Study (34) and in the 2-year Behavior Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial (35), patients with BMI ≥30 to ≤45 kg/m2 or BMI ≥27 to ≤45 kg/m2 and ≥1 weight-related comorbidity were placed on a lifestyle intervention program and randomized to receive placebo or lorcaserin 10 mg BID. After 1 year, placebo- and lorcaserin-treated patients lost 2.8% (2.9 kg) and 5.8% (5.8 kg) of their baseline weight, respectively, in the BLOSSOM trial, and 2.2% (2.2 kg) and 5.8% (5.8 kg), respectively, in the BLOOM trial. In the BLOOM trial, at the end of year 1, lorcaserin-treated patients were rerandomized to either placebo or continuation on the drug for the second year of the study. By the end of year 2, patients rerandomized to placebo gained weight to the level of patients treated throughout with lifestyle intervention alone, and there was slight weight regain in the lorcaserin-treated patients, such that weight loss from baseline was 5.5% (5.6 kg), compared with 2.4% (2.4 kg) in placebo-treated patients. In the third phase III trial, BLOOM-DM (36), overweight or obese subjects (BMI ≥27 to ≤45 kg/m2) with T2DM treated with metformin, a sulfonylurea, or both, were randomized to placebo, lorcaserin 10 mg every day, or lorcaserin 10 mg BID, and the patients were then followed for 1 year. All subjects were managed to standard of care for their respective comorbidities, including T2DM, and received dietary and lifestyle counseling. Weight loss from baseline was 4.5% (4.7 kg) in patients treated with lorcaserin 10 mg BID and 1.5% (1.6 kg) in the diabetics randomized to the lifestyle intervention and placebo groups.
Lorcaserin-assisted weight loss led to improvements in cardiometabolic disease when compared with placebo. In the BLOOM trial, lorcaserin improved glycemia and insulin sensitivity (fasting glucose and insulin), lipids (total and low-density-lipoprotein cholesterol, triglycerides), blood pressure, and CVD biomarkers (CRP, fibrinogen), although there was partial deterioration in glycemia and lipids with weight regain in the second year (35). In the BLOSSOM trial, patients receiving lorcaserin experienced a decrease in triglycerides but no significant improvements in glycemia or blood pressure (34). In the BLOOM-DM study, lorcaserin lowered HbA1c from a baseline of 8.1 to 7.2%, compared with a decrease from 8.0 to 7.6% in the placebo group, despite the fact that significantly more lorcaserin-treated patients were able to decrease diabetes medications (36).
BMI-CENTRIC MODEL AND ITS LIMITATIONS
The recent approval of safe and effective weight-loss medications, together with advances in lifestyle therapy and bariatric surgery, have provided clinicians with expanded therapeutic options and enabled the evolution of algorithms for the “medicalization” of obesity management. Ideally, medical models would identify those patients who would most benefit from the various treatment options in an evidence-based approach that optimizes the benefit/risk ratio and patient outcomes in a cost-effective manner. Because pharmacotherapy and surgery entail risk, these therapies would be initiated and intensified as a function of disease severity, comorbidities, and mortality risk. The current prevailing model for obesity management is BMI-centric, as typified by the National Heart, Lung, and Blood Institute Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (7). In this algorithm, it is the presenting BMI value that largely dictates indications for medical and surgical interventions, as exemplified by all patients with a BMI ≥30 kg/m2 being candidates for pharmacotherapy and those with a BMI ≥40 kg/m2 being eligible for bariatric surgery. These guidelines are reflected in the FDA-approved prescribing information for phentermine/topiramate ER and lorcaserin, which are indicated in all patients with a BMI ≥30 kg/m2 (11). At these BMI levels, medical and surgical therapies are indicated, without reference to the presence or absence of obesity complications.
