Abstract
Exercise training (ExT) normalizes the increased sympathetic outflow in heart failure (HF), but the mechanisms are not known. We hypothesized ExT would normalize the augmented glutamatergic mechanisms mediated by NMDA receptors within the PVN that occurs with HF. Four groups of rats were used: 1) Sham Sedentary (Sed); 2) Sham ExT; 3) HF Sed; and 4) HF ExT. HF was induced by left coronary artery ligation, and ExT consisted of three weeks of treadmill running. In α-chloralose-urethane-anesthetized rats, the increase in renal sympathetic nerve activity (RSNA) in response to the highest dose of NMDA (200 pmol) injected into the PVN in the HF Sed group was approximately twice that of the Sham Sed group. In the HF ExT group the response was not different from the Sham Sed or Sham ExT groups. Relative NMDA receptor subunit NR1 mRNA expression was 63% higher in the HF Sed group compared to the Sham Sed group, but in the HF ExT group was not different from the Sham Sed or Sham ExT groups. NR1 receptor subunit protein expression was increased 87% in the HF Sed group compared to the Sham Sed group but in the HF ExT group was not significantly different from the Sham Sed or Sham ExT groups. Thus, one mechanism by which ExT alleviates elevated sympathetic outflow in HF may be through normalization of glutamatergic mechanisms within the PVN.
Keywords: paraventricular nucleus, heart failure, NMDA receptors, exercise training, sympathetic nerve activity
Introduction
A characteristic feature of heart failure (HF) is increased sympathoexcitation, which correlates with the severity of the disease as well as complications and mortality (28). The source of the increased sympathoexcitation associated with HF is not entirely understood, although several lines of evidence point to a role for the central nervous system (4, 16, 19). Sinoaortic (4) or cardiovagal (19) denervation does not normalize plasma norepinephrine (NE) levels in denervated compared to intact HF dogs. Plasma NE is also not altered in HF dogs by β-adrenergic blockade (19). Stimulation of the aortic depressor nerve produces altered lumbar sympathetic nerve activity in rats with HF compared to sham rats (16). Taken together these data indicate an alteration within the central nervous system in HF.
The paraventricular nucleus (PVN) of the hypothalamus is reciprocally connected to other areas of the brain involved in control of cardiovascular function (18) and contains preautonomic neurons which project to sympathetic preganglionic neurons within the intermediolateral cell column of the spinal cord both directly and indirectly via the rostral ventrolateral medulla (40). Previous data from this laboratory suggest that the increased activity of PVN neurons associated with HF (32) is due to an increase in glutamatergic mechanisms within the PVN (21). The increase in renal sympathetic nerve activity (RSNA) in response to NMDA injected into the PVN is greater in HF rats than in Sham (21), which correlates with an increased expression of the NR1 subunit of the NMDA receptor within the PVN (21).
Exercise training (ExT) in human HF patients increases survival, decreases complications, and decreases muscle sympathetic nerve activity (13). In a rapid pacing model of HF in rabbits, ExT decreases RSNA to that of normal rabbits (23). However, the mechanism by which ExT normalizes sympathetic outflow in HF is not known. In the current study, we hypothesized that one mechanism by which ExT restores sympathetic outflow to normal levels in HF is by normalizing the increased glutamatergic mechanisms within the PVN. Specifically, we wanted to determine whether ExT attenuated the increased RSNA response to NMDA injected into the PVN and whether ExT normalized the elevated expression of the NR1 subunit of the NMDA receptor in HF rats.
Methods
Animals
Male Sprague-Dawley rats weighing 220 to 280 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. Protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee and were in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals. Rats were given rat chow and water ad libitum and were housed in a room with a 12-h light-dark cycle. Rats were allowed to acclimatize for one week prior to cardiac surgery.
Induction of heart failure
Rats were randomly assigned to either the sham-operated control group or the HF group. HF was induced by ligation of the left coronary artery as has been described previously (32). Left ventricular dysfunction was assessed using hemodynamic and anatomic criteria. Echocardiograms were performed before and after the three-week ExT period. Left ventricular end-diastolic pressure was measured following the ExT period using a Mikro-Tip catheter (Millar Instruments, Houston, TX) inserted into the left ventricle via the right carotid artery at the time of the terminal experiment. To measure infarct size, the heart was dissected free of adjacent tissues, and the atria were removed. The right ventricle was opened with a lengthwise incision such that the heart was flattened with the left ventricle lying in the middle with the right ventricle on either side of it. The right ventricle was removed and the remaining left ventricle laid flat. A digital image of the left ventricle was captured using a Kodak DC290 digital camera (Kodak, Rochester, NY) and the infarcted area and total left ventricle area quantified using SigmaScan Pro. Infarct size (%) was found by dividing the size of the infarcted area by the total size of the left ventricle. Rats with elevated left ventricular end-diastolic pressure (LVEDP; ≥15 mm Hg) and infarct size >30% of total left ventricle wall were considered to be in HF. Urine NE concentration was measured by radioenzymatic assay according to previously described protocols (36). Sensitivity of the assay was 1–2 pg of NE per 50 μl of diluted urine. Plasma angiotensin II (Ang II) was measured using an RIA (Alpco Diagnostics, Salem, NH). Sensitivity of the Ang II assay was 0.68 pg/ml.
