Abstract
To characterize daily variation of amino acids (AAs) and acylcarnitines (ACs) in response to feeding and activity, we measured serum metabolites at various times and after various activities during the day. Subjects were admitted overnight for serial serum sampling, collected in the evening (6–8pm, n=40), before rising from bed or eating (8AM, n=40), 1 hour after rising but before eating (9 AM, n=20), 1–2 hours after rising and breakfast (9–10 AM, n=40), and at noon (12 PM, n=20). Measurements of 15 AAs and 45 ACs were performed by quantitative tandem mass spectrometry using stable-isotope dilution. Coefficients of variation within and between patients were calculated for individual metabolite values and factors derived from principal components analysis. The change of state between timepoints was evaluated by nearest neighbor non-parametric analysis of values at one timepoint compared to the next subsequent value. Relative to baseline AM recumbent concentrations, AA concentrations rose after activity and feeding while AC concentrations rose after activity and decreased with feeding. Furthermore, for all AAs, ACs, and their factors, biological variation was quantifiably evident and distinct from daily variation. This study confirms the daily variation of AAs and provides the first report of daily variation for a large panel of ACs. Although standardization of sample collection is highly desirable to control for daily variation (within a subject due to activity or feeding), this study demonstrated measurable biological variability (across subjects) suggesting that non-standardized sample collections could potentially provide insights into specific AA and AC metabolic pathways and disease mechanisms.
Keywords: daily variation, acylcarnitines, amino acids, metabolomics, biomarkers
1 Introduction
Metabolomics as a global profiling tool has expanded greatly in recent years, with investigators from research fields such as diabetes, cancer and infectious disease using this approach in applications ranging from generating hypotheses to dissecting molecular mechanisms (Bain et al. 2009; Oakman et al. 2011; Vinayavekhin et al. 2010). The characterization of amino acids and acylcarnitines has proved especially valuable in the screening of newborns for inborn errors of metabolism (Millington and Stevens, 2011; De Jesus et al. 2010) Much of the strength of the findings in these and future studies relies on detecting subtle changes in the concentrations of metabolites, many of which are present at extremely low levels. Assessing the significance of these differences can be difficult, particularly against multiple background sources of variability. In this study we sought to characterize the daily variability due to activity and feeding across a range of metabolites of interest, namely a panel of amino acids and acylcarnitines, with the long-term goal of informing the interpretation of results from other studies with varying sample collection times and protocols.
Amino acids (AAs) are the agents of protein metabolism, while acylcarnitines (ACs) are derived from oxidation of a variety of metabolic fuels, including prominently, β-oxidation of fatty acids (FAs). Acyl carnitines are formed to facilitate transport of long-chain fatty acids into mitochondria. They are also formed by equilibration of mitochondrial acyl CoAs with their cognate acylcarnitines through the action of carnitine acyltransferase enzymes. While often treated as separate pathways, AA and FA circuits are increasingly being considered in tandem, for example with respect to cardiovascular disease (Shah et al. 2009; Shah et al. 2010), and for development of biomarkers and mechanistic insights in diabetes and metabolic syndrome (Newgard et al. 2010).
Metabolite levels can fluctuate in response to daily activity, including feeding (Feigin et al. 1967; Eriksson et al. 1989). As metabolic profiling is applied more widely, it is becoming possible to detect even subtle variation in the levels of numerous metabolites simultaneously. Consequently, to advance the biological understanding of metabolites, it is necessary to determine the extent of biological variation attributable to variation between individuals in contrast to the extent of daily variation within individuals, attributable to activity, rest, feeding, and circadian rhythms. For the purposes of this work, the term daily variation is used throughout to refer generally to all the sources of systematic variation within individuals during the usual events (fasting, feeding, activity, inactivity, circadian influences) of a typical day.
In order to ascertain the degree and direction of these changes in energy metabolism, and in anticipation of continuing investigation into the interaction of these agents, amino acids and acylcarnitines were measured at various time points during the day, including before and after physical activity and a mixed-nutrient meal. The primary goal of this study was to evaluate the potential daily variation of 15 AAs and 45 ACs, each aggregated separately into single factor scores, while the secondary analysis considered the daily variation of individual metabolites. We hypothesized that AA and AC factors derived from principal components analysis would provide a means of assessing the overall daily variation of these metabolic intermediates. Characterizing potential sources of variability of the metabolites will provide guidance for design of studies to assess biological and disease-related variation and facilitate differentiation of effects attributable to different health states or interventions from those due to daily and analytical or technical variation.
2 Materials and Methods
2.1 Participants
Participants in this study were recruited through the Duke Rheumatology outpatient clinic and had knee OA as previously described (Criscione et al. 2005, Gordon et al. 2008). Patients were excluded for rheumatoid arthritis, systemic lupus erythematosus, psoriasis, gout, or hemachromatosis, and had no history, signs or symptoms of liver disease or cancer. Informed consent was obtained from all participants and all procedures were previously approved by the Institutional Review Board of Duke University Medical Center. A total of 40 subjects in two cohorts of 20 subjects each were admitted overnight to the General Clinical Research Center of Duke University to undergo serial serum sampling.
2.2 Serum Sampling
In the first cohort of 20 subjects, blood was collected in the evening after eating dinner (T3, 8 PM), before rising from bed or eating (T0, 8AM), 2 hours after rising and breakfast (T1, 10 AM), and at noon before eating lunch (T2, 12 PM). In the second cohort of 20 subjects, to discriminate between the effects of feeding and activity upon first rising in the morning, blood was collected at similar time points (T3, T0, T1) and, in addition, 1 hour after rising but before eating (T1A, 9 AM, n=20) instead of at noon. Because the study conditions were otherwise identical, the data were merged over the five timepoints: T0 n=40; T1a n=20; T1 n=40; T2 n=20; T3 n=40. Morning activity consisted of the usual activities of daily living, and individuals were asked to be seated for no longer than 30 minutes at a time. After blood collection, at each time point, 7.5 mL blood was collected and allowed to coagulate for thirty minutes. Serum was isolated, divided into aliquots, immediately frozen at −20°C, then transferred to −80°C for long-term storage. Subjects chose their meals from a standardized menu which included protein, carbohydrate, and fat. Breakfast items and amounts consumed were recorded, with the average breakfast consisting of 30.5 g protein, 60.8 g carbohydrates and 25.5 g fat. Participants were allowed to take all of their usual prescription medications, including non-steroidal anti-inflammatory drugs.