Several salient considerations point to the inadequacy of a BMI-centric model. First, due to considerations of safety and cost, it is neither desirable nor feasible to treat all overweight and obese patients with medical or surgical therapy, which is underscored by the fact that this encompasses 70% of U.S. adults (2). Any intervention entails risk, and medical and surgical treatments should be targeted to those patients who will derive the greatest benefits from the intervention and not necessarily everyone above a certain baseline BMI level. Second, treatment should not be based solely on the reduction in fat mass for a primary cosmetic outcome. The degree of weight loss that can predictably be achieved with medical, lifestyle, and surgical therapies will not achieve ideal body weight in the vast majority of patients. An average weight loss of ~10%, achievable with the new weight-loss drugs, will not suffice cosmetically or even bring many patients below the BMI threshold for obesity (i.e., BMI <30 kg/m2); however, this degree of weight loss is sufficient to exert powerful benefits regarding obesity complications. This brings us to the third point, which is that moderate weight loss (~10%) is sufficient to lower fasting glucose and insulin, enhance insulin sensitivity, reduce blood pressure, lower triglycerides, raise HDL-c, decrease levels of hepatic transaminases, prevent progression to diabetes, lower HbA1c in patients with T2DM while at the same time reducing the requirements for diabetes medications, and improve biomarkers of cardiovascular risk, such as CRP, fibrinogen, and adiponectin (4,9,12–16,25,27–36). In patients with T2DM, weight loss of ~10% can improve control of glycemia and blood pressure, concomitant with reductions in the dose and number of diabetes and hypertension medications (15,25,29,30,36,37). Also, in patients with severe obstructive sleep apnea, weight loss from diet (38) or phentermine/topiramate ER (31) can markedly reduce the apnea-hypopnea index. Clearly, weight loss can be used to promote the health of individuals by ameliorating obesity complications. The final point is that not all patients with obesity have complications. Up to 30% of patients with obesity have been observed to be insulin sensitive without cardiometabolic disease and thus may not progress to diabetes or CVD (39,40). In all these aspects, a BMI-centric approach to obesity management does not discriminate between obese patients with and without complications and fails to identify those patients who will benefit most from weight-loss therapy.
A COMPLICATIONS-CENTRIC MODEL FOR OBESITY TREATMENT
In the general approach to the overweight/obese patient, clinicians must identify those who will benefit most from therapy, establish therapeutic targets and goals, and identify the modality and intensity of treatment in order to optimize the benefit/risk ratio and achieve the best outcomes for patients. As alluded to above, the patients that will benefit most from treatment have obesity-related complications that can be ameliorated by weight loss. A complications-centric medical model, rather than a BMI-centric model, is more rationally designed to achieve these goals.
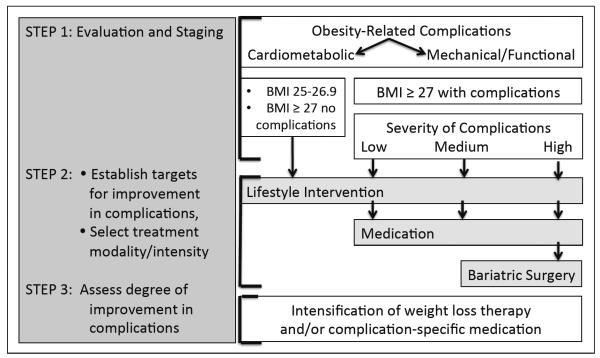
As shown in Figure 1, patients are evaluated for the presence and severity of complications in step 1. The comorbidities of obesity can be classified into two general categories, namely, those that relate to insulin resistance and cardiometabolic disease and those that relate to the mechanical or functional consequences of excess body weight. Not all patients with obesity have cardiometabolic disease or mechanical complications; therefore, the first step in a complications-centric approach is to evaluate the patient for the presence and severity of obesity complications in order to develop an appropriate therapeutic strategy. In patients with cardiometabolic disease or risk factors, the objective of weight-loss therapy is to reduce the risk of future T2DM and CVD and to treat patients with overt diabetes and hypertension. This includes insulin-resistant patients with traits that comprise the diagnosis of metabolic syndrome (elevated waist circumference, fasting glucose, blood pressure, and triglycerides, and low HDL-c), patients with prediabetes, and those who have progressed to type 2 diabetes or CVD. The clinician should evaluate patients for metabolic syndrome (41) and prediabetes (42), as this effectively identifies individuals at high risk of future diabetes and CVD. However, metabolic syndrome and prediabetes have high specificity but low sensitivity for identifying patients with insulin resistance and cardiometabolic disease (43,44), and these entities alone will not identify significant proportions of at-risk patients. Various indices using information from history and physical examination (45–49) or commercial products that employ clinical laboratory assays (50–53) can also be used to stage risk in insulin-resistant patients, whether or not they meet the diagnostic criteria for metabolic syndrome or prediabetes. The initial evaluation should also screen for other disease entities that will be affected by weight loss, including nonalcoholic fatty liver disease and sleep apnea. Finally, obese patients should be evaluated for mechanical complications, such as problematic degenerative joint disease, gastroesophageal reflux, stress incontinence, and immobility/disability.