Exercise training (ExT)
Three weeks following coronary artery ligation surgery, rats were randomly assigned to either ExT or sedentary (Sed) groups to produce four total experimental groups: Sham Sed, HF Sed, Sham ExT, and HF ExT. For ExT, rats ran on a motor-driven treadmill (Columbus Instruments, Columbus, OH) for a period of three weeks according to a modified protocol of Musch and Terrell (27). Renal nerve recording or NR1 expression experiments were performed six to eight weeks following coronary artery ligation surgery, and thus the ExT period took place during the final three weeks of the progression of HF. Initially, low speed (10 m/min) and grade (0%) and short duration (10 min/day) were used to familiarize the rats with running on the treadmill. The speed, duration, and grade were gradually increased to 20–25 m/min, 60 min/day, and 5–10%, respectively, to ensure that a significant endurance effect was produced. This level of exercise is considered moderate for the sham rats. Only rats that ran steadily with little or no prompting were used in the study. To ensure a similar level of ExT between groups, citrate synthase activity assays on the soleus muscle were performed following the protocol of Srere (44).
General surgery for hemodynamic and RSNA measurements and microinjection
Experiments were performed six to eight weeks after HF or sham surgery. Rats were anesthetized with urethane (0.75 g/kg IP) and α-chloralose (70 mg/kg IP). The left femoral vein was cannulated with polyethylene tubing (PE-50) for injection of supplemental anesthesia. The left femoral artery was cannulated and connected via a pressure transducer (Gould P23 1D) to a computer-based data recording and analyzing program (PowerLab) to record mean arterial blood pressure (MAP) and heart rate (HR).
The left kidney was exposed through a retroperitoneal flank incision. A branch of the renal nerve was isolated from fat and connective tissue. The central end of the nerve was placed on thin bipolar platinum electrodes. The nerve-electrode junction was fixed and electrically insulated from surrounding tissues with a Wacker Silgel mixture (604 and 601). The electrical signal was amplified with a Grass amplifier with high- and low-frequency cutoffs of 1000 Hz and 100 Hz, respectively. The rectified output from the amplifier was displayed using the PowerLab system to record and integrate the raw nerve discharge. Basal nerve activity was determined at the beginning of the experiment, and background noise was determined by nerve activity recorded at the end of the experiment (after the rat was euthanized). The nerve activity during the experiment was calculated by subtracting the background noise from the recorded value. The RSNA response to injection of drugs into the PVN was expressed as a percentage change from the basal value.
For placement of microinjection cannulas into the PVN, the anesthetized rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujanga, CA). A longitudinal incision was made on the head and bregma was exposed. A small burr hole was made in the skull to allow access to the PVN. The coordinates for the PVN, determined using the Paxinos and Watson Atlas (33), were 1.5 mm posterior to bregma, 0.4 mm lateral to midline, and 7.8 mm ventral to the dura. A thin needle (0.5 mm OD, 0.1 mm ID) connected to a 0.5 μl microsyringe (Hamilton, Reno, NV) was lowered into the PVN. N-methyl-d-aspartic acid (NMDA; Calbiochem, La Jolla, CA) was injected into the PVN in three doses (50, 100, and 200 pmol in 50–200 nl) in random order. Each animal received all three doses of NMDA. Subsequent injections were made at least 20 min after prior injections to allow MAP, HR, and RSNA to return to basal levels.
Brain histology
Following microinjection experiments, Chicago blue dye (50 nl) was injected into the brain to histologically verify that the injection site was located within the PVN. The brains were removed, fixed in 10% formalin for at least 24 h, sectioned (30 μm) on a crytostat, and processed for histology. The sections were mounted on gel-coated microscope slides and stained using 1% neutral red. The location of the injection was visualized on a microscope, and injections with terminations within the boundaries of the PVN were considered to be appropriately targeted to the PVN. Injections located outside of the PVN were excluded from data for the PVN and were analyzed as anatomical controls.
Micropunch of the PVN and isolation of mRNA for real-time RT-PCR and protein for Western blot measurements
The following experiments were performed in a separate group of animals from those used in the PVN microinjection experiments described above. After the animal was euthanized, the brain was removed and quickly frozen on dry ice. Six serial coronal sections (100 μm) were cut through the hypothalamus at the level of the PVN using a cryostat, and, following the Palkovits technique (29), the PVN was bilaterally punched using a diethylpyrocarbonate (DEPC)-treated blunt 18-gauge needle attached to a syringe, such that there were 12 total punches per brain. For each brain, six of the punches were placed in 500 μl Tri-Reagent (MRC, Cincinnati, OH), followed by sonication and extraction of mRNA following the manufacturer's instructions. Briefly, the homogenate was separated into organic and aqueous phases by the addition of bromochloropropane and subsequent centrifugation. The RNA, contained in the aqueous phase, was precipitated with isopropanol, washed with ethanol, and solubilized in 10 μl DEPC-treated water. The other six punches for each brain were placed in 100 μl protein extraction buffer (1 M Tris, 0.5 M EDTA, 10% SDS, Triton-X-100, and 100 mM phenylmethylsulfonyl fluoride), sonicated, and incubated for 30 min at 37° C to extract the protein. The supraoptic nucleus (SON), lateral hypothalamus (LH), and dorsal cortex were punched at the same rostro-caudal level as the PVN to serve as anatomical control regions from the same cross-sectional slices.
