Abstract
Ophiocordycipitaceae is a diverse family comprising ecologically, economically, medicinally, and culturally important fungi. The family was recognized due to the polyphyly of the genus Cordyceps and the broad diversity of the mostly arthropod-pathogenic lineages of Hypocreales. The other two cordyceps-like families, Cordycipitaceae and Clavicipitaceae, will be revised taxonomically elsewhere. Historically, many species were placed in Cordyceps, but other genera have been described in this family as well, including several based on anamorphic features. Currently there are 24 generic names in use across both asexual and sexual life stages for species of Ophiocordycipitaceae. To reflect changes in Art. 59 in the International Code of Nomenclature for algae, fungi, and plants (ICN), we propose to protect and to suppress names within Ophiocordycipitaceae, and to present taxonomic revisions in the genus Tolypocladium, based on rigorous and extensively sampled molecular phylogenetic analyses. When approaching this task, we considered the principles of priority, monophyly, minimizing taxonomic revisions, and the practical utility of these fungi within the wider biological research community.
Keywords: arthropod-pathogens, Article 59, new combinations, nomenclature, Ophiocordycipitaceae, Tolypocladium
BACKGROUND
The revision of Art. 59 in the International Code of Nomenclature for algae, fungi, and plants (ICN; McNeill et al. 2012) has created a major task for mycologists, who must now reconcile under one name various possible names existing for different morphs of the same species of fungus (Hibbett & Taylor 2013). Groups have already begun to propose names which should be protected or suppressed within Hypocreales in accordance with the ‘one fungus one name’ policy (Geiser et al. 2013, Rossman et al. 2013, Leuchtmann et al. 2014, Johnston et al. 2014, Kepler et al. 2014) and others are in progress. Here, we seek to retain names in Ophiocordycipitaceae with the goal of harmonizing priority, monophyly, simplicity of taxonomic revisions, and minimization of disruption to the research community.
The family Ophiocordycipitaceae was described by Sung et al. (2007) to accommodate species that were determined to be phylogenetically distinct from Cordycipitaceae and Clavicipitaceae s.str. Asexual morphologies in Ophiocordycipitaceae show a tremendous range of variation, some of which are restricted in their phylogenetic distribution while others are often found in disparate lineages. For example, Verticillium is a common asexual morph of many species in several hypocrealean families, including Ophiocordycipitaceae, Cordycipitaceae and Clavicipitaceae (see Zare et al. 2000, Sung et al. 2001, 2007, and Gams & Zare 2001).
Ophiocordyceps is the most speciose genus of the family, and was described originally by Petch (1931a) for species of Cordyceps that have septate ascospores that do not disarticulate into part-spores at maturity and asci with inconspicuous apical caps (Petch 1931a, 1933). Kobayasi (1941) later used Ophiocordyceps as a subgeneric classification of the genus Cordyceps, but Sung et al. (2007) restored Ophiocordyceps to the rank of genus to include those Cordyceps species within Ophiocordycipitaceae forming a sister clade with the genus Elaphocordyceps (see below). The type of the genus is O. blattae, a rarely collected cockroach pathogen for which no culture or molecular data are available.
Asexual generic names associated with Ophiocordyceps include Sorosporella, the oldest name still in use for species in the clade, Hirsutella, Hymenostilbe, Stilbella, Syngliocladium, and Paraisaria. Hirsutella species typically produce one to several conidia in a limited mucus droplet borne on basally subulate phialides that taper into slender necks (Gams & Zare 2003). Hymenostilbe was proposed by Petch (1931b), and there is some evidence to support restricting its use within the genus Ophiocordyceps to the ‘O. sphecocephala clade’, most species of which sporulate from adult insects (Sung et al. 2007, Luangsa-ard et al. 2011a). These taxa produce conidia singly from multiple denticles on conidiogenous cells forming a palisade-like layer along the entire outer surface of synnemata (Mains 1950). The Stilbella morphology has been applied broadly among species associated with Ophiocordyceps, as well as to fungi later reclassified in other genera (Seifert 1985, Gräfenhan et al. 2011). Stilbella species often produce aggregate synnemata with a fertile, terminal head of conidia. Syngliocladium spp. often have laterally arising conidiophores similar in morphology to the hypocreaceous asexual morph Gliocladium, and they may be either synnematous or mononematous on their arthropod hosts (Petch 1932, Hodge et al. 1998). Sorosporella, a chlamydospore producing spore state, has been linked as a synasexual morph of Syngliocladium (Speare 1917, 1920), but the two morphologies are not always produced by all species (Hodge et al.1998, Evans & Shah 2002). Species of Paraisaria possess feathery synnemata which fruit from arthropod hosts, and several species have been linked via cultural and molecular data to the O. gracilis clade (Samson & Brady 1983, Sung et al. 2007, Evans et al. 2010). Names of genera associated with Ophiocordyceps whose types are located outside of Hypocreales include Tilachlidiopsis and Podonectria, members of the Agaricomycetes and Dothideomycetes, respectively (Rossman 1978, Stalpers et al.1991, Hughes et al. 2001, Boonmee et al. 2011). Despite the large number of taxa associated with Ophiocordyceps, a lack of support for internal nodes resulting in equivocal topologies has limited inferences about relationships within the genus in previous studies (Sung et al.2007).
The most notable species in the Ophiocordyceps clade is O. sinensis, which is nearly double the price of gold by weight (Stone 2008, Shrestha & Bawa 2013) and the subject of intense research, especially in China (Shrestha et al. 2010, Hu et al. 2013, Ren & Yao 2013, Bushley et al. 2013a, etc.). Almost exclusively found parasitizing the larvae of ghost moths (Hepialidae: Thitarodes) in the alpine and sub-alpine pastures of the Tibetan plateau and the Himalayas, this species is undergoing heavy, possibly unsustainable, and destructive harvesting (Cannon et al. 2009, Shrestha & Bawa 2013).
The recently described genus Elaphocordyceps is typified by E. ophioglossoides, one of the first Cordyceps species to be described. Species in Elaphocordyceps are mostly parasites of the ectomycorrhizal truffle genus Elaphomyces (Ascomycota, Eurotiales). The majority of Elaphocordyceps species have no known asexual morph, but where known they produce ones which are verticillium-like or Tolypocladium (Sung et al. 2007). There are a few Elaphocordyceps species known to be entomopathogens, including three cicada pathogens (E. inegoensis, E. paradoxa, and E. toriharamontana), and one beetle pathogen, E. subsessilis (syn.Tolypocladium inflatum) (Hodge et al. 1996, Sung et al. 2007). Tolypocladium inflatum (a name conserved by the rejection of Pachybasium niveum; Dreyfuss & Gams 1994), is a medicinally important fungus and the subject of much research due to its production of the immunosuppressant drug, cyclosporin A (Survase et al. 2011, Bushley et al. 2013b). The other species of Tolypocladium have no known sexual morphs and have mainly been isolated from soil (Gams 1971, Bissett 1983) or observed parasitizing rotifers or insects (Barron 1980, 1981, 1983, Samson & Soares 1984, Weiser et al. 1991). The asexually typified genus Chaunopycnis is also related to this clade (Bills et al. 2002) and has been isolated mainly from soil samples (Gams 1980, Bills et al. 2002), although one species was isolated from epilithic Antarctic lichens (Möller & Gams 1993). The similarity of conidiogenesis between Chaunopycnis and Tolypocladium was noted in the original description of Chaunopycnis (Gams 1980), and its phialides often taper in a manner similar to those of Tolypocladium. Interestingly, these two genera have also been linked by their shared production of cyclosporin A (Traber & Dreyfuss 1996). Two of the described Chaunopycnis species produce loosely enclosed conidiomata, a morphology not seen in other members of the clade or within Ophiocordycipitaceae as a whole.