Fig. 1.
A Complications-centric approach to obesity treatment. The figure shows the basic elements of a complications-centric approach to obesity treatment. The presence and severity of complications that can be ameliorated by weight loss are the critical determinants for the selection of treatment modality and intensity. The BMI cutoff of 27 kg/m2 reflects the U.S. Food and Drug Administration indication threshold for medications at which point expanded treatment options are available to the clinician. BMI = body mass index.
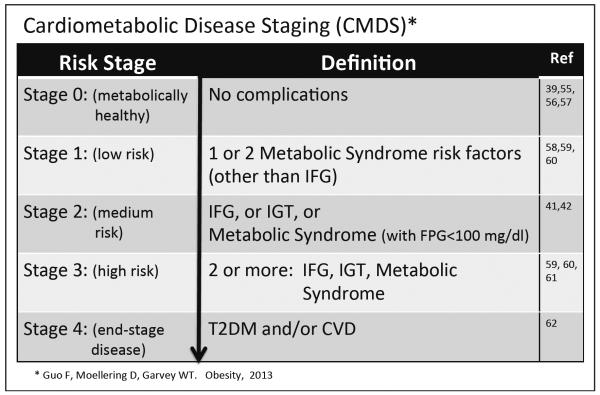
There are two paradigms that have been developed for staging the severity of obesity-associated comorbidities that can be used to guide the modality and intensity of therapy. The Edmonton Obesity Staging System incorporates an assessment of cardiometabolic disease, psychological issues, and mechanical complications (54,55). The Edmonton system features 5 stages, beginning with the metabolically healthy obese (stage 0) and progressing to stage 1, which includes all patients with insulin resistance, prediabetes, metabolic syndrome, and mild functional impairment. Stage 2 patients have diabetes and/or moderate functional impairment, whereas stage 3 patients have CVD events and severe functional impairment. Stage 4 patients are determined to be “end-stage” regarding both cardiometabolic disease and functional status. The staging system has been shown to discriminate increasing risk for all-cause mortality using National Health and Nutrition Examination Survey (NHANES) data (55). We have proposed a second paradigm, Cardiometabolic Disease Staging (CMDS) (56), as shown in Fig. 2. The 5 stages of CMDS were constructed based on established physiologic and epidemiologic observations, which take into account: (1) the presence of the metabolically healthy obese (stage 0) (39,55–57); (2) the fact that patients with one or two risk factors are at increased risk of diabetes and CVD (58–60), even if they do not meet the criteria for metabolic syndrome or prediabetes (stage 1); (3) the documented risk conferred by the isolated presence of metabolic syndrome or impaired fasting glucose or impaired glucose tolerance (stage 3) (41,42); (4) the augmented risk of diabetes and CVD in patients with both metabolic syndrome and prediabetes (59–61) (stage 3); and (5) the observation that T2DM is a CVD risk equivalent (62) (stage 4). Advancement from CMDS stage 0 to 4 was validated to predict increasing risk of diabetes, based upon data from the national Coronary Artery Risk Development in Young Adults study cohort, and was validated to predict the risk of all-cause and CVD mortality based upon NHANES data (56). The CMDS is a more granular differentiation of risk for all-cause mortality as well as risk for future diabetes and CVD mortality, as all patients in CMDS stages 1, 2, and 3 would fall into stage 1 in the Edmonton system. Thus, CMDS utilizes information that is readily available to the clinician in the context of routine clinical practice to quantitatively stratify risk for both diabetes and CVD.