2.3 Metabolite Measurement and Statistical Analysis
Serum concentrations of 15 amino acids (AAs), and 45 acylcarnitines (ACs) were measured using a targeted, mass-spectrometry based platform as previously described (Newgard et al. 2009; Huffman et al. 2009). Specifically, amino acids and acylcarnitines were analyzed by flow injection electrospray ionization tandem mass spectrometry and quantified by isotope or pseudo-isotope dilution using methods (Wu et al., 2004; An et al., 2004; Millington and Stevens, 2011) based on those previously developed for fast ion bombardment tandem mass spectrometry (Millington and Chace, 1992; Chace et al., 1993). Briefly, plasma samples were spiked with a cocktail of heavy-isotope internal standards (Cambridge Isotope Laboratories, MA, USA; C-D-N, Isotopes, Canada) and deproteinated with methanol. The methanol supernatants were dried and esterified with either acidified methanol or butanol for acylcarnitine or amino acid analysis, respectively. Mass spectra for acylcarnitine and amino acid esters were obtained using precursor ion and neutral loss scanning methods, respectively. The spectra are acquired in a multi-channel analyzer (MCA) mode to improve signal-to-noise. The data were acquired using a Micromass Quattro Micro™ system equipped with a model 2777 autosampler, a model 1525 HPLC solvent delivery system and a data system controlled by MassLynx 4.0 operating system (Waters, Milford, MA). Ion ratios of analyte to respective internal standard computed from centroided spectra were converted to concentrations using calibrators constructed from authentic aliphatic acylcarnitines and amino acids (Sigma, MO, USA; Larodan Sweden) and Bovine Adult Serum (Sigma, MO, USA). Leucine and isoleucine are reported as a single analyte because they are not resolved by this MS/MS method. It should also be noted that the Leu/Ile values include contributions from allo-isoleucine and hydroxyproline. Under normal circumstances these isobaric amino acids contribute little to the signal attributed to Leu/Ile (Chace et al., 1995). In addition, the acidic conditions used to form butyl esters results in partial hydrolysis of glutamine to glutamic acid and of asparagine to aspartate. Accordingly, values reported as Glu/Gln or Asp/Asn are not meant to signify the molar sum of glutamate and glutamine, or of aspartate and asparagine, but rather measure the amount of glutamate or aspartate plus the contribution of the partial hydrolysis reactions of glutamine and asparagine.
We elected to define a practical LLOQ for these flow injection analyses as that concentration whereby repetitive measurement of a quality control sample gave CV greater than 15%. For AC and AA assays we determined that the CV rapidly increased for concentrations below 0.25 μM for acetyl carnitine, below 0.05 μM for the remaining acylcarnitines, below 5 μM for glycine and alanine, and below 1 μM for the remaining amino acids. Per the assay design, these levels correspond to approximately 5% of the relevant stable isotope-labeled internal standard employed in the assay. All measured concentrations of amino acids were above the practical LLOQ. However, the majority (58%) of the acylcarnitine concentrations were below the practical LLOQ. All values for acylcarnitines, including those below the practical LLOQ and zero values (194 of 7200 total measurements), were included in the statistical analyses to evaluate the daily variation, if any, of the minor metabolites.
Variability in this sample was assessed by calculating % coefficient of variation (%CV) as 100*standard deviation/mean. Total, or between-subject, variability (the combination of biological and daily variation) was calculated based on all values for all individuals and time points for each analyte. Within-subject (daily) variability was calculated based on concentrations over time for each individual.
To take advantage of the correlation between the measures of AAs and ACs and to reduce the Type-I error risks inherent in tests of so many measures, principal components analysis (PCA) was used to reduce the sets of 15 amino acids and 45 acylcarnitines each to a single AA or AC factor. Since the distribution of AAs and ACs demonstrated right skew, these measures were log-transformed. The factor scoring algorithm was developed on the baseline (T0) values only for a single factor separately for the AAs and ACs. At each subsequent timepoint, for each subject, the values were normalized to the baseline means and standard deviations, and the scoring algorithm applied to compute expected 1-factor AA and AC solutions. Thus, changes across time are expressed in units of standard deviation (SD) relative to this baseline. The Signed Rank test was used to evaluate if the change relative to baseline was significantly different from zero. Statistical analysis was performed using SAS v9.2 (SAS Institute, Cary, North Carolina).
Daily variation for each AA and AC analyte was assessed by calculating, for each subject, the ratio of the analyte concentration at each time point relative to its nearest previous time point, using log-transformed (ln) values. Median values are presented and the Signed Rank test was used to evaluate if the overall ratio between neighboring time points was significantly different from 1.0. No correction for multiple testing was employed for this exploratory analysis.
3 Results
3.1 Study Participants
The total study sample of 40 subjects included 65% females, 79.5% Caucasians and 22.5% African Americans. The mean (range, SD) age was 69 years (50–89, 9.8) and BMI was 32.8 kg/m2 (21.3 – 59.85, 8.9).
3.2 Serum Concentrations and Variability of Metabolites
Although amino acid concentrations varied widely between individuals, all measured concentrations were in reported physiological ranges (Eriksson et al. 1989, Shah et al. 2009, Redman et al. 2011) (Table 1). Concentrations of the 45 acylcarnitines also varied widely, were in reported physiological ranges (Shah et al. 2009, Redman et al. 2011) and were present at much lower concentrations than the amino acids (Table 2). For all 15 amino acids and 45 acylcarnitines, variation (expressed as %CV) between individuals was greater than variation within an individual. Variation was similar for short chain (containing aliphatic chains of less than 6 carbons), medium-chain (6–12 carbons), long-chain (greater than 12 carbons) and very long chain ACs (greater than 22 carbons).
Table 1.