The relationships among the species of the Purpureocillium clade were recently reviewed by Luangsa-ard et al. (2011b). The genus was proposed to encompass taxa closely related to Purpureocillium lilacinum (syn. Paecilomyces lilacinus) and consists of species with purple-hued conidia, including Nomuraea atypicola and Isaria takamizusanensis. The type of Nomuraea is N. rileyi (syn. N. prasina), which has recently been synonymized with Metarhizium (Kepler et al. 2014). The type of Isaria is a member of Cordycipitaceae (Gams et al. 2005, Hodge et al. 2005, Luangsa-ard et al. 2011b). While N. atypicola and I. takamizusanensis have not been addressed taxonomically, other studies found close relationships between these taxa and Purpureocillium (Sung et al.2007, Perdomo et al. 2013). Nomuraea atypicola is the asexual morph of C. cylindrica (Hywel-Jones & Sivichai 1995), the only sexual morph described for this clade and one of the “residual” Cordyceps s. lat. left without reassignment to any phylogenetically redefined genus by Sung et al. (2007).
Nematode pathogens have been described in many genera throughout Hypocreales. The largest and oldest of these is the asexually typified genus Harposporium. Most Harposporium species produce crescent-shaped or helicoid conidia that are ingested by their hosts and become lodged in the upper portions of the digestive tract (Barron 1977). Conidia are produced on spherical conidiogenous cells, and several species are known to produce hirsutella-like synasexual morphs (Hodge et al. 1997, Chaverri et al. 2005, Li et al. 2005). While the majority of Harposporium species are known from nematodes, these fungi are common in the soil and several studies have reported an entomopathogenic ecology as well (e.g., Shimazu & Glockling 1997, Evans & Whitehead 2005). In 2005, Chaverri et al. reported the asexual-sexual morph connection between Harposporium and Podocrella, an arthropod-pathogenic genus. Several researchers initially described nematophagous taxa in the originally plant-pathogenic genus Meria (Vuillemin 1896, Drechsler 1941), but this genus was found to be polyphyletic (Gams & Jansson 1985), and for this reason Drechmeria was erected for the nematophagous meria-like taxa in Hypocreales. The type of Drechmeria, D. coniospora, has cone-shaped conidia whose conidiogenous cells are not basally swollen as in Harposporium. One protozoan-infecting species of Drechmeria, D. harposporioides, produces crescent-shaped conidia similar to those of Harposporium (Barron & Szijarto 1982). Haptocillium was erected for asexual nematode pathogens bearing verticillate phialides and whose conidia are not ingested but adhere to the surface of their hosts (Zare & Gams 2001).
Polycephalomyces represents a diverse clade that is currently incertae sedis within Hypocreales, as its placement has lacked support in previous molecular studies (Kepler et al. 2013). Of particular uncertainty was whether Polycephalomyces and its closest related taxon, C. pleuricapitata, formed a sister clade to Ophiocordycipitaceae, or if it was more closely related to Clavicipitaceae. Many morphological characters are shared between Ophiocordycipitaceae and Polycephalomyces. For example, numerous species in both clades produce hirsutella-like anamorphs with conidia often borne in a slimy mass (Seifert 1985). In addition, sexual sporing structures of Polycephalomyces often possess a wiry, tough, carbonaceous stipe which is a common morphology of Ophiocordyceps (Kepler et al. 2013). Many species within this genus are known mycoparasites of other hypocrealean entomopathogens and myxomycetes, but there are also several species of entomopathogens. Cordyceps pleuricapitata was deemed a residual species of Cordyceps of uncertain placement by Kepler et al. (2013), due to a lack of statistical support joining that species and Polycephalomyces.
In this paper we expand the taxon sampling presented in Sung et al. (2007) by 222 hypocrealean isolates. This includes sexual and asexual states which provide the framework for addressing the nomenclatural issues demanded by changes to the most recent ICN.
MATERIALS AND METHODS
Sequences from five nuclear loci, including the small and large subunits of the rDNA (SSU and LSU), the transcription elongation factor-1α (TEF), and the first and second largest subunits of RNA polymerase II (RPB1 and RPB2) were used for phylogenetic analyses. DNA extraction and PCR amplification were carried out as previously described (Kepler et al. 2013). Sequencing reactions were performed at the University of Washington High-Throughput Genomics Center (Seattle, WA) with the primers used for the initial amplifications. All other sequences were collected from GenBank. Efforts were made for all specimens to have data for at least three of the five genes to be considered in our analyses. However, certain taxa for which only one or two genes were available were included due to the importance in addressing the taxonomic issues at hand (Table 1).
Table 1.
Specimen information and GenBank accession numbers for sequences used in this study.
Raw sequences were processed, aligned, and gaps excluded as in Kepler et al. (2013), using the programs MAFFT v. 6 (Katoh et al. 2002, Katoh & Toh 2008), Geneious v. 7.0.6 (Biomatters, available http://www.geneious.com), and Gblocks (Talavera & Castresana 2007). The final alignment length was 4570 nucleotides - 1023 for SSU, 879 for LSU, 987 for TEF, 646 for RPB1, and 1035 for RPB2. RAxML v. 7.6.6 (Stamatakis 2006) was used to perform Maximum likelihood (ML) estimation of the phylogeny with 500 bootstrap replicates on the concatenated dataset using eleven data partitions. These included one each for SSU and LSU, and three for each of the three codon positions of the protein coding genes, TEF, RPB1, and RPB2. The GTR-GAMMA model of nucleotide substitution was used.