Fig. 2.
The Cardiometabolic Disease Staging System. The figure delineates criteria for stages 0 to 4 of Cardiometabolic Disease Staging (CMDS) (56). The risks for future diabetes, all-cause mortality, and cardiovascular disease mortality have been validated to increase progressively with each advancing stage. The CMDS can be used by clinicians to estimate the severity of cardiometabolic disease as a guide to the selection of treatment modality and intensity for obesity. CVD = cardiovascular disease; FPG = fasting plasma glucose; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; T2DM = type 2 diabetes mellitus.
Two aspects of CMDS are deserving of consideration. First, risk staging requires a 2-hour glucose value during an oral glucose tolerance test (OGTT). This is necessary for accurate and comprehensive diagnoses of prediabetes and diabetes. HbA1c was not employed because we (63) and others (64,65) have shown that HbA1c has low sensitivity for these diagnoses and is responsible for a high false-negative rate among patients diagnosed using the gold standard measures of fasting glucose combined with 2-hour glucose values. Elevated 2-hour glucose is also a strong independent risk factor for CVD (66,67). This underscores the contention that both fasting and 2-hour OGTT glucose values are important clinical parameters in evaluating obese and overweight patients for weight-loss therapy in the context of a complications-centric medical model. Secondly, BMI was not included in the determination of cardiometabolic disease risk. In addition to the fact that insulin resistance exists largely independent of generalized adiposity (68,69) and that BMI is a poor independent predictor of CVD (70–73), adjustment for BMI did not substantially alter risks predicted by CMDS (56) or the Edmonson Obesity Staging System (55). In contrast, waist circumference, which is a strong independent predictor of insulin resistance and CVD (70–73), is incorporated into CMDS.
Step 2 in the complications-centric model is to identify targets for improvements in the complications that can be ameliorated by weight loss and to select the modality and intensity of therapy to generate sufficient weight loss to achieve these targets. All three treatment approaches for obesity are characterized by a wide range of intensity that can be employed to achieve a greater or lesser degree of weight loss. Furthermore, there is a dose-response relationship between weight loss and improvements in cardiometabolic disease. For example, following both lifestyle intervention in the diabetes prevention program and in clinical trials employing phentermine/topiramate ER, prevention of future diabetes was progressive until maximal benefits were achieved at ~10 to 15% weight loss (14,30). In contrast, in the Look Action for Health in Diabetes (AHEAD) Study (15), improvements in HbA1c, fasting glucose, triglycerides, HDL-c, and blood pressure were progressive up to >15% weight loss, without evidence of a threshold effect; with these latter parameters, the more weight loss the better. The baseline BMI and the number of pounds lost are less important than the presence and severity of complications at baseline and the degree of improvement in these complications with the ensuing weight loss following initiation of weight-loss therapy.
In Step 3, patients are re-evaluated for improvements in complications after equilibrating at a lower body weight. If the targets for improvement in complications are not reached, then the weight-loss therapy should be intensified, for example, by proceeding to a more highly structured intensive lifestyle therapy program or increasing the daily treatment dose of phentermine/topiramate ER 7.5 mg/46 mg to the top dose of 15 mg/92 mg. Alternatively, additional medication could be employed that is specifically targeted to the complication.
The American Association of Clinical Endocrinologists (AACE) has proposed a complications-centric medical model for obesity (74). In this scenario, the presence and severity of complications dictate the aggressiveness of the intervention and the rational application of recent advances in lifestyle therapy, medications, and bariatric surgery. The baseline BMI is less important than the presence or absence of obesity-associated complications, and the number of pounds lost is less germane than whether the degree of weight loss achieved has been sufficient to produce the desired improvement in complications. Therapeutic interventions are intensified based on efficacy and safety commensurate with the severity of complications and risk of morbidity and mortality. The complications-centric approach is designed to optimize the benefit/risk ratio of treatment, thus enhancing patient outcomes and the cost-effectiveness of the intervention.
