Abstract
Importance:
Although white matter hyperintensities (WMH) are associated with risk for Alzheimer’s disease (AD), it is unknown whether they represent an independent source of impairment or whether they interact with known markers of disease. Here, we examined the important question of whether WMH predict aggressive cognitive decline, either independently or interacting with markers of AD-related neurodegeneration, in individuals at risk for AD.
Objective:
To examine the degree to which WMH predict aggressive cognitive decline among individuals with mild cognitive impairment (MCI), either independently or by modifying the effects of entorhinal cortex volume (ECV), a marker of AD-related neurodegeneration.
Design/Setting/Participants:
The Alzheimer’s Disease Neuroimaging Initiative is a longitudinal study with 6-month follow-up visits. Three hundred thirty-two subjects (mean±SD age=74.6±7.4, 118 women) of a total of 374 participants diagnosed with MCI were included. Participants were excluded if they did not have longitudinal data, APOE genotype data, or had evidence of supratentorial infarct.
Main outcome measures:
A decline in Mini Mental State Examination (MMSE) score of 3 points over 6 months or 6 points over one year between consecutive visits was defined as “aggressive” decline. White matter hyperintensity volume and ECV were entered as predictors in Cox Proportional Hazards models and Wilcoxon-Breslow tests to examine their impact on this outcome, adjusting for sex, age, education, and apoE status.
Results:
Greater WMH volume at baseline, APOE ε4 status, and smaller ECV at baseline were associated with increased risk of aggressive decline (HR=1.23, p-value=0.01, HR=1.4, p-value=0.04; HR=0.66, p-value <0.001 respectively). White matter hyperintensity volume modified the effect of ECV on aggressive decline risk: individuals with high ECV and low WMH were at particularly low likelihood of decline (X2=15, p=0.001).
Conclusions:
White matter hyperintensity burden and ECV predict rapid cognitive decline among individuals with MCI both additively and multiplicatively.
Introduction
Despite contemporary models of Alzheimer’s disease (AD) pathogenesis, which emphasize the precipitating role of β amyloid and subsequent neurodegenerative changes due to tau pathology 1, small vessel cerebrovascular disease has emerged as an important driver of risk and clinical expression of the disease. We previously showed that individuals with prevalent AD and those at risk for AD have increased burden of small vessel cerebrovascular changes, visualized as increased white matter hyperintensities (WMH) on T2-weighted magnetic resonance imaging (MRI)2. Increased WMH burden also predicts incident AD 3 and individuals with evidence of amyloidosis are more likely to exhibit symptoms of dementia if they have substantial WMH burden 4. The degree to which WMH burden contributes to clinically meaningful decline in individuals at risk for AD remains an important question.
The diagnostic category of Mild Cognitive Impairment (MCI) refers to the intermediate stage of a three part journey that begins with normal cognitive aging and ends with dementia due to AD5. Individuals with MCI have objective evidence of cognitive impairment but without the functional impairment that interferes with their daily activities5. When clinically defined, there is much heterogeneity in the rate of cognitive decline among individuals with MCI, with some progressing quite precipitously while others remain cognitively stable and functionally unimpaired 6. Work that has examined clinical outcomes in MCI tends to focus on “conversion” to AD, where the threshold between the two is defined by a switch from a functionally unimpaired state to a cognitive syndrome defined by functional impairment 7.
The Mini Mental State Examination (MMSE) 8 is one of the most common tools used by clinicians to follow patients’ progression over time. Many efforts to classify progression rates in MCI as well AD stages relied on the MMSE and have shown great heterogeneity, possibly due to real individual difference in progression rates, biased sampling in terms of baseline characteristics, and/or floor and ceiling effects of the scales administrated. Operational definitions of MMSE drop thresholds to assess “rapidity” or “aggressiveness” of progression have been long debated: declines of 3 9, 4 or 7 points/year 10 and 3 points/6 months 11 have been proposed as classification criteria. However, much confusion exists not only in terms of cut-offs but also regarding baseline level of impairment and observational periods; previous studies applied these thresholds to a wide range of baseline MMSE 11,12 including mild to moderate AD across a large range of follow-up periods (MMSE decline in 6 to 24 12 months, escalating cognitive impairment in three years13 or survival time of less than 4 years14). A recent study, based on extensive review of previous studies 15, suggested that a decline of 6 points/year on the MMSE should be used as the optimal threshold to define rapidly progressive AD.