Real-time RT-PCR measurement of NR1 mRNA
Following extraction of mRNA, samples underwent reverse transcription for 40 min at 37° C in the presence of 1.5 M random hexamers and 100 U MMLV-RTase. Real-time RT-PCR measurements were made using the iCycler iQ Multicolor Real-Time Detection System with output to a computer-based acquisition system (Bio-Rad). The protocol consisted of denaturation (95° C for 3 min), amplification and quantification repeated 50 times (95° C for 10 s, 55° C for 45 s), denaturation at 95° C for 1 min, reannealing at 55° C for 1 min, and a melt curve (55°–95° C with a heating rate of 0.5° C per 10 s). The reaction mixture consisted of S YBR Green Supermix (Bio-Rad), 300 nM sense primer, 300 nM antisense primer, DEPC-treated H2O, and the cDNA template of interest. For NR1, the sense primer was 5'-ATAGTGACAATCCACCAAGAGCC and the antisense primer 5'-GTAGCTCGCCCATCATTCCGTT. For rpl19, used as the reference gene, the sense primer was 5'CCCCAATGAAACCAACGAAA, and the antisense primer was 5'ATGGACAGTCACAGGCTTC. Relative expression of NR1 was calculated using the Pfaffl equation which relates expression of the target gene (NR1) to expression of a reference gene (rpl19) (34).
Western blot measurement of NR1 protein
The total protein concentration from the extracted protein described above was measured using a BCA Assay Kit (Pierce, Rockford, IL). Samples were adjusted to contain the same concentration of total protein, and then equal volumes of 2X 4% SDS sample buffer were added. The samples were boiled for 3 min and then loaded onto a 7.5% SDS-PAGE gel (40 μg/20 μl per well). Gels were subjected to electrophoresis at 40 mA/gel for 60 min. The fractionated proteins on the gel were electrophoretically transferred to a PVDF membrane (Millipore, Billerica, MA) at 300 mA for 90 min. The membrane was probed with primary antibody: rabbit anti-NR1 (1:500 dilution, Santa Cruz, CA), rabbit anti-NR2B (1:500, Santa Cruz), or rabbit anti-β-Tubulin (1:2,000, Santa Cruz), washed with TBST, and then probed with secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, 1:5,000 dilution, Pierce). An enhanced chemiluminescence substrate (Pierce) was applied to the membrane for 5 min followed by a 30 second exposure within an Epi Chemi II Darkroom (UVP BioImaging, Upland, CA) for visualization using the Worklab digital imaging system. Kodak 1D software was used to highlight the bands and quantify the signal. The expression of NR1 or NR2B was calculated as the ratio of intensity of the NR1 or NR2B band, respectively, relative to the intensity of the β-Tubulin band.
Data Analysis
Data are presented as mean ± SE. The data were subjected to two-way ANOVA followed by comparison for individual group differences using the Newman-Keuls test (7). Statistical significance was indicated by a value of P<0.05.
Results
General Data
Table 1 presents baseline MAP and HR data as well as morphological characteristics and left ventricular function data among the four experimental groups. The data represent mean values from animals used for RSNA experiments and animals in which mRNA and protein expression within the PVN were measured. Baseline RSNA (int.RSNA) was not different between groups, although baseline RSNA varies from animal to another and was thus not expected to be different in these animals. Only rats with ≥30% infarct of the left ventricular wall were included in the study. Five rats had infarct sizes <30% and were excluded from data analysis. Sham rats had no visible myocardial damage. LVEDP was significantly increased in the HF Sed rats compared to both Sham groups and the HF ExT group. HF Sed rats had significantly lower dP/dt compared to Sham rats, which was partially improved by ExT. While LVEDP and dP/dt were only partially normalized by ExT, the values in the HF ExT rats were nonetheless significantly different from those in either of the Sham groups. Ejection fraction was also significantly lower in both HF groups compared to the Sham groups and was not altered by ExT. Taken together, these data confirm that rats in the HF groups were experiencing cardiac dysfunction and that ExT did not normalize cardiac function.
Table 1.
Baseline and left ventricular function data
| Sham Sed (n=16) | HFSed (n=16) | Sham ExT (n=16) | HFExT (n=16) | |
|---|---|---|---|---|
| Body weight (g) | 395±12 | 392±11 | 347±8# | 377±7* |
| MAP (mm Hg) | 91±1 | 92±4 | 95±3 | 88±5 |
| HR (beats/min) | 335±18 | 310±12 | 325±15 | 323±10 |
| Int.RSNA, μV•s | 50.8+8.0 | 69.1+19.8 | 55.0+10.3 | 70.6+10.7 |
| Infarct size (% of left ventricle) | 0 | 37±2* | 0 | 39±2* |
| Ejection fraction (%) | 81±2 | 50±4* | 83±2 | 54±5* |
| LVEDP (mm Hg) | 4.8±0.5 | 24.8±2.2* | 3.6±0.6 | 18.8±2.4*# |
| dP/dt (mm Hg/sec) | 8841±503 | 4419±515* | 8653±348 | 6146±413*# |
| CS activity (μmol/g/min) | 6.0±0.5 | 6.0±0.7 | 9.7±2.0* | 7.7±0.6* |
Data are means±SE. MAP, mean arterial pressure; HR, heart rate; int.RSNA, integrated renal sympathetic nerve activity; LVEDP, left ventricular end-diastolic pressure; CS, citrate synthase.
p<0.05 vs Sham;
p<0.05 vs Sed.