Application to Prediabetes and T2DM
Perhaps the greatest potential benefit of a complications-centric approach, in terms of public health and containment of heath care costs, is the use of weight loss to prevent diabetes in high-risk individuals (6). Weight loss produced by lifestyle intervention (14,16), bariatric surgery (22–24), or medications (9,29) has been shown to prevent or delay progression to diabetes. For example, in clinical trials, patients with metabolic syndrome and/or prediabetes who were treated with phentermine/topiramate ER plus lifestyle modification experienced an ~80% reduction in the progression to diabetes over 2 years when compared with patients randomized to placebo plus lifestyle modification (29,30). Given the high cost of diabetes care and the morbidity and mortality that accompany this disease, the targeted treatment of overweight/obese patients with metabolic syndrome and prediabetes would have a pronounced impact in reducing the burden of diabetes.
The new weight-loss drugs have been approved to treat obese patients, including those with diabetes. The benefits of weight loss in T2DM have been well documented. In short, weight loss, whether induced by diet and exercise (15), bariatric surgery (22–24), or medications (33,36), can improve control of glycemia, blood pressure, and lipids while at the same time reducing the need for other medications to specifically treat these metabolic abnormalities. The clinical trials programs for both phentermine/topiramate ER (33) and lorcaserin (36) included studies in T2DM and consistently demonstrated lower HbA1c with medication-assisted weight loss, together with a reduced need for medications in actively managed patients, when compared with patients treated with lifestyle modification alone. It could be argued that weight-loss medications should be considered for any overweight or obese patient with overt T2DM who fail to achieve moderate weight loss (i.e., ~10%) with lifestyle modification. Although additional clinical trials involving diabetes are necessary, weight-loss drugs could be effective second-line drugs in patients who fail metformin, or weight-loss drugs could be used in conjunction with metformin as initial dual therapy or as first-line therapy in newly diagnosed patients. Indeed, these drugs could change the landscape of how we therapeutically approach T2DM and expand the notion of “diabetes drugs” beyond those that act primarily to increase insulin secretion and action. The comprehensive algorithm for diabetes recently advocated by AACE (74) not only established a complications-centric model for the treatment of obesity but also incorporated the obesity-treatment algorithm, including medication-assisted weight loss, in the treatment of prediabetes and T2DM.
Cost-Effectiveness and Health Care Policy
A complications-centric model for obesity assures that treatment is targeted to those patients who will benefit most from weight loss, specifically, those patients with complications that are remediable through weight loss. Such a model will promote the cost-effectiveness of medical care for the obese patient. Studies in middle-aged adults (75) and in the older Medicare population (76) have demonstrated cost savings resulting from both transient and permanent moderate weight loss equal to what can often be achieved with the combination of lifestyle intervention plus weight-loss medications. In addition, the cost of quality-of-life year gained as a result of weight loss achieved through diet and exercise was shown to be favorable in the Diabetes Prevention Program (77). A complications-centric model would further enhance cost savings over those predicted in these studies by targeting medical and surgical care to those patients based on the presence and severity of complications, as opposed to the indiscriminate application of therapy in all obese subjects based only on BMI. Health care policy will need to integrate coverage of the costs of obesity therapy if we are to reduce the burden of this disease in our society. Payers will more readily accept these costs in health care systems if they are confident that the interventions will be targeted to obese patients based on the health benefits of weight loss. Hopefully, the implementation of a complications-centric algorithm will accelerate the covered access to obesity care. This model, which emphasizes weight loss as a tool to ameliorate the cardiometabolic and mechanical complications of obesity, will serve to optimize the benefit/risk ratio and achieve the best outcomes in overweight/obese patients in a manner that considers both patient safety and the cost of therapy.