Concentrations, variability, and contribution to the 1-factor solution of 15 amino acids.
| Amino Acid | Median (SD) Concentration* μM | %CV Between Individuals | Mean %CV (Range) Within Individuals | Mean %CV Analytical Variability | Factor Loading |
|---|---|---|---|---|---|
| 1. Glycine | 313.2 (73.4) | 23.0 | 10.9 (3.1 – 27.8) | 3.2 | 0.53 |
| 2. Alanine | 481.6 (104.8) | 21.5 | 14.4 (5.1 – 31.4) | 5.1 | 0.53 |
| 3. Serine | 116.6 (24.8) | 21.2 | 11.5 (1.9 – 27.5) | 7.2 | 0.49 |
| 4. Proline | 231.6 (63.5) | 26.9 | 17.9 (5.3 – 32.4) | 2.9 | 0.66 |
| 5. Valine | 275.4 (72.7) | 25.8 | 14.6 (5.7 – 27.4) | 6.2 | 0.68 |
| 6. Leucine/Isoleucine | 196.1 (62.4) | 30.2 | 21.6 (4.0 – 51.2) | 5.2 | 0.65 |
| 7. Methionine | 31.4 (8.4) | 26.4 | 21.9 (5.3–46.5) | 6.6 | 0.80 |
| 8. Histidine | 86.7 (20.0) | 22.5 | 10.2 (3.3 – 24.4) | 12.2 | 0.55 |
| 9. Phenylalanine | 76.2 (16.0) | 20.5 | 14.2 (4.4 – 26.1) | 3.0 | 0.75 |
| 10. Tyrosine | 82.7 (21.4) | 25.3 | 17.2 4.8 – 35.0) | 3.1 | 0.71 |
| 11. Aspartate/Asparagine | 21.4 (6.0) | 26.9 | 20.9 (7.4 – 65.8) | 8.1 | 0.23 |
| 12. Glutamate/Glutamine | 100.0 (27.5) | 26.5 | 14.2 (2.1 – 31.8) | 10.5 | 0.36 |
| 13. Ornithine | 77.5 (19.7) | 25.7 | 16.8 (2.1 – 49.5) | 7.6 | 0.48 |
| 14. Citrulline | 41.3 (13.6) | 32.1 | 13.9 (5.7 – 28.0) | 7.4 | 0.26 |
| 15. Arginine | 125.8 (26.6) | 20.9 | 13.1 (4.8 – 29.2) | 12.5 | 0.55 |
Variability was expressed as %CV, calculated as 100*SD/mean. Lower limits of quantification were 5 μM for glycine and alanine, and 1 μM for remaining amino acids. Variability between individuals, or total variability, was calculated based on all values (all subjects at all time points) obtained in the study. Variability within individuals was calculated based on determining each individual’s %CV over four sample collection time points. Analytical variability, or error attributable to the instrument, was determined by measuring two quality control mixtures (diluent spiked with high or low concentrations of each AA) each day before and after assaying samples. %CV=100*standard deviation/mean
Table 2.
Concentrations, variability, and contribution to the 1-factor solution of 45 acylcarnitines.
| Acylcarnitine | Median (SD) Concentration* nM | %CV Between Individuals | Mean %CV (Range)Within Individuals | Mean %CV Analytical Variability | Factor Loading |
|---|---|---|---|---|---|
| 1. Acetyl carnitine, C2 | 6811 (2797) | 38.0 | 20.5 (3.8–52.5) | 6.1 | 0.60 |
| 2. Propionyl carnitine, C3 | 452.6 (187.4) | 39.2 | 26.4 (8.2–61.1) | 7.9 | 0.07 |
| 3. Butyryl/Isobutyryl carnitine, C4/Ci4 | 246.4 (119.7) | 44.3 | 20.5 (7.6–38.7) | 8.8 | 0.27 |
| 4. Tiglyl carnitine, C5:1 | 84.8 (24.4) | 28.3 | 23.4 (7.8–42.3) | 0.10 | |
| 5. Isovaleryl/3-Methylebutyryl/2-Methylbutyryl carnitine, C5’s | 176.7 (77.1) | 40.7 | 29.2 (5.4–58.0) | 7.8 | 0.24 |
| 6. β-hydroxy butyryl carnitine, C4OH | 48.2 (30.2) | 57.3 | 40.4 (8.6–88.8) | 0.28 | |
| 7. Hexanoyl carnitine, C6 | 60.8 (57.8) | 93.0 | 92.6 (14.8–200) | 36.8 | −0.05 |
| 8. 3-Hydroxy-isovaleryl/Malonyl carnitine, C5OH/C3DC | 59.5 (27.6) | 45.8 | 33.0 (8.0–88.8) | 0.12 | |
| 9. Methylmalonyl/ Succinyl carnitine, Ci4DC/C4DC | 54.5 (19.4) | 35.2 | 23.3 (3.1–45.7) | 0.41 | |
| 10. Octenoyl carnitine, C8:1 | 283.0 (107.7) | 35.6 | 14.8 (4.2–27.4) | 0.56 | |
| 11. Octanoyl carnitine, C8 | 135.1 (101.0) | 62.0 | 43.7 (15.7–92.1) | 5.5 | 0.67 |
| 12. Glutaryl carnitine, C5DC | 44.7 (16.9) | 34.6 | 23.8 (6.1–68.6) | 0.40 | |
| 13. Adipoyl carnitine, C6DC | 47.2 (22.0) | 42.9 | 21.8 (4.4–48.3) | 0.51 | |
| 14. Decatrienoyl carnitine, C10:3 | 103.3 (50.5) | 45.0 | 17.0 (4.1–34.5) | 0.51 | |