Additionally, citrate synthase activity is presented in Table 1. Citrate synthase activity of the soleus muscle was significantly higher in both ExT groups compared to the Sed groups, demonstrating a significant effect of ExT. ExT had a similar effect in the two groups in that there was no significant difference between Sham ExT and HF ExT.
Figure 1 illustrates plasma Ang II and urinary NE data. Plasma Ang II levels were increased in HF Sed compared to Sham Sed but were not different in HF ExT compared to Sham Sed or Sham ExT, indicating that ExT normalized the elevated plasma Ang II associated with HF (Fig. 1A). Urinary NE levels were elevated in HF Sed rats compared to Sham Sed rats, indicative of increased overall sympathoexcitation. In HF ExT rats, urinary NE levels were not different from Sham Sed or Sham ExT, suggesting that ExT normalized the increased overall sympathetic outflow associated with HF (Fig. 1B).
Figure 1.
A, Plasma Ang II and B, urine NE. Data are means ± SE. Ang II, Angiotensin II; NE, norepinephrine. *p<0.05 vs Sham; #p<0.05 vs Sed.
Figure 2 is a schematic illustrating the location of injection sites of NMDA into the PVN. Of 30 microinjections, 24 were located within the PVN, and 6 were outside of the PVN and therefore analyzed separately as anatomical controls. Administration of NMDA into these sites (anatomical controls) did not produce the typical increases in RSNA in response to NMDA injected into the PVN.
Figure 2.

Injection sites within and around the PVN. Schematic representations of serial coronal sections from the rostral (−1.4) to caudal (−2.1) regions of the PVN. The distance (mm) is shown for each section according to the Paxinos and Watson atlas (33). Each filled square represents the site of termination of an injection within the PVN. Each plus sign represents the site of termination of an injection outside of the PVN, the data from which was not included for analysis. AH, anterior hypothalamus; f, fornix; and 3V, third ventricle.
Microinjection of NMDA into the PVN
Figure 3A illustrates examples of responses of RSNA, MAP, and HR to microinjection of the highest dose of NMDA (200 pmol) into the PVN in each of the four experimental groups. NMDA injected into the PVN elicited dose-dependent increases in RSNA in all groups as shown in the group data in Figure 3B. At the lowest dose of NMDA (50 pmol), there was a trend for the RSNA response to be potentiated in the HF Sed group. However, the mean response in that group was not significantly different from that in the Sham Sed group. Similarly, there was a trend for the RSNA response to 50 pmol NMDA in the HF ExT group to be reduced compared to the HF Sed group although there was not a statistically significant difference between the two groups. At the two higher doses of NMDA, the increase in RSNA was significantly potentiated in the HF Sed group consistent with previous work (21). At 100 pmol, the response in the HF Sed rats was significantly higher than Sham Sed and Sham ExT. The response to 100 pmol in the HF ExT group was significantly lower than HF Sed and not different from either Sham group.
Figure 3.
RSNA, MAP, and HR responses to NMDA injected into the PVN. A, Segments of original recordings from individual rats from each experimental group showing responses of renal sympathetic nerve activity (RSNA), integral of RSNA (int.RSNA), arterial blood pressure (AP), and heart rate (HR) to different doses of NMDA injected into the PVN. B, Mean changes in RSNA following injections of NMDA into the PVN.
At the highest dose of NMDA (200 pmol), the response in the HF Sed group (93 ± 13%, n=6) was significantly higher (p<0.05) than Sham Sed (45 ± 2%, n=6) and Sham ExT (33 ± 9%, n=6). In the HF ExT group, this response (39 ± 5%, n=6) was significantly lower (p<0.05) than in the HF Sed group and not different from either of the Sham groups. These data indicate that ExT normalizes the RSNA response to NMDA microinjection into the PVN in rats with HF.
Figure 4A illustrates the increase in MAP in response to NMDA injected into the PVN. At 100 pmol NMDA, the increase in MAP in the HF Sed group was significantly higher compared to the Sham Sed and Sham ExT groups. The increase in MAP in the HF ExT group was significantly attenuated compared to the HF Sed group and not different from either of the Sham groups. At the highest dose of NMDA (200 pmol), the change in MAP in the HF Sed group was 23 ± 5 mmHg (n=6), significantly higher than the MAP change in the Sham Sed (10 ± 2 mmHg, n=6) and Sham ExT (13 ± 2 mmHg, n=6) groups (p<0.05). In the HF ExT group the response (13 ± 2 mmHg, n=6) was significantly lower than the HF Sed group (p<0.05) and not different from either Sham group. These data indicate that the increase in MAP in response to NMDA injected into the PVN is augmented in rats with HF but is normalized by ExT.
Figure 4.
A, Mean changes in MAP following injections of NMDA into the PVN. B, Mean changes in HR following injections of NMDA into the PVN. *P<0.05 vs sham within the same dose of NMDA; #P<0.05 vs Sed within the same dose of NMDA.
Figure 4B shows the increase in HR in response to NMDA injected into the PVN. Although the HR responses tended to be higher in the HF Sed group compared to the other groups, the values did not reach statistical significance at the two lower doses of NMDA (50 and 100 pmol). At the highest dose of NMDA (200 pmol), the increase in HR in the HF Sed group (31 ± 5 beats/min, n=6) was significantly higher than the responses in the Sham ExT (10 ± 7 beats/min, n=6) and HF ExT groups (9 ± 6 beats/min, n=6) but was not statistically different from that of the Sham Sed group (18 ± 2 beats/min, n=6).