CONCLUSION
The BMI-centric approach, using baseline BMI as the principal determinant of indications for therapy, fails to target therapy to those obese patients who will benefit most from weight loss. In contrast, a complications-centric medical model will earmark the modality and intensity of the therapeutic intervention based on the presence and severity of complications that can be ameliorated by weight loss. CMDS uses data readily available to the clinician to predict risk for future diabetes as well as all-cause and CVD mortality and can be used as a guide to treatment intensity for obesity based on the risk and severity of cardiometabolic disease. The complications-centric approach to “medicalizing” obesity care employs weight loss primarily as a tool to treat obesity-related complications and promotes the optimization of health outcomes, the benefit/risk ratio, and the cost-effectiveness of therapy.
ACKNOWLEDGMENT
The author wishes to gratefully acknowledge research support from the Merit Review program of the Department of Veterans Affairs, National Institutes of Health (DK-038765 and DK-083562) and the UAB Diabetes Research Center (P60-DK079626).
DISCLOSURE Dr. Garvey is an advisor for Alkermes Plc, Daiichi-Sankyo Inc, LipoScience, VIVUS Inc, Janssen Pharmaceuticals, Novo Nordisk, Eisai, AstraZeneca/Bristol-Myers-Squibb, and Tethys. He is a speaker for Merck & Company and Amylin Pharmaceuticals Inc and holds stock in Bristol-Myers Squibb Company, Isis/Genzyme, Merck, Pfizer Inc, Eli Lilly and Company, and VIVUS Inc. He has received research support from Amylin, Merck, and Weight Watchers International Inc.
Abbreviations
- BID
twice a day
- BLOOM
Behavior Modification and Lorcaserin for Overweight and Obesity Management
- BLOSSOM
Behavior Modification and Lorcaserin Second Study for Obesity Management
- BMI
body mass index
- CMDS
cardiometabolic disease staging
- CRP
C-reactive protein
- CVD
cardiovascular disease
- ER
extended release
- FDA
U.S. Food and Drug Administration
- HbA1c
hemoglobin A1c
- HDL-c
high-density-lipoprotein cholesterol
- T2DM
type 2 diabetes mellitus
REFERENCES
- 1.Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists' position statement on obesity and obesity medicine. Endocr Pract. 2012;18:642–648. doi: 10.4158/EP12160.PS. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219–229. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Ryan DH. Medical therapy for the patient with obesity. Circulation. 2012;125:1695–1703. doi: 10.1161/CIRCULATIONAHA.111.026567. [DOI] [PubMed] [Google Scholar]
- 5.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Heart, Lung, and Blood Institute . The Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Institutes of Health; Bethesda, MD: 1998. NIH publication 98-4083. [Google Scholar]
- 8.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1:352–354. doi: 10.1136/bmj.1.5588.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 10.James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 11.Qsymia® [package insert], VIVUS Inc, Mountain View, CA. Belviq® [package insert] Arena Pharmaceuticals, Zofingen; Switzerland: [Google Scholar]
- 12.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 13.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing RR, Lang W, Look Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindström J, Ilanne-Parikka P, Peltonin M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 20.Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378:1485–1492. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene LF, Malpede CZ, Henson CS, Hubbert KA, Heimburger DC, Ard JD. Weight maintenance 2 years after participation in a weight loss program promoting low-energy density foods. Obesity (Silver Spring) 2006;14:1795–1801. doi: 10.1038/oby.2006.207. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien PE, Macdonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257:87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 23.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 24.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract. 2013;19:337–372. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvey WT. Phentermine and topiramate extended-release: a new treatment for obesity and its role in a complications-centric approach to obesity medical management. Expert Opin Drug Saf. 2013;12:741–756. doi: 10.1517/14740338.2013.806481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan J, Astrup AV, Day WW. Effects of phentermine and extended release topiramate alone and in combination on cardiovascular risk factors. Diabetologia. 2012;55(suppl 1):S285. [Google Scholar]
- 27.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Sliver Spring) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 29.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Amer J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garvey WT, Bowden CH. Diabetes prevention in subjects with prediabetes using controlled-release phenter-mine/topiramate (PHEN/TPM CR) over 2 years. Diabetes. 2012;61(suppl 1):A517. [Google Scholar]