Regardless of the threshold chosen, the value of this approach is that it clusters patients within discrete classes of progression by defining a “rapid” event as a MMSE point drop that deviates from what is the average points drop in AD (2.5/year) and obviously in MCI (1/year). This approach may be ideal for clinical practice because it provides an operational definition that can be applied to an individual patient.
The purpose of this study was to examine whether WMH predict the rapidity of cognitive decline in MCI as measured by clinically-defined categorical changes in MMSE scores over time. We hypothesized that WMH burden would predict clinical outcomes independent of a measure of entorhinal cortex atrophy, a putative biological marker of neurodegeneration due to AD 1,16, and APOE-ε4, a well-known genetic risk factor for AD. We also explored whether WMH volume and entorhinal cortex atrophy interact to predict clinical outcome.
Materials and Methods
ADNI
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO and ADNI-2. To date these three protocols have recruited over 1500 adults, ages 55 to 90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
Subjects
Data from subjects diagnosed with MCI (n=374) were downloaded from the ADNI database (www.loni.usc.edu/ADNI) and included demographic, genetic, and data from structural MRI scans. All participants included had amnestic MCI. The ADNI study was designed to parallel procedures employed in a clinical trial and thus only included participants who were in good medical health. Importantly, individuals were excluded from participation if they had a significant vascular disease risk history, defined as a modified Hachinski score17 greater than 4. Diagnosis of MCI was based on standard research criteria and included age between 55 and 90, a memory complaint (study subject or informant), objective evidence of abnormal memory, Clinical Dementia Rating (CDR) score of 0.5, with a Memory Box score of at least 0.5, MMSE score between 24 and 30 (inclusive), general cognition preserved such that a diagnosis of AD could not be made, stable medication, and not depressed (Geriatric Depression Scale18 score of less than 6). Recruitment and diagnostic procedures have been reported in detail previously 19.
For the current analyses, participants were included if they had diagnosis of MCI at baseline, demographic variables, APOE genotype, assessment of MRI predictors selected for this study. Twenty-eight subjects were excluded because of radiological evidence of supratentorial infarcts. An additional 14 subjects were excluded if that had outlying values (i.e., greater than 3 standard deviations deviating from the mean) for any of the primary variables or if they did not have longitudinal data. Thus, the final number of participants included in the analysis was 332.
Clinical Outcome
Due to unequally spaced MMSE assessment in ADNI follow-up visits (the scale was administrated at screening visits, 6, 12, 18, 24, 36, 48 months) and to missing visits, a progression rate of 3 points drop/6 months or 6 points drops/year was considered as the event outcome, indicating “rapid progression,” in the survival analyses as defined in previous studies 15. None of the included subjects showed a final MMSE below 10 points (i.e. what traditionally defines “severe stage”)
Neuroimaging acquisition
A standardized MRI protocol for image acquisition was implemented across ADNI sites, which was validated across platforms 20. All data acquisition was performed on 1.5 Tesla systems. T1-weighted volumetric magnetization prepared rapid gradient echo sequences were acquired in the sagittal orientation. A proton density/T2-weighted fast spin echo sequence was acquired in the axial orientation. Sites included in the ADNI protocol were required to pass rigorous scanner validation tests and scan acquisitions for each subject included a fluid-filled phantom. Details of the validation procedures are provided elsewhere 20 (www.loni.usc.edu/ADNI).
White matter hyperintensity quantification
White matter hyperintensity volumetric quantification has been described in detail elsewhere 21,22. Briefly, the T1-, T2-, and PD-weighted MRI scans were co-registered and skull-stripped 23,24. After bias field correction 25, WMH were detected in minimum deformation template space at each voxel based on corresponding PD, T1, and T2 intensities, the prior probability of WMH, and the conditional probability of WMH based on the presence of WMH at neighboring voxels. Labeled voxels were summed and multiplied by voxel dimensions to yield total WMH volumes. White matter hyperintensity volumes estimated with this method agreed strongly with WMH volumes estimated from fluid attenuated inverse recovery MRI in a large, diverse elderly sample 22.