Expression of NR1 subunit mRNA and protein within the PVN
NR1 mRNA expression, measured by real-time RT-PCR, is shown in Figures 5 and 6. Examples of original real-time RT-PCR traces comparing, on the top, Sham Sed and HF Sed, and, on the bottom, HF Sed and HF ExT are shown in Figure 5. The traces for the reference gene rpl19 are shown on the left side of each panel and the traces for the target gene NR1 are shown on the right side of each panel. Figure 6 shows the composite real-time RT-PCR data for the four experimental groups. Relative NR1 expression was significantly increased in the HF Sed group compared to the Sham Sed group. However, in the HF ExT group, relative NR1 expression was significantly lower than HF Sed and not different from Sham Sed or Sham ExT. These data indicate that ExT normalizes NR1 mRNA expression within the PVN in rats with HF.
Figure 5.
mRNA expression of NMDA receptor NR1 subunit in punched PVN samples measured by real-time RT-PCR. Expression was measured by determining at which cycle the fluorescence of each sample rose above threshold (Ct). Lower Ct values correspond to higher expression. Examples of original real-time RT-PCR traces comparing NR1 mRNA expression. Top panel, Sham Sed and HF Sed; bottom panel, HF Sed and HF ExT. rpl19 (reference gene) traces showing no difference between groups are shown in the insets in the upper left of each graph.
Figure 6.
Mean data of relative expression of NR1 mRNA to rpl19 mRNA. *P<0.05 vs Sham; #P<0.05 vs Sed.
NR1 protein expression, measured by Western blot, is shown in Figure 7. Sample gels showing NR1 and β-Tubulin protein in the four experimental groups are presented in Figure 7A. Figure 7B shows the composite data for NR1 expression within the PVN, calculated as the ratio of the density of the NR1 band to the density of the β-Tubulin band. The level of NR1 protein expression in the HF Sed group was significantly higher than in the Sham Sed group. In the HF ExT group, NR1 protein expression was significantly lower than in the HF Sed group and was not different from either the Sham Sed group or the Sham ExT group.
Figure 7.
Protein expression of NMDA receptor NR1 subunit in punched PVN samples measured by Western blot. A, Example of visualized electrophoresis bands of NR1 and β-Tubulin protein. B, Mean data of band densities of NR1 relative to β-Tubulin. *P<0.05 vs Sham; #P<0.05 vs Sed.
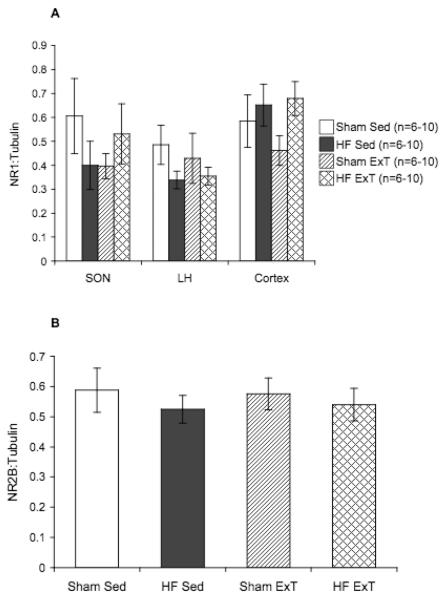
Figure 8 shows NR1 protein expression within the SON, LH, and cortex, and NR2B protein expression within the PVN. SON, LH, and cortex samples were punched from the same coronal sections as the PVN to be used as control areas. There were no significant differences in NR1 protein expression within the SON, LH, or cortex among all of the experimental groups (Figure 8A), indicating that the changes in NR1 expression by HF and ExT are specific to the region of the PVN. There were also no significant differences in PVN NR2B protein expression among the four experimental groups (Figure 6B), suggesting that the changes in expression within the PVN are specific for the NR1 subunit. Overall, these data show that ExT normalizes the increased expression of NR1 within the PVN in rats with HF.
Figure 8.
Control protein expression of NMDA receptor subunits. A. Protein expression of NR1 subunit in punched SON, LH, and cortex samples measured by Western blot. No significant differences between groups. B. NMDA receptor subunit NR2B protein expression in punched PVN samples measured by Western blot. No significant differences between groups.
Discussion
In the present study we found that ExT normalizes the potentiated increase in RSNA in response to NMDA microinjected into the PVN in rats with HF. Further, while expression of the NR1 subunit of the NMDA receptor within the PVN was increased in HF rats in agreement with previous data, the current results demonstrate that NR1 expression in the PVN of HF ExT rats was not different from that in Sham Sed or Sham ExT rats. This normalization of NR1 expression within the PVN suggests a mechanism by which the RSNA responses to NMDA injected into the PVN are normalized by ExT in HF rats. Taken together, these results indicate that one mechanism by which ExT normalizes sympathetic outflow in HF is through normalization of glutamatergic mechanisms within the PVN.
The coronary artery ligation model of HF has been used extensively by this laboratory and others (35, 51). The utility of this model as a simulation of heart failure is demonstrated by increased left ventricular end-diastolic pressure, decreased dP/dt of the left ventricle, decreased ejection fraction, and greater than 30% infarct size at the time of the experiment, 6 to 8 weeks following the coronary artery ligation procedure. The advantage of using this model as opposed to other models of HF, such as ventricular pacing, is that ligation of the coronary artery mimics blockage of the artery, commonly seen in patients with HF. Additionally, in the current study, we found that ExT only partially improved the increased LVEDP and the decreased dP/dt and did not improve the decreased ejection fraction associated with HF, indicating that ExT did not normalize cardiac function per se in this model of HF.