- 31.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/ topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35:1529–1539. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2. Am J Cardiol. 2013;111:1131–1138. doi: 10.1016/j.amjcard.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Troupin B, Peterson C, Najarian T, Tam P, Day W. Treatment with VI-0521 (phentermine and topiramate) leads to one year durable glycemic benefit and weight loss in subjects with type 2 diabetes. Diabetologia. 2009;52(suppl 1):S77. [Google Scholar]
- 34.Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 35.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 36.O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 37.Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review) J Diabetes Complications. 2013 May 28; doi: 10.1016/j.jdiacomp.2013.04.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 40.Blüher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes. 2012;19:341–346. doi: 10.1097/MED.0b013e328357f0a3. [DOI] [PubMed] [Google Scholar]
- 41.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 42.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y, Kwon S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care. 2004;27:978–983. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 44.Qiao Q, DECODE Study Group Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia. 2006;49:2837–2846. doi: 10.1007/s00125-006-0438-6. [DOI] [PubMed] [Google Scholar]
- 45.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 46.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 48.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 49.Bang H, Edwards AM, Bomback AS, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151:775–783. doi: 10.1059/0003-4819-151-11-200912010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodge AM, Jenkins AJ, English DR, O'Dea K, Giles GG. NMR-determined lipoprotein subclass profile predicts type 2 diabetes. Diabetes Res Clin Pract. 2009;83:132–139. doi: 10.1016/j.diabres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Frazier-Wood AC, Garvey WT, Dall T, Honigberg R, Pourfarzib R. Opportunities for using lipoprotein subclass profile by nuclear magnetic resonance spectroscopy in assessing insulin resistance and diabetes prediction. Metab Syndr Relat Disord. 2012;10:244–251. doi: 10.1089/met.2011.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolberg JA, Gerwien RW, Watkins SM, Wuestehube LJ, Urdea M. Biomarkers in Type 2 diabetes: improving risk stratification with the PreDx® Diabetes Risk Score. Expert Rev Mol Diagn. 2011;11:775–792. doi: 10.1586/erm.11.63. [DOI] [PubMed] [Google Scholar]
- 53.Shafizadeh TB, Moler EJ, Kolberg JA, et al. Comparison of accuracy of diabetes risk score and components of the metabolic syndrome in assessing risk of incident type 2 diabetes in Inter99 cohort. PLoS One. 2011;6:e22863. doi: 10.1371/journal.pone.0022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009;33:289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 55.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:E1059–E1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity. 2013 Jul 26; doi: 10.1002/oby.20585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogorodnikova AD, Kim M, McGinn AP, Munter P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–659. doi: 10.1038/oby.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 59.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart Study The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Grundy SM, Wang W, et al. Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. 2007;153:552–558. doi: 10.1016/j.ahj.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 61.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA. 2001;285:2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 62.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 63.Guo F, Zhang W, Garvey WT. Differentially segmented association between HbA1c and CHD risks and FPG across ethnicities. (late breaking poster) Diabetes. 2012;61(suppl 1):LB16. [Google Scholar]
- 64.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care. 2011;34:84–89. doi: 10.2337/dc10-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3:661–667. doi: 10.1161/CIRCOUTCOMES.110.957936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 67.DECODE Study Group. the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 68.Liao Y, Kwon S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care. 2004;27:978–983. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 69.Lara-Castro C, Garvey WT. Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab. 2004;89:4197–4205. doi: 10.1210/jc.2004-0683. [DOI] [PubMed] [Google Scholar]
- 70.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 71.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 73.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 74.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 75.Dall TM, Zhang Y, Zhang S, et al. Weight loss and lifetime medical expenditures: a case study with TRICARE prime beneficiaries. Am J Prev Med. 2011;40:338–344. doi: 10.1016/j.amepre.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 76.Thorpe KE, Yang Z, Long KM, Garvey WT. The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3:7. doi: 10.1186/2191-1991-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]