| 15. Decadienoyl carnitine, C10:2 | 37.6 (18.0) | 43.5 | 29.3 (6.6–79.5) | 0.64 | |
| 16. Decenoyl carnitine, C10:1 | 150.6 (90.0) | 52.8 | 38.5 (9.1–86.0) | 0.77 | |
| 17. Decanoyl carnitine, C10 | 167.7 (126.5) | 61.7 | 46.4 (13.0–124.0) | 6.5 | 0.72 |
| 18. 3-Hydroxy-decanoyl/ Suberoyl carnitine, C10-OH/C8-DC | 31.0 (20.0) | 57.0 | 35.4 (12.3–90.6) | 0.60 | |
| 19. Dodecenoyl carnitine, C12:1 | 99.2 (58.8) | 52.5 | 39.3 (15.3–111.1) | 0.77 | |
| 20. Lauroyl carnitine, C12 | 78.0 (40.1) | 48.5 | 37.0 (15.1–85.7) | 7.6 | 0.84 |
| 21. 3-Hydroxy-dodecanoyl/ Sebacoyl carnitine, C12-OH/ C10-DC | 7.7 (5.7) | 63.5 | 41.9 (2.3–89.2) | 0.68 | |
| 22. Tetradecadienoyl carnitine, C14:2 (μM) | 33.8 (24.8) | 62.7 | 47.6 (16.3–109.1) | 0.79 | |
| 23. Tetradecenoyl carnitine, C14:1 | 66.6 (40.1) | 56.5 | 39.9 (12.3–118.8) | 0.83 | |
| 24. Myristoyl carnitine, C14 | 26.4 (11.4) | 41.9 | 33.2 (8.8–65.3) | 4.6 | 0.70 |
| 25. 3-Hydroxy-tetradecenoyl carnitine, C14:1-OH | 12.8 (6.0) | 42.5 | 30.2 (10.0–63.3) | 0.69 | |
| 26. 3-Hydroxy-tetradecanoyl/ Dodecanedioyl carnitine, C14-OH/C12-DC | 8.6 (6.0) | 60.6 | 40.4 (14.0–77.6) | 0.59 | |
| 27. Palmitoyl carnitine, C16 | 71.1 (18.9) | 25.6 | 18.3 (4.8–41.7) | 3.9 | 0.51 |
| 28. 3-Hydroxy-hexadecanoyl/ Tetradecanedioyl carnitine, C16-OH/C14-DC | 4.4 (8.9) | 148.4 | 41.1 (9.6–80.1) | 0.52 | |
| 29. Linoleyl carnitine, C18:2 | 60.8 (22.1) | 34.8 | 22.9 (77–42.5) | 0.60 | |
| 30. Oleyl carnitine, C18:1 | 121.4 (38.3) | 31.7 | 19.5 (3.3–42.6) | 0.75 | |
| 31. Stearoyl carnitine, C18 | 36.2 (11.5) | 31.1 | 20. (4.4–47.4)6 | 6.4 | 0.36 |
| 32. 3-Hydroxy-octadecenoyl carnitine, C18:1-OH | 6.1 (4.1) | 58.9 | 42.5 (12.6–12.7) | 0.50 | |
| 33. 3-Hydroxy-octadecanoyl/ Hexadecanedioyl carnitine, C18-OH/C16-DC | 6.8 (7.4) | 96.3 | 40.2 (11.9–107.7) | 0.54 | |
| 34. Arachidoyl carnitine, C20 | 5.6 (3.2) | 51.7 | 41.2 (8.3–88.3) | 0.05 | |
| 35. Octadecenedioyl carnitine, C18:1-DC | 7.7 (8.0) | 82.9 | 36.0 (7.3–82.9) | 0.54 | |
| 36. 3-Hydroxy-eicosanoyl/Octadecanedioyl carnitine, C20-OH/C18-DC | 8.1 (10.6) | 102.9 | 32.3(4.5–70.) | 0.50 | |
| 37. Docosanoyl carnitine, C22 | 4.3 (2.7) | 61.5 | 51.4 (9.7–114.4) | 0.08 | |
| 38. 3-Hydroxy-cis-5-octenoyl/ Hexenedioyl carnitine, C8:1-OH/C6:1-DC | 27.4 (12.6) | 41.7 | 29.5 (7.1–67.1) | 0.16 | |
| 39. Heptanedioyl carnitine, C7-DC | 0.0 (20.2) | 140.0 | 130.9 (39.6–200.0) | −0.06 | |
| 40. Octenedioyl carnitine, C8:1-DC | 36.2 (15.5) | 40.8 | 31.5 (11.8–71.3) | 0.59 | |
| 41. Hexadecadienoyl carnitine, C16:2 | 7.5 (5.5) | 64.8 | 50.3 (11.5–119.4) | 0.62 | |
| 42. Palmitoleoyl carnitine, C16:1 | 22.2 (11.2) | 47.1 | 36.0 (10.1–71.6) | 0.66 | |
| 43. 3-Hydroxy-palmitoleoyl/ cis-5-Tetradecenedioyl carnitine, C16:1-OH/C14:1-DC, | 6.4 (3.7) | 52.8 | 46.1 (11.4–91.1) | 0.45 | |
| 44. 3-Hydroxy-linoleyl carnitine, C18:2-OH | 7.7 (3.0) | 37.2 | 31.6 (10.3–71.0) | 0.26 | |
| 45. Arachidonoyl carnitine, C20:4 | 7.1 (3.4) | 45.0 | 32.7 (7.3–80.7) | 0.26 |
Variability was expressed as %CV, calculated as 100*SD/mean. Lower limits of quantification were 0.25 μM for AC1 (acetyl carnitine) and 0.05 μM for the remaining ACs. Variability between individuals, or total variability, was calculated based on all values (all subjects at all time points) obtained in the study. Variability within individuals was calculated based on determining each individual’s %CV over four sample collection time points. Analytical variability, or error attributable to the instrument, was determined by measuring two quality control mixtures (diluent spiked with high or low concentrations of 11 ACs) each day before and after assaying samples. %CV=100*standard deviation/mean.