RESULTS AND DISCUSSION
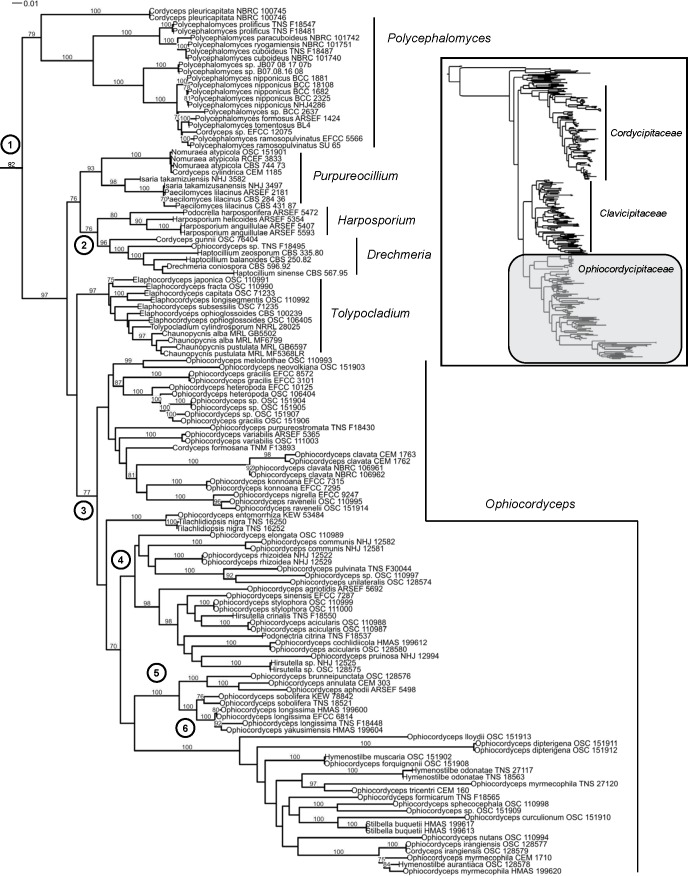
Our results are in agreement with the overall phylogenetic structure of the order Hypocreales put forth by Sung et al. (2007). Nomenclatural issues for taxa in the other two families of cordyceps-like organisms, Cordycipitaceae and Clavicipitaceae, will be presented elsewhere or have already been published (Leuchtmann et al. 2014, Kepler et al. 2014). Based on this exhaustive phylogenetic reconstruction (Fig. 1), we recognize six genera within Ophiocordycipitaceae Ophiocordyceps, Tolypocladium, Purpureocillium, Harposporium, Drechmeria, and Polycephalomyces (Table 2). This framework will provide clarity for researchers, ease of communication for instructors, and phylogenetic taxonomy around which to investigate the evolution of life histories (e.g. morphology, ecology).
Fig. 1.
ML tree of Ophiocordycipitaceae obtained using RAxML to analyze the concatenated five gene dataset (SSU, LSU, TEF, RPB1, and RPB2). Proposed genus level names to protect are delimited, but names of individual species have not been changed on the leaves of the tree, to demonstrate the diversity of taxa sampled. Values above branches represent MLBP proportions greater than or equal to 70 % from 500 replicates. Inset tree shows the larger phylogeny of Hypocreales.
Table 2.
Proposed list of generic names in Ophiocordycipitaceae to be protected and their competing synonyms. Names to be protected are in bold type, and names previously synonymized are in blue.
| Proposed to protect | Proposed to suppress |
|---|---|
| Ophiocordyceps Petch, Trans. Br. Mycol. Soc. 16: 74 (1931). | Sorosporella Sorokin Zentbl. Bakt. ParasitKde., Abt. II 4: 644 (1888). |
| Type: O. blattae Petch 1931. | Type: S. agrotidis Sorokin 1888. |
| Hirsutella Pat., Revue Mycol. 14: 67 (1892). | |
| Type: H. entomophila Pat. 1892. | |
| Didymobotryopsis Henn., Hedwigia 41: 149 (1902). | |
| Type: D. parasitica Henn. 1902. | |
| Mahevia Lagarde, Archs Zool. Exp. Gen. 56: 292 (1917). | |
| Type: M. guignardii (Maheu) Lagarde 1917. | |
| Synnematium Speare, Mycologia 12: 74 (1920). | |
| Type: S. jonesii Speare 1920. | |
| Trichosterigma Petch, Trans. Br. Mycol. Soc. 8: 215 (1923). | |
| Type: T. clavisporum Petch 1923. | |
| Didymobotrys Clem. & Shear, Gen. Fungi: 228 (1931). | |
| Type: D. parasitica (Henn.) Clem. & Shear 1931. | |
| Troglobiomyces Pacioni, Trans. Br. Mycol. Soc. 74: 244 (1980). | |
| Type: T. guignardii (Maheu) Pacioni 1980. | |
| Hymenostilbe Petch, Naturalist (Hull), ser. 3, 1931: 101 (1931). | |
| Type: H. muscaria Petch 1931. | |
| Syngliocladium Petch, Trans. Br. Mycol. Soc. 17: 177 (1932). | |
| Type: S. aranearum Petch 1932. | |
| Cordycepioideus Stifler, Mycologia 33: 83 (1941). | |
| Type: C. bisporus Stifler 1941. | |
| Paraisaria Samson & B.L. Brady, Trans. Br. Mycol. Soc. 81: 285 (1983). | |
| Type: P. dubia (Delacr.) Samson & B.L. Brady 1983. | |
| Purpureocillium Luangsa-ard et al., FEMS Microbiol Lett 321: 144 (2011). | |
| Type: P. lilacinum (Thom) Luangsa-ard et al. 2011 (syn. Penicillium lilacinum Thom 1920). | |
| Tolypocladium W. Gams, Persoonia 6: 185 (1971). | Chaunopycnis W. Gams, Persoonia 11: 75 (1980). |
| Type: T. inflatum W. Gams 1971. | Type: C. alba W. Gams 1980. |
| Elaphocordyceps G.H. Sung & Spatafora, Stud. Mycol. 57: 36 (2007). | |
| Type: E. ophioglossoides (Ehrh. ex J.F. Gmel.: Fr.) G.H. Sung et al. 2007. | |
| Harposporium Lohde, Tagbl. Versamml. Ges. Deutsch. Naturf. 47: 206 (1874). | Polyrhina Sorokin, Annls Sci. Nat., Bot., sér 6, 4: 65 (1876). |
| Type: H. anguillulae Lohde 1874. | Type: P. multiformis Sorokin 1876. |
| Podocrella Seaver, Mycologia 20: 57 (1928). | |
| Type: P. poronioides Seaver 1928. | |
| Atricordyceps Samuels, N.Z. Jl. Bot. 21: 174 (1983). | |
| Type A. harposporifera Samuels 1983. | |
| Drechmeria W. Gams & H.-B. Jansson, Mycotaxon 22: 36 (1985). | Haptocillium W. Gams & Zare, Nova Hedwigia 73: 334 (2001). |
| Type: D. coniospora (Drechsler) W. Gams & H.-B. Jansson 1985 (syn. Meria coniospora Drechsler 1941). | Type: H. balanoides (Drechsler) Zare & W. Gams 2001. |
| Polycephalomyces Kobayasi, Sci. Rep. Tokyo Bunrika Daig., sect. B 5: 245 (1941). | Blistum B. Sutton, Mycol. Pap. 132: 16 (1973). |
| Type: P. formosus Kobayasi 1941. | Type: B. tomentosum (Schrad.) B. Sutton 1973. |
TAXONOMY
Ophiocordyceps Petch 1931
Ophiocordyceps sensu Sung et al. (2007) is resolved as a well-supported (MLBP=77) clade (Fig. 1, Node 3). This clade is speciose, diverse, and almost exclusively comprises insect pathogens. In spite of increased taxon sampling, current reconstructions fail to find strong statistical support at the internal nodes, and therefore we refrain from defining infrageneric groupings (Fig. 1). While Sorosporella is the oldest name for any members in this clade, there are only two described species, and Evans & Shah (2002) argued Sorosporella should be synonymized with Syngliocladium instead of being recognized as a an asexual morph, as Synnematium was previously treated with respect to Hirsutella (Evans & Samson 1982). We propose, therefore, to suppress the use of Sorosporella for this clade. Hirsutella is the next oldest name, but the type, H. entomophila, which was described growing from adult Coleoptera, has not been sampled and no culture of this species is available. Sung et al. (2007) argued that the Hirsutella morphology was phylogenetically informative for the ‘O. unilateralis group’ which they resolved as paraphyletic, a topology recovered in the current analyses as well (Fig 1, Nodes 4 and 5). However, the Hirsutella morphology is observed in other clades (e.g. Harposporium, Polycephalomyces, Clavicipitaceae), and while it is difficult to place the type species based on morphology alone, it appears from its original description to be morphologically and ecologically (as a parasite of adults) similar to species of Hymenostilbe found in the ‘O. sphecocephala’ clade and not Hirsutella of the ‘O. unilateralis group’ (Patouillard 1892). Another reason for suppressing the use of Hirsutella for this clade is the larger number of new combinations that would have to be made – 178 for Ophiocordyceps vs. 77 for Hirsutella – as the vast majority of species encompassed here are currently described as Ophiocordyceps. Also, preservation of the name “cordyceps” within the name of O. sinensis is considered paramount given its economic, medicinal, and cultural importance in addition to being the most widely known and researched species in the clade (Shrestha et al. 2010).