We also analyzed the efficacy of our regimen of ExT. Citrate synthase activity is commonly used as a marker for the metabolic capacity of muscle (43) and is increased in skeletal muscle by exercise (11). We measured citrate synthase activity in the soleus muscle in all four groups and found that it was increased in both of the ExT groups compared to the Sed groups, indicating not only an effect of the ExT itself but also a similar level of ExT in the Sham ExT and HF ExT groups.
The PVN has a major role within the forebrain with a role in direct regulation of sympathetic outflow (48). Preautonomic neurons originating in the dorsal and lateral parvocellular divisions of the PVN project to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord directly (40) and indirectly via the rostral ventrolateral medulla (40). Importantly, spinally-projecting preautonomic PVN neurons have been shown to be sensitive to NMDA (5, 37). The PVN is involved in fluid balance and vasopressin release (48), receives afferent input from cardiac vagal neurons (24), and is reciprocally connected to other cardiovascular control regions within the brain (18). In normal animals, stimulation of the PVN increases discharge of renal sympathetic nerves (17), whereas the activity of PVN neurons is increased in HF rats (32). Given these characteristics of the PVN, as well as the increase in sympathetic outflow (10, 31) and salt and water retention in HF (8), there is good evidence that the PVN is involved in the increased sympathetic activity associated with HF (30). The results of the current study are in agreement with this idea; the increase in sympathetic nerve activity in response to NMDA injected into the PVN, as well as the expression of NR1 within the PVN, is increased in HF rats compared to sham rats.
In our NMDA microinjection experiments, it is possible that the spread of NMDA reached areas surrounding the PVN. Histological examination of the spread of dye injected at the end of each experiment revealed that the injections were limited to the PVN, although it is possible that the areas of spread of NMDA and dye did not precisely overlap. Thus, while the spread of dye was limited to the PVN, it is possible that NMDA affected regions outside the PVN, notably the dorsomedial nucleus of the hypothalamus (DMH), which is located rostrally to the PVN (33) and also has a significant role in modulation of sympathetic nerve activity, MAP, and HR (6). In our experiments in which the injection was found to be located outside of the PVN, the typical increase in RSNA, MAP, and HR in response to NMDA was not observed, indicating that the areas affected by NMDA in these animals were not involved in control of RSNA, MAP, or HR. This evidence indicates that the increases in RSNA, MAP, and HR in response to NMDA microinjection were most likely mediated by neurons located within the PVN.
A functional NMDA receptor comprises the NR1 subunit and at least one NR2 subunit (9, 45). In the present study we showed that expression of the NR1 subunit increases in the PVN in HF, and this increase is prevented with ExT. We also measured protein expression of an NR2 subunit that is abundant in the PVN, NR2B. Previous data from our laboratory indicate that expression of NR2B within the PVN is not altered by HF (21). In the current experiments, our data indicate that NR2B also is not altered by ExT. This suggests that changes in NMDA subunit expression, with implications for NMDA receptor function, may be limited to NR1. It is possible, however, that expression of at least one other NR2 subunit may be altered by HF and/or ExT. We did not examine expression of other NR subunits (NR2A, NR2C, NR2D, NR3A, or NR3B). The NR2A subunit is also expressed within the PVN, albeit less densely than NR2B (14). While the NR2C and NR2D subunits are also expressed within the PVN, the expression of the NR2B subunit is considerably greater, particularly in neurons within the parvocellular subdivisions (14). Nonetheless, it is possible that HF and/or ExT alter the expression of one or more of these subunits to affect the function of NMDA receptors, which remains to be examined.
In addition, while we did not measure glutamate levels in the current study, we have previously demonstrated (21) that glutamate levels within the
PVN are unaltered by HF. Thus, the increased glutamatergic response within the PVN in HF is due to increased expression of NR1 and not to a change in concentration of the receptor agonist.
We also measured NR1 protein expression within the SON, another area within the hypothalamus important in cardiovascular control, and found that it was not significantly changed in HF (in agreement with previous data (21)), nor as a result of ExT. This suggests that the changes in NR1 expression in hypothalamic cardiovascular control areas are selective for specific areas such as the PVN. Additionally, we measured NR1 protein expression within the lateral hypothalamus, which is adjacent to the PVN, and in the cortex, to serve as anatomic controls and found that NR1 expression was not changed in either region as a result of HF or ExT, further lending support to our conclusion that changes in NR1 expression in HF and/or with ExT are anatomically specific to the PVN. Furthermore, when NMDA was injected into an area adjacent to the PVN, little or no change in RSNA was observed, indicating that the alterations in NMDA-mediated RSNA responses are specific and limited to the PVN.