3.3 Daily Variation of Metabolites
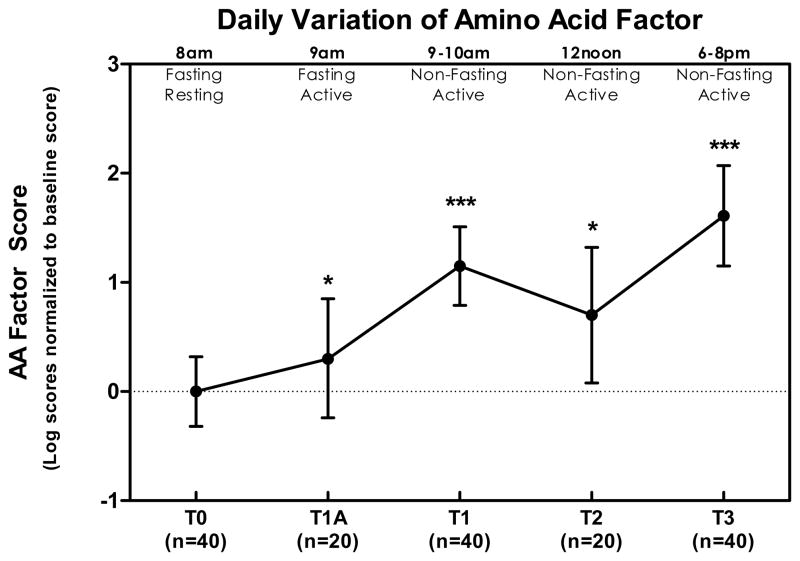
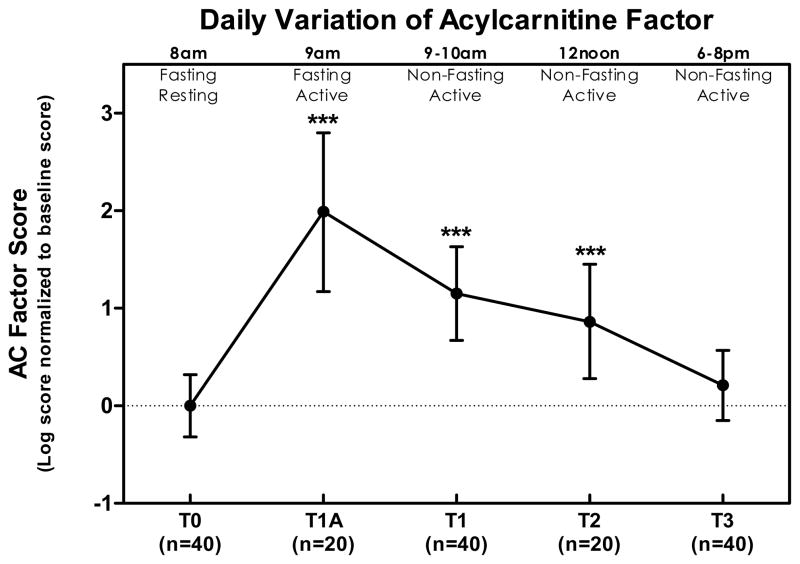
The goal of our primary analysis was to evaluate daily variation of amino acids and acylcarnitines in the aggregate. As described in the Methods, factor scores for AAs and ACs were computed for each subject at each timepoint based on all analytes at baseline and their respective factor loadings (AAs Table 1, ACs Table 2). The AA factor displayed a statistically significant increase with initial morning activity (an average 0.3 SD increase relative to baseline, p=0.04) and feeding (1.15 SD increase relative to baseline, p<0.0001), remained elevated through the middle of the day (0.7 SD above baseline, p = 0.019) and finally reached a peak in the evening (1.61 SD above baseline, p <0.0001) (Figure 1). The AC factor exhibited the largest change with initial morning activity, first increased (1.99 SD increase relative to baseline, p<0.0001), then decreased after breakfast (to 1.15 SD above baseline, p < 0.001), decreased further by midday (to 0.86 above baseline, p = 0.0056), and by evening returned to a value not significantly different from baseline (0.21 SD above baseline, p = 0.3).
Figure 1. Daily variation of 1-factor solution scores for 15 amino acids (AA) at various time points as a ratio of the baseline value.
All values are normalized to concentrations at T0 and represent natural log means and 95% confidence interval. Change from baseline was statistically significant at T1a (p = 0.04), T1 (p < 0.0001), T2 (p = 0.019), and T3 (p < 0.0001). Summarized in the figure as * p < 0.05, ** p < 0.01, and *** p < 0.001.
We also conducted secondary analyses of the daily variation of individual metabolites. These were assessed in two ways, by comparing each subject’s AA or AC concentrations at each time point: a) relative to baseline, and b) relative to its next subsequent next time point. While all 15 amino acids and 42 of the 45 acylcarnitines displayed significant changes relative to baseline at some point during the day, the more meaningful analysis was that comparing each time point to its next subsequent neighboring time point, as this allowed identification of events triggering changes of state in metabolite concentrations. Amino acids tended to increase after AM arising and/or with feeding (Table 3). Only ornithine remained statistically unchanged between time points, although levels gradually increased over the course of the day to eventually reach a level statistically higher than baseline. With acylcarnitines the greatest change was seen with beginning morning activity when 32 of 45 ACs demonstrated significant increases (maximal increase of 120%). This was followed by a significant decrease of 14 ACs after feeding (Table 4).
Table 3.
Within subject (daily) variation in 15 amino acids -nearest neighbor ratios.
| ||||
|---|---|---|---|---|
| 1. Glycine | 1.00 | 1.03 | 1.08 * | 0.99 |
| 2. Alanine | 1.02 | 1.18 *** | 1.15 *** | 1.05 * |
| 3. Serine | 1.06 | 1.12 ** | 1.02 | 1.04 |
| 4. Proline | 0.96 * | 1.20 *** | 0.98 | 1.12 * |
| 5. Valine | 1.00 | 1.08 * | 1.00 | 1.01 |
| 6. Leucine/Isoleucine | 1.02 | 1.16 *** | 1.01 | 1.03 |
| 7. Methionine | 1.01 | 1.13 *** | 1.02 | 0.94 |
| 8. Histidine | 0.99 | 1.05 * | 0.98 | 1.01 |
| 9. Phenylalanine | 1.04 * | 1.12 *** | 1.01 | 0.99 |
| 10. Tyrosine | 1.04 ** | 1.08 *** | 0.99 | 0.94 |
| 11. Aspartate/Asparagine | 1.15 *** | 1.05 | 1.04 | 1.08 |
| 12. Glutamate/Glutamine | 1.09 ** | 0.95 | 1.12 *** | 0.96 |
| 13. Ornithine | 0.99 | 1.02 | 0.92 | 0.98 |
| 14. Citrulline | 1.02 | 0.84 *** | 0.92 | 0.94 |
| 15. Arginine | 1.09 *** | 1.08 * | 1.11 * | 1.04 |
For each subject, the ratio of each time point relative to its previous time point was calculated using log-transformed (ln) values. Median values of the individual ratios are presented and P values (where the null hypothesis is that the overall ratio = zero) were generated by the Signed Rank Statistic. P values:
p<0.05,
p<0.01,
p<0.001.