At this time, we also propose to suppress the use of the other names proposed for taxa in this clade, including Hymenostilbe, Syngliocladium, and Paraisaria, because these names are younger, and they contain fewer associated taxa than either Ophiocordyceps or Hirsutella. Our results suggest the restriction of Hymenostilbe to the ‘O. sphecocephala clade’ (Fig. 1, Node 6) which occupies a long branch and has strong support (MLBP=100), however, because the other internal nodes of the clade do not receive support, we refrain from making this distinction now as it would result in a paraphyletic Ophiocordyceps. These analyses place one species of Stilbella, S. buquetii, in this clade, while other studies (Seifert 1985, Gräfenhan et al. 2011) have placed other Stilbella species in Nectriaceae, Bionectriaceae, or Polycephalomyces, and the current placement of Stilbella remains Hypocreales incertae sedis (Kirk et al. 2008). The type of Stilbella, a coprophile, has yet to be considered in a phylogenetic context, and for these reasons we do not address that name here, but reject the use of that name for this clade. Therefore, we propose to protect Ophiocordyceps as the genus name for the entire clade, while acknowledging that future studies including more data and taxonomic sampling may provide better resolution of the relationships within the genus and a narrower concept of Ophiocordyceps.
Tolypocladium W. Gams 1971
Tolypocladium is proposed for protection over the other two generic names in the clade, Elaphocordyceps and Chaunopycnis. The clade itself is well supported (MLBP=97) in this and other published analyses (Sung et al. 2007, Kepler et al. 2013). However, relationships between species in this clade are very sensitive to taxon sampling, and there is little bootstrap support for internal branches from the current data to justify more than one name for this clade. The asexual-sexual morph connection between Tolypocladium and some Elaphocordyceps species has been known for several years (Hodge et al. 1996), although where known most Elaphocordyceps spp. do not possess the morphology associated with Tolypocladium (Sung et al. 2007). While this may cause some short-term confusion, the alternative would be to name the clade Elaphocordyceps (which would cause the fewest name changes, 12 vs. 26 for Tolypocladium) and suppress Tolypocladium, a much more widely known, medicinally important, and older name, and therefore we find this a poor option. In this analysis the Chaunopycnis species sampled form a monophyletic clade which is the most divergent group within the clade. However, this may be the result of limited taxon and genetic sampling; only small subunit rDNA data for the sampled Chaunopycnis species was available for these analyses.
Here, we present a list of 26 new combinations within the genus Tolypocladium, which we emend to include species whose anamorphic forms do not possess inflated phialide bases, but that do form a single monophyletic clade encompassing a large number of truffle parasites, several insect pathogens, rotifer pathogens, and several fungi isolated to date only from soil.
Tolypocladium W. Gams, Persoonia 6: 185 (1971).
Synonyms: Chaunopycnis W. Gams, Persoonia 11: 75 (1980).
Elaphocordyceps G.H. Sung & Spatafora, Stud. Mycol. 57: 36 (2007).
Circumscription: The genus Tolypocladium is emended here to apply to all descendants of the node defined in the reference phylogeny (Fig. 1) as the terminal Tolypocladium clade. It is the least inclusive clade containing T. album, T. capitatum, T. cylindrosporum, T. fractum, T. inflatum, T. japonicum, T. longisegmentum, T. ophioglossoides, and T. pustulatum. No definitive synapomorphies are known for the clade. Morphologies associated with sexual reproductive states include robust stipitate stroma with clavate to capitate clava (e.g.T. capitatum) to highly reduced stroma comprising rhizomorphs and aggregated perithecia (e.g. T. inflatum); perithecia may be immersed and ordinal to the long axis of the stroma or superficial and produced on a highly reduced stromatic pad; asci are single-walled, long and cylindrical with a pronounced apical cap; ascospores are filiform, approximately as long as asci, septate and typically disarticulate into part-spores. Where known, asexual states include morphologies described as Tolypocladium sensu Gams (1970), Chaunopycnis sensu Gams (1979), or verticillium-like. Ecologies include parasites and pathogens of insects, rotifers and fungi, as well as, soil-inhabiting.
Type: Tolypocladium inflatum W. Gams 1971.
Tolypocladium inflatum W. Gams, Persoonia 6: 185 (1971), nom. cons.
Synonyms: Cordyceps subsessilis Petch, Trans. Brit. Mycol. Soc. 21: 39 (1937).
Elaphocordyceps subsessilis (Petch) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Cordyceps facis Kobayasi & Shimizu, Trans. Mycol. Soc. Japan 23: 361 (1982); as ‘Codyceps’.
Tolypocladium album (W. Gams) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808698
Basionym: Chaunopycnis alba W. Gams, Persoonia 11: 75 (1979).
Tolypocladium capitatum (Holmsk.: Fr.) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808699
Basionym: Clavaria capitata Holmsk., Beata Ruris Otia Fung. Dan. 1: 38 (1790).
Synonyms: Sphaeria capitata (Holmsk.: Fr.) Pers., Comm. Fung. Clav.: 13 (1797): Fr., Syst. Mycol. 2: 324 (1822).
Cordyceps capitata (Holmsk.: Fr.) Link, Handb. Erk. Gew. 3: 347 (1833).
Torrubia capitata (Holmsk.: Fr.) Tul. & C. Tul., Sel. Fung. Carpol. 3: 22 (1865).