The source(s) for the changes in NR1 expression within the PVN in HF and ExT are not entirely clear since there are multiple possibilities. Decreased cardiac output as a result of HF may increase stimulation of cardiopulmonary receptors, which send afferent neural input to the PVN. Humoral factors, which are both central and peripheral, are also changed in HF. For example, in the current study, plasma Ang II concentration is increased in HF Sed rats compared to Sham Sed rats, but in the HF ExT rats the level of plasma Ang II was not different from that in the Sham Sed or Sham ExT groups. The Ang II type I (AT1) receptor has been shown to participate in a variety of neuronal intracellular signaling pathways (for review see (46)) and thus may play a role in modulation of NR1 gene expression. Although neurons within the PVN cannot directly detect the concentration of Ang II in the plasma, these neurons receive input from circumventricular organs, notably the subfornical organ (SFO) (26). PVN-projecting SFO neurons increase firing in response to Ang II (1) and the SFO-PVN projection is angiotensinergic (2). It is possible that the augmented plasma Ang II associated with HF chronically increases firing of these PVN-projecting SFO neurons, which would restore the enhanced glutamatergic mechanisms within the PVN to control levels.
Another factor which may be involved in the alteration of glutamatergic mechanisms within the PVN is nitric oxide (NO), which acts as an unconventional neurotransmitter in the central nervous system (39) and has been shown to regulate gene expression (3). We have previously demonstrated that prior injection of L-NMMA into the PVN significantly augments the increase in RSNA in response to NMDA injected into the PVN (22), suggesting negative feedback from NO on the NMDA system within the PVN. We have also shown that the inhibitory effect of NO within the PVN is blunted in HF as is nNOS expression (50), but these are normalized by ExT (52). The loss of negative feedback by NO due to the downregulation of nNOS within the PVN in the HF state may remove an inhibitory modulation of NO on the expression of NR1. Thus it is possible that the decrease in NO mechanisms in HF may contribute to the increase in glutamatergic mechanisms within the PVN. The restoration of nNOS within the PVN by ExT thus may restore the NO-mediated negative feedback on glutamatergic mechanisms, normalizing NR1 expression and NMDA-mediated changes in RSNA. NMDA receptor activation in the NTS has been shown to increase NO release (25). Thus, if this system also exists within the PVN, another possibility is that the release of NO by NMDA receptor activation is altered by HF. However, to our knowledge, NMDA receptor activation has not been shown to regulate NO release within the PVN.
It is also possible that the effects of ExT on the PVN in HF are mediated by other mechanisms. For example, the mechanisms by which the exercise pressor reflex activates PVN neurons (20) and by which the reflex itself is altered in HF (42) are not understood, but it is possible that HF alters PVN neurons involved in the exercise pressor reflex. Other mechanisms that are altered by HF and ExT, such as baroreceptor (15), cardiopulmonary (36), chemoreceptor (47), or cardiac sympathetic afferent (49) reflexes, as well as input from central command (12) or from humoral factors such as atrial natriuretic factor (41) or vasopressin (38) may be involved as well.
In conclusion, the results of the present study demonstrate that ExT attenuates the potentiated increase in RSNA in response to NMDA injected into the PVN in HF rats. Normalization of expression of the NR1 subunit of the NMDA receptor may underlie this effect. Therefore, one mechanism by which ExT restores sympathetic outflow in HF is through normalization of glutamatergic mechanisms within the PVN.
Perspectives and significance
HF is commonly characterized by an overall increase in sympathoexcitation, an effect intended to compensate for the decrease in cardiac output but which ultimately exacerbates the condition. Uncovering the mechanism(s) involved in this increased sympathetic outflow may lead to improved treatment of this particular symptom of the disease, improving quality of life and survival. We have demonstrated, both previously and in the current work, that alterations within the glutamatergic system within the PVN play a role in the hyperactivity of the renal nerves in HF, thus identifying this systemas a potential therapeutic target. Furthermore, among the numerous beneficial effects of ExT on the HF state is alleviation of the increased sympathoexcitation. In the current set of experiments, we have shown that one of the ways ExT improves sympathetic outflow is through normalization of the enhanced glutamatergic mechanisms within the PVN. This understanding of the mechanisms of ExT-induced normalization of sympathoexcitation furthers our knowledge of the mechanisms underlying some of the symptoms of HF, offers a target in care of the disease, and emphasizes the importance of ExT in treatment of HF.
Acknowledgments
This work was supported by NIH grant HL62222 (K.P.P.) and American Heart Association predoctoral fellowship 05515518Z (A.C.K.). The technical assistance of Dr. Xuefei Liu, Dr. Kurtis Cornish, and Phyllis Anding is greatly appreciated.