Table 4.
Within subject (daily) variation in 45 acylcarnitines – nearest neighbor ratios
| ||||
|---|---|---|---|---|
| Individual Acylcarnitine | ||||
| 1. Acetyl carnitine, C2 | 1.21 ** | 1.02 | 1.06 | 0.98 |
| 2. Propionyl carnitine, C3 | 1.23 ** | 1.14 * | 1.31** | 0.99 |
| 3. Butyryl/Isobutyryl carnitine, C4/Ci4 | 1.08 | 0.85 | 1.08 | 0.95 |
| 4. Tiglyl carnitine, C5:1 | 1.15 | 1.10 | 0.94 | 0.94 |
| 5. Isovaleryl/3-Methylebutyryl/2-Methylbutyryl carnitine, C5’s | 0.97 | 1.08 | 1.30* | 1.05 |
| 6. β-hydroxy butyryl carnitine, C4OH | 0.97 | 1.33 * | 1.04 | 1.49 |
| 7. Hexanoyl carnitine, C6 | 1.48 * | 1.00 | 1.01 | 1.02 |
| 8. 3-Hydroxy-isovaleryl/Malonyl carnitine, C5OH/C3DC | 0.98 | 0.88 | 1.17 | 0.89 |
| 9. Methylmalonyl/ Succinyl carnitine, Ci4DC/C4DC | 1.05 | 1.13 | 1.10 | 1.03 |
| 10. Octenoyl carnitine, C8:1 | 1.17 *** | 0.86 ** | 1.01 | 0.97 |
| 11. Octanoyl carnitine, C8 | 2.16 *** | 0.61 *** | 0.75** | 0.63 |
| 12. Glutaryl carnitine, C5DC | 1.07 | 1.06 | 0.87 | 0.92 |
| 13. Adipoyl carnitine, C6DC | 1.19 *** | 0.86 | 1.03 | 0.82 |
| 14. Decatrienoyl carnitine, C10:3 | 1.15 *** | 0.95 | 1.08** | 0.97 |
| 15. Decadienoyl carnitine, C10:2 | 1.23 *** | 0.98 | 1.14 | 0.74 |
| 16. Decenoyl carnitine, C10:1 | 2.20 *** | 0.57*** | 0.85 | 0.84 |
| 17. Decanoyl carnitine, C10 | 2.34 *** | 0.51*** | 0.71** | 0.65** |
| 18. 3-Hydroxy-decanoyl/ Suberoyl carnitine, C10-OH/C8-DC | 1.53 *** | 0.75 | 0.96 | 0.78 |
| 19. Dodecenoyl carnitine, C12:1 | 1.94 *** | 0.62*** | 0.90 | 0.63** |
| 20. Lauroyl carnitine, C12 | 2.07 *** | 0.78** | 0.91 | 0.73** |
| 21. 3-Hydroxy-dodecanoyl/ Sebacoyl carnitine, C12-OH/ C10-DC | 1.71 ** | 0.86 | 0.88 | 0.69 |
| 22. Tetradecadienoyl carnitine, C14:2 (μM) | 2.19 *** | 0.62*** | 0.67** | 0.93 |
| 23. Tetradecenoyl carnitine, C14:1 | 1.74 *** | 0.71*** | 0.79 | 0.86 |
| 24. Myristoyl carnitine, C14 | 1.59 *** | 0.81* | 0.86 | 0.85 |
| 25. 3-Hydroxy-tetradecenoyl carnitine, C14:1-OH | 1.40 *** | 0.89 | 1.00 | 0.80 |
| 26. 3-Hydroxy-tetradecanoyl/ Dodecanedioyl carnitine, C14-OH/C12-DC | 1.40 ** | 0.87 | 0.83 | 0.89 |
| 27. Palmitoyl carnitine, C16 | 1.19 *** | 0.97 | 0.90 | 0.96 |
| 28. 3-Hydroxy-hexadecanoyl/ Tetradecanedioyl carnitine, C16-OH/C14-DC | 1.23 * | 1.17 | 0.96 | 0.90 |
| 29. Linoleyl carnitine, C18:2 | 1.24 *** | 0.75** | 0.82 | 1.25 |
| 30. Oleyl carnitine, C18:1 | 1.15 ** | 0.91* | 0.90 | 1.03 |
| 31. Stearoyl carnitine, C18 | 1.07 | 0.95 | 0.89 | 1.01 |
| 32. 3-Hydroxy-octadecenoyl carnitine, C18:1-OH | 1.36 * | 0.88 | 0.83 | 0.91 |
| 33. 3-Hydroxy-octadecanoyl/ Hexadecanedioyl carnitine, C18-OH/C16-DC | 1.13 | 1.04 | 1.15 | 0.82 |
| 34. Arachidoyl carnitine, C20 | 1.40 * | 0.90 | 1.15 | 0.93 |
| 35. Octadecenedioyl carnitine, C18:1-DC | 1.48 *** | 0.84 | 1.04 | 1.00 |
| 36. 3-Hydroxy-eicosanoyl/Octadecanedioyl carnitine, C20-OH/C18-DC | 1.43 *** | 0.91 | 1.14 | 0.91 |
| 37. Docosanoyl carnitine, C22 | 1.15 | 0.79 | 1.26 | 1.12 |
| 38. 3-Hydroxy-cis-5-octenoyl/ Hexenedioyl carnitine, C8:1-OH/C6:1-DC | 1.32 ** | 0.97 | 1.18* | 0.85 |
| 39. Heptanedioyl carnitine, C7-DC | 1.00 | 1.00 | 2.69** | 0.68 |
| 40. Octenedioyl carnitine, C8:1-DC | 1.42 *** | 0.80 | 0.96 | 0.75* |
| 41. Hexadecadienoyl carnitine, C16:2 | 1.42 * | 0.68 | 0.77 | 1.07 |
| 42. Palmitoleoyl carnitine, C16:1 | 1.46 *** | 0.81*** | 0.72 | 0.97 |
| 43. 3-Hydroxy-palmitoleoyl/ cis-5-Tetradecenedioyl carnitine, C16:1-OH/C14:1-DC, | 1.25 | 1.08 | 1.16 | 0.88 |
| 44. 3-Hydroxy-linoleyl carnitine, C18:2-OH | 1.06 | 0.97 | 0.97 | 0.95 |
| 45. Arachidonoyl carnitine, C20:4 | 1.12 * | 0.99 | 1.33 | 1.14 |
For each subject, the ratio of each time point relative to its previous time point was calculated using log-transformed (ln) values. Median values of the individual ratios are presented and P values (where the null hypothesis is that the overall ratio = zero) were generated by the Signed Rank Statistic. P values:
p<0.05,
p<0.01,
p<0.001.