Elaphocordyceps capitata (Holmsk.: Fr.) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Sphaeria agariciformis Bolton, Hist. Fung. Halifax: 130 (1789).
Cordyceps agariciformis (Bolton) Seaver, N. Amer. Fl. 3: 53 (1910).
Cordyceps canadensis Ellis & Everh., Bull. Torrey Bot. Club 25: 501 (1898).
Cordyceps capitata var. canadensis (Ellis & Everh.) Lloyd, Mycol. Writ. 5: 609 (1916).
Cordyceps nigriceps Peck, Bull. Torrey Bot. Club 27: 21 (1900).
Tolypocladium delicatistipitatum (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808700
Basionym: Cordyceps delicatistipitata Kobayasi,Bull. Natn. Sci. Mus., Tokyo 5 (2, no. 47): 79 (1960); as ‘delicatostipitata’.
Synonym: Elaphocordyceps delicatistipitata (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium fractum (Mains) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808701
Basionym: Cordyceps fracta Mains, Bull. Torrey. Bot. Club 84: 250 (1957).
Synonym: Elaphocordyceps fracta (Mains) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium inegoense (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808702
Basionym: Cordyceps inegoensis Kobayasi, Bull. Natn. Sci. Mus., Tokyo 6: 292 (1963
Synonyms: Elaphocordyceps inegoensis (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 37 (2007); as ‘inegoënsis’.
Tolypocladium intermedium (S. Imai) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808703
Basionym: Cordyceps intermedia S. Imai, Proc. Imp. Acad. Japan 10: 677 (1934).
Synonyms: Elaphocordyceps intermedia (S. Imai) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium intermedium f. michinokuense (Kobayasi & Shimizu) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808704
Basionym: Cordyceps intermedia f. michinokuensis Kobayasi & Shimizu, Bull. Natn. Sci. Mus., Tokyo, B 8: 116 (1982).
Synonym: Elaphocordyceps intermedia f. michinokuensis (Kobayasi & Shimizu) G.H. Sung et al., Stud. Mycol. 57: 37 (2007); as ‘michinokuënsis’.
Tolypocladium japonicum (Lloyd) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808705
Basionym: Cordyceps japonica Lloyd, Mycol. Writ. 6 (Letter 62): 913 (1920).
Synonyms: Elaphocordyceps japonica (Lloyd) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Cordyceps umemurae S. Imai, Trans. Sapporo Nat. Hist. Soc. 11: 32 (1930) [1929]; as ‘umemurai’.
Tolypocladium jezoense (S. Imai) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808706
Basionym: Cordyceps jezoensis S. Imai, Trans. Sapporo Nat. Hist. Soc. 11: 33 (1930) [1929]. Synonym: Elaphocordyceps jezoensis (S. Imai) G.H. Sung et al., Stud. Mycol. 57: 37 (2007); as ‘jezoënsis’.
Tolypocladium longisegmentum (Ginns) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808856
Basionym: Cordyceps longisegmentis Ginns, Mycologia 80: 219 (1988).
Synonym: Elaphocordyceps longisegmentis (Ginns) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium minazukiense (Kobayasi & Shimizu) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808857
Basionym: Cordyceps minazukiensis Kobayasi & Shimizu, Bull. Natn. Sci. Mus., Tokyo, B 8: 117 (1982).
Synonym: Elaphocordyceps minazukiensis (Kobayasi & Shimizu) G.H. Sung et al., Stud. Mycol. 57: 37 (1982).
Tolypocladium miomoteanum (Kobayasi & Shimizu) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808858
Basionym: Cordyceps miomoteana Kobayasi & Shimizu, Bull. Natn. Sci. Mus., Tokyo, B 8: 118 (1982).
Synonym: Elaphocordyceps miomoteana (Kobayasi & Shimizu) G.H. Sung et al., Stud. Mycol. 57: 37 (1982).
Tolypocladium ophioglossoides (Ehrh. ex J.F. Gmel.) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808859
Basionym: Sphaeria ophioglossoides Ehrh. ex J.F. Gmel., Syst. Na., 13th edn 2: 1474 (1792).
Synonyms: Sphaeria ophioglossoides Ehrh., Pl. Crypt. Exs. fasc. 16 no. 160 (1789); nom. inval. (Art. 38.1).
Cordyceps ophioglossoides (Ehrh. ex G.F. Gmel.) Link, Handb. Erk. Gew. 3: 347 (1833): Fr., Syst. Mycol. 2: 324 (1822).
Torrubia ophioglossoides (Ehrh. ex G.F. Gmel.) Tul. & C. Tul., Sel. Fung. Carp. 3: 20 (1865).
Elaphocordyceps ophioglossoides (Ehrh. ex G.F. Gmel.) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Clavaria parasitica Willd., Fl. Berol. Prodr.: 405 (1787).
Cordyceps parasitica (Willd.) Henn., Nerthus 6: 4 (1904).
Tolypocladium ophioglossoides f. album (Kobayasi & Shimizu ex Y.J. Yao) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808860
Basionym: Cordyceps ophioglossoides f. alba Kobayasi & Shimizu ex Y.J. Yao, Acta Mycol. Sin. 14: 257 (1995).
Synonym: Elaphocordyceps ophioglossoides f. alba (Koba-yasi & Shimizu ex Y.J. Yao) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium ophioglossoides f. cuboides (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808861
Basionym: Cordyceps ophioglossoides f. cuboides Kobayasi, Bull. Natn. Sci. Mus., Tokyo 5 (2, no. 47): 77 (1960).
Synonym: Elaphocordyceps ophioglossoides f. cuboides (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium ovalisporum (C. Möller & W. Gams) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808862
Basionym: Chaunopycnis ovalispora C. Möller & W. Gams, Mycotaxon 48: 442 (1993).
Tolypocladium paradoxum (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808863
Basionym:Cordyceps paradoxa Kobayasi, Bulletin of the Biogeogr. Soc. Jap. 9: 156 (1939).
Synonym: Elaphocordyceps paradoxa (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium pustulatum (Bills et al.) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808864
Basionym:Chaunopycnis pustulata Bills et al., Mycol. Progr. 1: 8 (2002).
Tolypocladium ramosum (Teng) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808865
Basionym: Cordyceps ramosa Teng, Sinensia 7: 810 (1936).
Synonym: Elaphocordyceps ramosa (Teng) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium rouxii (Cand.) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808866
Basionym: Cordyceps rouxii Cand., Mycotaxon 4: 544 (1976).
Synonym: Elaphocordyceps rouxii (Cand.) G.H. Sung et al., Stud. Mycol. 57: 37 (2007).
Tolypocladium szemaoense (M. Zang) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808867
Basionym:Cordyceps szemaoensis M. Zang, Acta Bot. Yunn. 23: 295 (2001).
Synonym: Elaphocordyceps szemaoensis (M. Zang) G.H. Sung et al., Stud. Mycol. 57: 38 (2007); as ‘szemaoënsis’.
Tolypocladium tenuisporum (Mains) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808868
Basionym: Cordyceps tenuispora Mains, Bull. Torrey Bot. Club 84: 247 (1957).