References
- 1.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res. 2001;921:78–85. doi: 10.1016/s0006-8993(01)03093-1. [DOI] [PubMed] [Google Scholar]
- 2.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 4.Brandle M, Patel KP, Wang W, Zucker IH. Hemodynamic and norepinephrine responses to pacing-induced heart failure in conscious sinoaorticdenervated dogs. JApplPhysiol. 1996;81:1855–1862. doi: 10.1152/jappl.1996.81.4.1855. [DOI] [PubMed] [Google Scholar]
- 5.Brown MH, Badura LL, Nunez AA. Evidence that neurons of the paraventricular nucleus of the hypothalamus with projections to the spinal cord are sensitive to the toxic effects of N-methyl aspartic acid. Neurosci Lett. 1987;73:103–108. doi: 10.1016/0304-3940(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Dampney RAL, Horiuchi J, Killinger S, Sheriff MJ, Tan PSP, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharm Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Saunders B, Trapp RG. Basic and Clinical Biostatistics. Appleton & Lange; Norwalk, CT: 1994. [Google Scholar]
- 8.DiBona GF, Herman PJ, Sawin LL. Neural control of renal function in edema-forming states. Am J Physiol. 1988;254:R1017–R1024. doi: 10.1152/ajpregu.1988.254.6.R1017. [DOI] [PubMed] [Google Scholar]
- 9.Dingledine R, Borges K, Bowie D, Traynelis S. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 10.Feng QP, Carlsson S, Thoren P, Hedner T. Characteristics of renal sympathetic nerve activity in experimental congestive heart failure. Acta Physiol Scand. 1994;150:259–266. doi: 10.1111/j.1748-1716.1994.tb09685.x. [DOI] [PubMed] [Google Scholar]
- 11.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol. 1975;228:1029–1033. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara K, Floras JS. After-effects of exercise on hemodynamics and muscle sympathetic nerve activity in young patients with dilated cardiomyopathy. BrHeart J. 1996;75:602–608. doi: 10.1136/hrt.75.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–362. [PubMed] [Google Scholar]
- 15.Higgins CB, Vatner SF, Eckberg DL, Braunwald E. Alterations in the baroreceptor reflex in conscious dogs with heart failure. J Clin Invest. 1972;51:715–724. doi: 10.1172/JCI106865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung R, Dibner-Dunlap ME, Gilles MA, Thames MD. Cardiorespiratory reflex control in rats with left ventricular dysfunction. AmJPhysiolHeart CircPhysiol. 1995;268:H218–H225. doi: 10.1152/ajpheart.1995.268.1.H218. [DOI] [PubMed] [Google Scholar]
- 17.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. AmJPhysiolRegulIntegrCompPhysiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 18.Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: Their possible involvement in neural control of the cardiovascular system in rats. Brain Res. 1985;329:205–212. doi: 10.1016/0006-8993(85)90526-8. [DOI] [PubMed] [Google Scholar]
- 19.Levett JM, Marinelli CC, Lund DD, Pardini BJ, Nader S, Scott BD, Augelli NV, Kerber RE, Schmid PG, Jr. Effects of β-blockade on neurohumoral responses and neurochemical markers in pacing-induced heart failure. AmJPhysiolHeart CircPhysiol. 1994;266:H468–H475. doi: 10.1152/ajpheart.1994.266.2.H468. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Hand GA, Potts JT, Mitchell JH. Identification of hypothalamic vasopressin and oxytocin neurons activated during the exercise pressor reflex in cats. Brain Res. 1997;752:45–51. doi: 10.1016/s0006-8993(96)01443-6. [DOI] [PubMed] [Google Scholar]
- 21.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. CircRes. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 22.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. AmJPhysiolHeart CircPhysiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- 23.Liu J-L, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 24.Lovick TA, Coote JH. Effects of volume loading on paraventriculo-spinal neurones in the rat. JAutonNervSyst. 1988;25:135–140. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo I, Hirooka Y, Hironaga K, Eshima K, Shigematsu H, Shihara M, Sakai K, Takeshita A. Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo. Am J Physiol. 2001;280:R1285–R1291. doi: 10.1152/ajpregu.2001.280.5.R1285. [DOI] [PubMed] [Google Scholar]
- 26.Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 27.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. AmJPhysiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 28.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 29.Palkovits M, Brownstein M. Brain microdissection techniques. In: Cuello AE, editor. Brain microdissection techniques. John Wiley & Sons; Chichester: 1983. [Google Scholar]
- 30.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 31.Patel KP, Zhang K, Carmines PK. Norepinephrine turnover in peripheral tissues of rats with heart failure. AmJPhysiolRegulIntegrCompPhysiol. 2000;278:R556–R562. doi: 10.1152/ajpregu.2000.278.3.R556. [DOI] [PubMed] [Google Scholar]
- 32.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. AmJPhysiolRegulIntegrCompPhysiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando: 1986. [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. CircRes. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 36.Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol. 2003;95:1883–1888. doi: 10.1152/japplphysiol.00486.2003. [DOI] [PubMed] [Google Scholar]
- 37.Porter JP. Contribution of spinal N-methyl-D-aspartic acid receptors to control of sympathetic outflow by the paraventricular nucleus. Brain Res Bull. 1993;32:653–660. doi: 10.1016/0361-9230(93)90169-c. [DOI] [PubMed] [Google Scholar]
- 38.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119:S47–S53. doi: 10.1016/j.amjmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Schuman EM, Madison DV. Nitric oxide and synaptic function. Ann Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 40.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 41.Shenker Y, Sider RS, Ostafin EA, Grekin RJ. Plasma levels of immunoreactive atrial natriuretic factor in healthy subjects and in patients with edema. J Clin Invest. 1985;76:1684–1687. doi: 10.1172/JCI112154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 43.Spina RJ, Chi MM-Y, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- 44.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 45.Sucher NJ, Awobuluyi M, Choi Y-B, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- 46.Summers C, Fleegal MA, Zhu M. Angiotensin AT1 receptor signalling pathways in neurons. Clin Exp Pharm Physiol. 2002;29:483–490. doi: 10.1046/j.1440-1681.2002.03660.x. [DOI] [PubMed] [Google Scholar]
- 47.Sun S-Y, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 48.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol. 1996;40:R751–R756. doi: 10.1152/ajpregu.1996.271.3.R751. [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, Li Y-F, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. AmJPhysiol. 2001;281:H995–1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. AmJPhysiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 52.Zheng H, Li Y-F, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]