4 Discussion
To our knowledge, this study represents the first report characterizing the daily variation of acylcarnitines, and confirms the systematic variation of amino acids in response to feeding and activity (Eriksson et al. 1989; Feigin et al. 1968; Maher et al. 1984). While previous studies have used a non-targeted approach in plasma (Park et al. 2009) or urine (Slupsky et al. 2007)without specifically distinguishing between the separate effects of nutrition and activity, the analysis employed herein did so using mass spectrometry to target a broad panel of specific metabolites in serum.
Changing energy demands at the beginning of the day likely account for the biphasic response in ACs following an overnight fast. The overnight fast would deplete glycogen stores and switch the body’s main fuel source to fatty acids thus raising acylcarnitine levels. The further increase in ACs following morning activity while fasting is consistent with continued fatty acid metabolism to meet energy demands. Consumption of a mixed meal would presumably cause blood glucose concentrations to rise, thereby leading to suppression of fatty acid oxidation via insulin-induced suppression of lipolysis, coupled with increases in tissue levels of malonyl CoA, leading to allosteric suppression of carnitine palmitoyltransferase I. The decrease in utilization of fatty acids would be expected to lead to decreased levels of a subset of acylcarnitines in our assay panel, particularly the even-chain species.
The pattern of daily responses seen in AAs contrasted with that for acylcarnitines. Six of fifteen AAs changed significantly with activity, but the change with feeding was greater, both in the number of AAs (eleven) that changed as well as the magnitude of response. Notably, feeding increased AA concentrations but decreased AC levels. The relatively smaller response of AAs to activity compared to that of ACs may be ascribed to the different roles these classes of metabolites play in energy balance. ACs are particularly sensitive to changes in fatty acid oxidation in the course of normal feeding/fasting cycles, whereas amino acids are particularly important as an oxidized fuel mainly in more prolonged fasting or starvation. As such, levels of ACs may display both greater fluctuations as well as more rapid responsiveness to the changing energy demands when compared to AAs, particularly at the start of the day.
While individual amino acids and acylcarnitines have distinct characteristics and functions, they also participate in larger metabolic networks. For example, beta oxidation involves the sequential cleavage of 2-carbon units, thus families of fatty acid species that are of various length can be considered part of a related cluster of metabolites. Similarly, groups of amino acids converge into common catabolic pathways. For example, glutamate and glutamine can be produced via the catabolism of numerous other AAs, which may explain the rise in glu/gln at T2 (noon) simultaneous with the fall of their precursors proline, histidine, and the branched chain amino acids valine, leucine, and isoleucine. Branched chain amino acids also contribute the amino groups necessary for alanine synthesis, which can then be converted to pyruvate for use in gluconeogenesis during times of fasting. In this report, single AA and AC factors generated from principal components analysis largely recapitulated the daily changes seen with individual metabolites described above, with the AC factor demonstrating the greatest change of state by rising with early morning activity and then falling with feeding, and the AA factor rising modestly with activity and more profoundly with feeding. This finding validates the use of single factors from principal components analysis in other studies which use metabolic profiling to create a composite picture of concurrent metabolic processes (Shah et al., 2009; Lum et al. 2011). Furthermore, this approach appears particularly well-suited for future studies focused on metabolic flexibility, generally understood as the capacity to appropriately match fuel oxidation with substrate availability (Galgani et al. 2008). The current report outlines a means by which numerous singular metabolite measurements (assessed between various change of state junctures instead of at isolated time points) can be aggregated into factor scores in order to elucidate complex substrate shifts in cells, systems and individuals.
Variability of these metabolites comes from several sources. Besides technical or analytical variability (which is of relatively less concern with a well-validated measurement platform such as mass spectrometry used here), we quantified total (biological and daily) and daily variability. In all cases, total variability exceeded daily variability, the difference of which can be ascribed to biological (between individuals) variability. While it is always preferable to adhere to standard sample collection conditions, this is not always possible, and analytes with substantial biological variation are potentially robust enough to yield meaningful findings in spite of collection inconsistencies. Although we observed significant daily variation in both AAs and ACs, we observed a portion of biological variation over and above this for all analytes in this study.
There were several limitations of this study. First, the population of 40 subjects was limited in size and was selected on the diagnosis of radiographic knee osteoarthritis. We cannot rule out the possibility that the presence of OA could have a specific and unique impact on either the levels of these metabolites or the body’s regulation of them during the course of the day or in response to feeding or activity. However, given the high prevalence of OA in the general population (12% for ages 25 years and older, Lawrence et al. 2008, and a lifetime risk of 44.7%, Gabriel and Michaud 2009), these results are likely generalizable to a large sector of the adult US population. Second, while the mixed meals served in the study generally reflect a typical diet in a developed western setting, the heterogeneity of nutrients prevented discrimination of the effects on AA and AC levels due to specific components such as carbohydrate, protein or fat. Finally, no statistical accommodations were made in the secondary analysis for the simultaneous analysis of multiple analytes, but this was warranted given the exploratory scope of this aspect of the study.
5 Conclusion
In conclusion, from this preliminary study it is evident that major classes of energy metabolites, including amino acids and acylcarnitines, vary significantly in response to normal daily activities including food intake. Relative to baseline AM recumbent concentrations, AA concentrations rose after activity and feeding while AC concentrations rose after activity and decreased with feeding. Furthermore, for all AAs, ACs, and their factors, biological variation was quantifiably evident and distinct from daily variation. Elucidating these variations in metabolites is an important step in understanding the greater complexities of energy balance.