Synonym: Elaphocordyceps tenuispora (Mains) G.H. Sung et al., Stud. Mycol. 57: 38 (2007).
Tolypocladium toriharamontanum (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808869
Basionym: Cordyceps toriharamontana Kobayasi, Bull. Natn. Sci. Mus., Tokyo 6: 305 (1963).
Synonym: Elaphocordyceps toriharamontana (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 38 (2007).
Tolypocladium valliforme (Mains) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808870
Basionym: Cordyceps valliformis Mains, Bull. Torrey Bot. Club 84: 250 (1957).
Synonym: Elaphocordyceps valliformis (Mains) G.H. Sung et al., Stud. Mycol. 57: 38 (2007).
Tolypocladium valvatistipitatum (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808871
Basionym: Cordyceps valvatistipitata Kobayasi, Bull. Natn. Sci. Mus., Tokyo 5(2, no. 47): 81 (1960); as ‘valvatostipitata’.
Synonym: Elaphocordyceps valvatistipitata (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 38 (2007).
Tolypocladium virens (Kobayasi) Quandt, Kepler & Spatafora, comb. nov.
MycoBank MB808872
Basionym: Cordyceps virens Kobayasi, J. Jap. Bot. 58: 222 (1983).
Synonym: Elaphocordyceps virens (Kobayasi) G.H. Sung et al., Stud. Mycol. 57: 38 (2007).
Purpureocillium Luangsa-ard et al. 2011
Our findings support those reported by Luangsa-ard et al. (2011b) for the Purpureocillium clade, and the change in Art. 59 allows for the inclusion of N. atypicola (syn. Cordyceps cylindrica) and Isaria takamizusanensis within this genus. Shared characters for this clade include purple-hued conidia and pathogenesis of arthropods, although P. lilacinum and P. lavendulum have been cultured from various substrates (Perdomo et al. 2013), and P. lilacinum can cause keratitis and other mycoses in humans and other vertebrates (Pastor & Guarro 2006, Rodríguez et al. 2010). Because this genus is well supported (MLBP=76) as sister to the nematode pathogen clade (Fig. 1), it is important to mention that P. lilacinum is frequently collected from nematodes (Luangsa-ard et al. 2011b), and has been used in the biocontrol of plant pathogenic nematodes (Kalele et al. 2006, Castillo et al. 2013).
Harposporium Lohde 1874 and Drechmeria W. Gams & H.-B. Jansson 1985
Our analyses reconstruct a well-supported (MLBP=76) monophyletic origin of the mostly nematophagous clade of Ophiocordycipitaceae (Fig. 1 Node 2). Within this clade, there is strong phylogenetic support for two clades: one containing Harposporium and Podocrella, and the other consisting of Drechmeria, Haptocillium, and Cordyceps gunnii. The relationship between Harposporium and Podocrella has already been described (Chaverri et al. 2005), but the revision of Art. 59 requires that one name be chosen for this genus. Harposporium is an older name, and the morphology of at least somewhat crescent-shaped conidia is a shared character for this clade. Suppression of Podocrella also requires the fewest taxonomic revisions (3 vs 30). For these reasons, we propose to protect Harposporium over Podocrella (Table 2).
Within the other nematophagous subclade, Drechmeria is an older name than Haptocillium, and the isolate included in these analyses is nested within the Haptocillium isolates sampled. For this reason, we propose to protect Drechmeria over Haptocillium. The inclusion of C. gunnii in this clade also provides a name for this residual taxon of Cordyceps. Most species however, are nematophagous (C. gunnii being the exception), and conidia may be cone-shaped, formed on conidiogenous cells in rosettes or verticils, or in the case of C. gunnii, paecilomyces-like. We did not have access to molecular data from D. harposporioides, but given our finding that the two nematophagous clades in Ophiocordycipitaceae are monophyletic in origin, it will be interesting to see if this species, a protozoan pathogen with helical conidia, is truly a member of the Drechmeria clade or in fact a species within Harposporium that simply lacks the basally swollen conidiogenous cells.
Polycephalomyces Kobayasi 1941
This study is the first to have definitive ML support (MLBP=82) for the sister relationship between the Polycephalomyces clade and Ophiocordycipitaceae (Fig. 1 Node 1). Support for this relationship remains even with the exclusion of C. pleuricapitata, which is on an early-diverging, long branch within the clade. Two options remain to deal with this finding. Either a new family must be erected to account for this clade, or Polycephalomyces and related taxa must be moved into Ophiocordycipitaceae. We propose to accept Polycephalomyces and C. pleuricapitata in Ophiocordycipitaceae, where it will be the earliest diverging lineage of the family. The taxonomy of C. pleuricapitata will be addressed elsewhere.
CONCLUSIONS
We present a concise, thorough, phylogenetically relevant, and taxonomically accurate revision of the family Ophiocordycipitaceae with the aim of complying with the changes to Art. 59 of the ICN. With the criteria of naming monophyletic taxa, and where possible, of adhering to priority while avoiding changes that would be disruptive to the wider community of researchers, we have proposed to protect six genera within Ophiocordycipitaceae, including incorporation of the genus Polycephalomyces within the family. We have also formally revised the genus Tolypocladium, to reflect the nomenclature suggested by our results.
Acknowledgments
We would like to acknowledge the following individuals for their contribution to this manuscript: Priscila Chaverri, Mingjun Chen, Tsuyoshi Hosoya, Jae-Mo Sung, and James White. An NSF Graduate Research Fellowship supported C.A.Q.