Figure 2. Daily profile of 1-factor solution scores for 45 acylcarnitines (AC) at various time points as a ratio of the baseline value.
All values are normalized to concentrations at T0 and represent natural log means and 95% confidence interval. Change from baseline was statistically significant at T1a (p < 0.0001), T1 (p < 0.0001), T2 (p = 0.0056), but not T3 (p = 0.3). Summarized in the figure as * p < 0.05, ** p < 0.01, and *** p < 0.001.
Acknowledgments
This study was supported by the National Institute on Aging at the National Institutes of Health (Claude D. Pepper Older Americans Independence Centers 5P30 AG028716): National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (RO1 AR48769): and the National Center for Research Resources at the National Institutes of Health (MO1-RR-30), supporting the Duke General Clinical Unit where this study was conducted. The final publication is available at Springer via http://dx.doi.org/DOI10.1007/s11306-011-0345-9.
We are appreciative of the time contributed by study participants.
Abbreviations
- AC
Acylcarnitine
- AA
amino acid
- CV
coefficient of variation
- FA
fatty acids
- LLOQ
lower limit of quantification
- MS
mass spectrometry
- OA
osteoarthritis
- PCA
principal components analysis
- SD
standard deviation
Footnotes
Disclosure statement
The authors declare that they have no conflict of interest.
Author contributions
DKT performed data analysis, participated in conceptual design and key discussions and wrote and edited the manuscript. RS and CFP participated in data analysis, participated in key discussions, and edited the manuscript. JRB and RDS performed primary sample analyses, participated in key discussions and edited the manuscript. CBN participated in key discussions and edited the manuscript. VBK designed the study, oversaw performance of all aspects of the study, participated in key discussions, and edited the manuscript.
References
- An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabete research: moving from information to knowledge. Diabetes. 2009;58(11):2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace DH, Hillman SL, Millington DS, et al. Rapid dignosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem. 1995;41:62–68. [PubMed] [Google Scholar]
- Chace DH, Millington DS, Terada N, et al. Rapid diagnois of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem. 1993;39:66–71. [PubMed] [Google Scholar]
- Criscione LG, Elliott AL, Stabler TV, Jordan JM, Pieper CF, Kraus VB. Variation of serum hyaluronan with activity in individuals with knee osteoarthritis. Osteoarthritis and Cartilage. 2005;13:837–840. doi: 10.1016/j.joca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- De Jesus VR, Chace DH, Lim TH, Mei JV, Hannon WH. Comparison of amino acids and acylcarnitines assay methods used in newborn screening assays by tandem mass spectrometry. Clinica Chimica Acta. 2010;411:684–689. doi: 10.1016/j.cca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Eriksson T, Voog L, Walinder J, Eriksson TE. Diurnal rhythm in absolute and relative concentrations of large neutral amino acids in human plasma. Journal of Psychiatric Research. 1989;23(3/4):241–249. doi: 10.1016/0022-3956(89)90029-0. [DOI] [PubMed] [Google Scholar]
- Feigin RD, Klainer AS, Beisel WR. Circadian periodicity of blood amino-acids in adult man. Nature. 1967;215:512–514. doi: 10.1038/215512b0. [DOI] [PubMed] [Google Scholar]
- Feigin RD, Klainer AS, Beisel WR. Factors affecting circadian periodicity of blood amino acids in man. Metabolism. 1968;17(9):764–775. doi: 10.1016/0026-0495(68)90026-7. [DOI] [PubMed] [Google Scholar]
- Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and co-morbidity of the rheumatic diseases. Arthritis Research and Therapy. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CD, Stabler TV, Kraus VB. Variation in osteoarthritis biomarkers from activity not food consumption. Clinica Chimica Acta. 2008;398:21–26. doi: 10.1016/j.cca.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KM, Shah SH, Bain JR, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis and Rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Sloane R, Huffman KM, et al. Plasma acylcarnitines are associated with physical performance in elderly men. Journal of Gerontology Medical Sciences. 2011;66A (5):548–553. doi: 10.1093/gerona/glr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher TJ, Glaeser BS, Wurtman RJ. Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake. American Journal of Clinical Nutrition. 1984;39:722–729. doi: 10.1093/ajcn/39.5.722. [DOI] [PubMed] [Google Scholar]
- Millington DS, Chace DH. Carnitine and acylcarnitines in metabolic disease diagnosis and management. In: Desiderio DM, editor. Mass Spectrometry: Clinical and Biomedical Applications. New York: Plenum Press; 1992. pp. 199–219. [Google Scholar]
- Millington DS, Stevens RD. Acylcarnitines: analysis in plasma and whole blood using tandem mass spectrometry. Methods Mol Biol. 2011;708:55–72. doi: 10.1007/978-1-61737-985-7_3. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman C, Tenori L, Biganzoli L, et al. Uncovering the metabolic fingerprint of breast cancer. International Journal of Biochemistry and Cell Biology. 2011;43 (7):1010–20. doi: 10.1016/j.biocel.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim SB, Wang B, et al. Individual vriation in macronutrient regulation measured by proton magnetic resonance spectroscopy of human plasma. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;297:R202–R209. doi: 10.1152/ajpregu.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Huffman KM, Landerman LR, et al. Effect of caloric restriction with and without exercise on metabolic intermediates in onobese men and women. J Clin Endocrinol Metab. 2011;96 (2):E312–321. doi: 10.1210/jc.2010-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with corony artery disease and risk of subsequent cardiovascular events. Circulation: Cardiovascular Genetics. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- Shah SH, Hauser ER, Bain JR, et al. High heritability of metabolimic profiles in families burdened with premature cardiovascular disease. Molecular Systems Biology. 2009;5:258. doi: 10.1038/msb.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slupsky CM, Rankin KN, Wagner J, et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Analytical Chemistry. 2007;79 (18):6995–7004. doi: 10.1021/ac0708588. [DOI] [PubMed] [Google Scholar]
- Vinayavekhin N, Homan EA, Saghatelian A. Exploring disease through metabolomics. ACS Chemical Biolog. 2010;5 (1):91–103. doi: 10.1021/cb900271r. [DOI] [PubMed] [Google Scholar]
- Wu JY, Kao HJ, Li, et al. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]