REFERENCES
- Barron GL. (1977) The Nematode-destroying Fungi. [Topics in Mycobiology no. 1.] Guelph, ON: Canadian Biological Publications [Google Scholar]
- Barron GL. (1980) Fungal parasites of rotifers: a new Tolypocladium with underwater conidiation. Canadian Journal of Botany 58: 439–442 [Google Scholar]
- Barron GL. (1981) Two new fungal parasites of bdelloid rotifers. Canadian Journal of Botany 59: 1449–1455 [Google Scholar]
- Barron GL. (1983) Structure and biology of a new Tolypocladium attacking bdelloid rotifers. Canadian Journal of Botany 61: 2566–2569 [Google Scholar]
- Barron GL, Szijarto E. (1982) A new hyphomycete parasitic on the ciliated protozoans Vorticella and Opercularia. Canadian Journal of Botany 60: 1031–1034 [Google Scholar]
- Bills GF, Polishook JD, Goetz MA, Sullivan RF, White JF., Jr. (2002) Chaunopycnis pustulata sp. nov., a new clavicipitalean anamorph producing metabolites that modulate potassium ion channels. Mycological Progress 1: 3–17 [Google Scholar]
- Bissett J. (1983) Notes on Tolypocladium and related genera. Canadian Journal of Botany 61: 1311–1329 [Google Scholar]
- Boonmee S, Zhang Y, Chomnunti P, Chukeatirote E, Tsui CKM, Bahkali AH, Hyde KD. (2011) Revision of lignicolous Tubeufiaceae based on morphological reexamination and phylogenetic analysis. Fungal Diversity 51: 63–102 [Google Scholar]
- Bushley KE, Li Y, Wang WJ, Wang XL, Jiao L, Spatafora JW, Yao YJ. (2013a). Isolation of the MAT1-1 mating type idiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biology 117: 599–610 [DOI] [PubMed] [Google Scholar]
- Bushley KE, Raja R, Jaiswal P, Cumbie JS, Nonogaki M, Boyd AE, Owensby CA, Knaus BJ, Elser J, Miller D, Di Y, McPhail KL, Spatafora JW. (2013b) Draft genome sequence of the Cyclosporin producing fungus Tolypocladium inflatum reveals complex patterns of secondary metabolite evolution and expression. PLoS Genetics 9: e1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon PF, Hywel-Jones NL, Maczey N, Norbu L, Samdup T, Lhendup P. (2009) Steps towards sustainable harvest of Ophiocordyceps sinensis in Bhutan. Biodiversity and Conservation 18: 2263–2281 [Google Scholar]
- Castillo JD, Lawrence KS, Kloepper JW. (2013) Biocontrol of the reniform nematode by Bacillus firmus GB-126 and Paecilomyces lilacinus 251 on cotton. Plant Disease 97: 967–976 [DOI] [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ, Hodge KT. (2005) The genus Podocrella and its nematode-killing anamorph Harposporium. Mycologia 97: 433–443 [DOI] [PubMed] [Google Scholar]
- Drechsler C. (1941) Some hyphomycetes parasitic on free-living terricolous nematodes. Phytopathology 31: 773–802 [Google Scholar]
- Dreyfuss M, Gams W. (1994) Proposal to reject Pachybasium niveum Rostr. in order to retain the name Tolypocladium inflatum W. Gams for the fungus that produces cyclosporin. Taxon 43: 660–661 [Google Scholar]
- Evans HC, Samson RA. (1982) Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems 1. The Cephalotes (Myrmicinae) complex. Transactions of the British Mycological Society 79: 431–453 [Google Scholar]
- Evans HC, Shah PA. (2002) Taxonomic status of the genera Sorosporella and Syngliocladium associated with grasshoppers and locusts (Orthoptera: Acridoidea) in Africa. Mycological Research 106: 737–744 [Google Scholar]
- Evans HC, Whitehead PF. (2005) Entomogenous fungi of arboreal Coleoptera from Worcestershire, England, including the new species Harposporium bredonense. Mycological Progress 4: 91–99 [Google Scholar]
- Evans HC, Groden E, Bischoff JF. (2010) New fungal pathogens of the red ant Myrmica rubra from the UK and implications for ant invasions in the USA. Fungal Biology 114: 451–466 [DOI] [PubMed] [Google Scholar]
- Gams W. (1971) Tolypocladium, eine Hyphomycetengattung mit geschwollenen Phialiden. Persoonia 6: 185–191 [Google Scholar]
- Gams W. (1980) Chaunopycnis alba, gen. et sp. nov., a soil fungus intermediate between Moniliales and Sphaeropsidales. Persoonia 11: 75–79 [Google Scholar]
- Gams W, Jansson H-B. (1985) The nematode parasite Meria coniospora Drechsler in pure culture and its classification. Mycotaxon 22: 33–38 [Google Scholar]
- Gams W, Hodge KT, Samson RA, Korf RP, Seifert KA. (2005) (1684) Proposal to conserve the name Isaria (anamorphic fungi) with a conserved type. Taxon 54: 537 [Google Scholar]
- Gams W, Zare R. (2001) A revision of Verticillium section Prostrata. III. Generic classification. Nova Hedwigia 72: 329–337 [Google Scholar]
- Gams W, Zare R. (2003) A taxonomic review of the clavicipitaceous anamorphs parasitizing nematodes and other microinvertebrates. In: Clavicipitalean Fungi: evolutionary biology, chemistry, biocontrol and cultural Impacts (White JF, jr., Bacon CW, Hywel-Jones NL, Spatafora JW, eds): 17–73 New York: Marcel Dekker [Google Scholar]
- Geiser DM, Aoki T, Bacon CW, Baker SE, Bhattacharyya MB, et al. (2013) One Fungus, One Name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 103: 400–408 [DOI] [PubMed] [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, Seifert KA. (2011) An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Taylor JW. (2013) Fungal systematics: is a new age of enlightenment at hand? Nature Reviews Microbiology 11: 129–133 [DOI] [PubMed] [Google Scholar]
- Hodge KT, Gams W, Samson RA, Korf RP, Seifert KA. (2005) Lectotypification and status of Isaria Pers.: Fr. Taxon 54: 485–489 [Google Scholar]
- Hodge KT, Humber RA, Wozniak CA. (1998) Cordyceps variabilis and the genus Syngliocladium. Mycologia 90: 743–753 [Google Scholar]
- Hodge KT, Krasnoff SB, Humber RA. (1996) Tolypocladium inflatum is the anamorph of Cordyceps subsessilis. Mycologia 88: 715–719 [Google Scholar]
- Hodge KT, Viaene NM, Gams W. (1997) Two Harposporium species with Hirsutella synanamorphs. Mycological Research 101: 1377–1382 [Google Scholar]
- Hu X, Zhang Y, Xiao G, Zheng P, Xia Y, Zhang X, St. Leger RJ, Liu X, Wang C. (2013) Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chinese Science Bulletin 58: 2846–2854 [Google Scholar]
- Hughes KW, Petersen RH, Johnson JE, Moncalvo JM, Vilgalys R, Redhead SA, Thomas T, McGhee LL. (2001) Infragenic phylogeny of Collybia s. str. based on sequences of ribosomal ITS and LSU regions. Mycological Research 105: 164–172 [Google Scholar]
- Hywel-Jones NL, Sivichai S. (1995) Cordyceps cylindrica and its association with Nomuraea atypicola in Thailand. Mycological Research 99: 809–812 [Google Scholar]
- Kalele DN, Affokpon A, Coosemans J. (2006) Efficacy of Paecilomyces lilacinus strain 251 against root knot nematodes in tomato under greenhouse conditions. Communications in Agricultural and Applied Biological Sciences 72: 209–213 [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Kepler RM, Ban S, Nakagiri A, Bischoff J, Hywel-Jones N, Owensby CA, Spatafora JW. (2013) The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: an application of One Fungus One Name. Fungal Biology 117: 611–622 [DOI] [PubMed] [Google Scholar]
- Kepler RM, Humber RA, Bischoff JF, Rehner SA. (2014) Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia doi:10.3852/13-319. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. (eds) (2008) Ainsworth & Bisby’s Dictionary of the Fungi. 10th edn Wallingford: CAB International [Google Scholar]
- Kobayasi Y. (1941) The genus Cordyceps and its allies. Science Reports of the Tokyo Bunrika Daigaku, sect. B, 84 (5): 53–260 [Google Scholar]
- Leuchtmann A, Bacon CW, Schardl CL, White JF, jr, Tadych M. (2014) Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia doi:10.3852/13-251. [DOI] [PubMed] [Google Scholar]
- Li X, Luo H, Zhang K. (2005) A new species of Harposporium parasitic on nematodes. Canadian Journal of Botany 83: 558–562 [Google Scholar]
- Luangsa-ard JJ, Houbraken J, van Doorn T, Hong S-B, Borman AM, Hywel-Jones NL, Samson RA. (2011b) Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiology Letters 321: 141–149 [DOI] [PubMed] [Google Scholar]
- Luangsa-ard JJ, Ridkaew R, Tasanathai K, Thanakitpipattana D, Hywel-Jones NL. (2011a) Ophiocordyceps halabalaensis: a new species of Ophiocordyceps pathogenic to Camponotus gigas in Hala Bala Wildlife Sanctuary, Southern Thailand. Fungal Biology 115: 608–614 [DOI] [PubMed] [Google Scholar]
- Mains EB. (1950) Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycologia 42: 566–588 [Google Scholar]
- McNeill J. (2012) Guidelines for requests for binding decisions on application of the Code. Taxon 61: 477–478 [Google Scholar]
- Möller C, Gams W. (1993) Two new hyphomycetes isolated from Antarctic lichens. Mycotaxon 48: 441–450 [Google Scholar]
- Pastor FJ, Guarro J. (2006) Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clinical Microbiology and Infection 12: 948–960 [DOI] [PubMed] [Google Scholar]
- Patouillard NT. (1892) Une Clavariée entomogène. Revue Mycologique 14: 67–70 [Google Scholar]
- Perdomo H, Cano J, Gené J, García D, Hernández M, Guarro J. (2013) Polyphasic analysis of Purpureocillium lilacinum isolates from different origins and proposal of the new species Purpureocillium lavendulum. Mycologia 105: 151–161 [DOI] [PubMed] [Google Scholar]
- Petch T. (1931a) Notes on entomogenous fungi. Transactions of the British Mycological Society 16: 55–75 [Google Scholar]
- Petch T. (1931b) New species of Cordyceps, collected during the Whitby foray. The Naturalist, Hull 1931: 101–103 [Google Scholar]
- Petch T. (1932) A list of the entomogenous fungi of Great Britain. Transactions of the British Mycological Society 17: 170–178 [Google Scholar]
- Petch T. (1933) Notes on entomogenous fungi. Transactions of the British Mycological Society 18: 48–75 [Google Scholar]
- Ren SY, Yao YJ. (2013) Evaluation of nutritional and physical stress conditions during vegetative growth on conidial production and germination in Ophiocordyceps sinensis. FEMS Microbiology Letters 346: 29–35 [DOI] [PubMed] [Google Scholar]
- Rodríguez MM, Pastor FJ, Serena C, Guarro J. (2010) Efficacy of voriconazole in a murine model of invasive paecilomycosis. International Journal of Antimicrobial Agents 35: 362–365 [DOI] [PubMed] [Google Scholar]
- Rossman AY. (1978) Podonectria, a genus in the Pleosporales on scale insects. Mycotaxon 7: 163–182 [Google Scholar]
- Rossman AY, Seifert KA, Samuels GJ, Minnis AM, Schroers H-J, Lombard L, Crous PW, Põldmaa K, Cannon PF, Summerbell RC, Geiser DM, Zhuang W, Hirooka Y, Herrera C, Salgado-Salazar C, Chaverri P. (2013) Genera in Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales) proposed for acceptance or rejection. IMA Fungus 4: 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RA, Brady BL. (1983) Paraisaria, a new genus for Isaria dubia, the anamorph of Cordyceps gracilis. Transactions of the British Mycological Society 81: 285–290 [Google Scholar]
- Samson RA, Soares GG., jr (1984) Entomopathogenic species of the hyphomycete genus Tolypocladium. Journal of Invertebrate Pathology 43: 133–139 [Google Scholar]
- Seifert KA. (1985) A monograph of Stilbella and some allied hyphomycetes. Studies in Mycology 27: 1–234 [Google Scholar]
- Shimazu M, Glockling SL. (1997) A new species of Harposporium with two spore types isolated from the larva of a cerambycid beetle. Mycological Research 101: 1371–1376 [Google Scholar]
- Shrestha B, Bawa KS. (2013) Trade, harvest, and conservation of caterpillar fungus (Ophiocordyceps sinensis) in the Himalayas. Biological Conservation 159: 514–520 [Google Scholar]
- Shrestha B, Zhang WM, Zhang YJ, Liu XZ. (2010) What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? Mycology 1: 228–236 [Google Scholar]
- Speare AT. (1917) Sorosporella uvella and its occurrence in cutworms in America. Journal of Agricultural Research 8: 189–194 [Google Scholar]
- Speare AT. (1920) Further studies of Sorosporella uvella, a fungous parasite of noctuid larvae. Journal of Agricultural Research 18: 399–439 [Google Scholar]
- Stalpers JA, Seifert KA, Samson RA. (1991) A revision of the genera Antromycopsis, Sclerostilbum, and Tilachlidiopsis (hyphomycetes). Canadian Journal of Botany 69: 6–15 [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Stone R. (2008) Last stand for the body snatcher of the Himalayas? Science 322: 1182. [DOI] [PubMed] [Google Scholar]
- Sung G-H, Spatafora JW, Zare R, Hodge KT, Gams W. (2001) A revision of Verticillium sect. Prostrata. II. Phylogenetic analyses of SSU and LSU nuclear rDNA sequences from anamorphs and teleomorphs of the Clavicipitaceae. Nova Hedwigia 72: 311–328 [Google Scholar]
- Sung G-H, Hywel-Jones NL, Sung J-M, Luangsa-ard JJ, Shrestha B, Spatafora JW. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Survase SA, Kagliwal LD, Annapure US, Singhal RS. (2011) Cyclosporin A - A review on fermentative production, downstream processing and pharmacological applications. Biotechnology Advances 29: 418–435 [DOI] [PubMed] [Google Scholar]
- Talavera G, Castresana J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56: 564–577 [DOI] [PubMed] [Google Scholar]
- Traber R, Dreyfuss MM. (1996) Occurrence of cyclosporins and cyclosporin-like peptolides in fungi. Journal of Industrial Microbiology 17: 397–401 [Google Scholar]
- Vuillemin P. (1896) Les Hypostomacées, nouvelle famille de champignons parasites. Bulletin de la Société des Sciences de Nancy, sér. 2 14: 15–67 [Google Scholar]
- Weiser J, Matha V, Jegorov A. (1991) Tolypocladium terricola sp. n., a new mosquito-killing species of the genus Tolypocladium Gams (hyphomycetes). Folia Parasitologica 38: 363–369 [PubMed] [Google Scholar]
- Zare R, Gams W, Culham A. (2000) A revision of Verticillium sect. Prostrata. I. Phylogenetic studies using ITS sequences. Nova Hedwigia 71: 465–480 [Google Scholar]
- Zare R, Gams W. (2001) A revision of Verticillium section Prostrata. VI. The genus Haptocillium. Nova Hedwigia 73: 271–292 [Google Scholar]