Entorhinal cortex volume
Structural MRI parcellation and segmentation data were downloaded from the ADNI website. ADNI structural MRI data were analyzed with FreeSurfer version 4.3 (https://surfer.nmr.mgh.harvard.edu) at University of California, San Francisco after the T1-weighted MRI scans were converted to NiFTI format and pre-processed at Mayo Clinic26. For the purposes of the current analyses, we focused on entorhinal cortex volumes.
Covariates and APOE genotyping
Gender, education and age at baseline were included as covariates in all the models presented. ApoE genotyping was based on allelic combinations of SNPs rs7412 and rs429358.
Statistical analysis
Cox proportional hazards models and Wilcoxon-Breslow test were constructed to examine the impact of baseline WMH volume on clinical outcome. Visits that occurred outside the tolerance range of ± 2 months per expected visit agenda were excluded. A total 48 months follow-up period was defined for our analysis. As secondary analyses, both ECV and WMH were dichotomized in order to define low and high WMH load and ECV volume subgroups.
Enter selection method was carried out to identify independent factors prognostic for survival: total WMH together with age, sex and education as covariates were included in the primary analysis. Secondary analyses were carried out including also well-established risk factors for conversion and rapid progression: APOE ε4 status and entorhinal cortex volume.
In order to weigh more the early occurrence of the defined event, survival curves constructed through Wilcoxon-Breslow test were computed contrasting four sub-populations (high vs. low entorhinal cortex groups stratified by high and low WMH groups, both defined by median split).
The importance of a prognostic variable was assessed via Wald-type test statistics, the hazard ratio, and its 95% confidence interval for survival. Alpha levels were set a priori at 0.05. Additional sensitivity analysis was performed through 1000 bootstrap generated simulation datasets to confirm the results obtained in the Cox regression model.
RESULTS
Descriptive data for participants’ characteristics are presented in Table 1. Subjects labeled as rapid progressors did not differ from the rest of the sample in terms of sex distribution, age at baseline, and number of years of education.
Table 1.
Patients demographic.
| ADNI MCIs (n= 332) | |
|---|---|
| Male/female | 219/118 |
| Age at baseline (sd) | 74.6 (7.4) |
| Education (sd) | 15.6 (3) |
| Any apoε4 (%) | 52,5% |
| White Matter Hyperintensity (log10 transformed) | |
| Entorhinal cortex volume (sd) | 1650.46 (385) |
| Subjects matching “rapid progression” definition (%) |
49 |
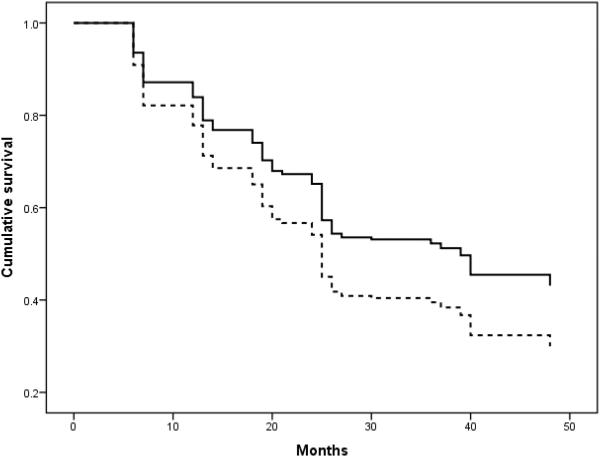
Higher WMH volume at baseline was associated with an increased risk of rapid decline over the follow-up period (HR=1.23, CI: 1.05-1.43, p-value=0.01). None of the other covariates included reliably predicted rapid decline. Figure 1 displays the cumulative survival of individuals with high and low amounts of WMH.
FIGURE 1.
Cumulative survival of individuals with the highest quartile of WMH (dotted lines) versus the individuals in the lowest three quartiles of WMH (solid line)
When APOE ε4 status and ECV were included in the model, total WMH remained a predictor with similar magnitude (HR=1.2, CI: 1.02-1.4, p-value=0.03). In the full model, both ApoE and ECV predicted outcomes (HR=1.4, p-value=0.03; HR=0.66, p-value <0.001 respectively).
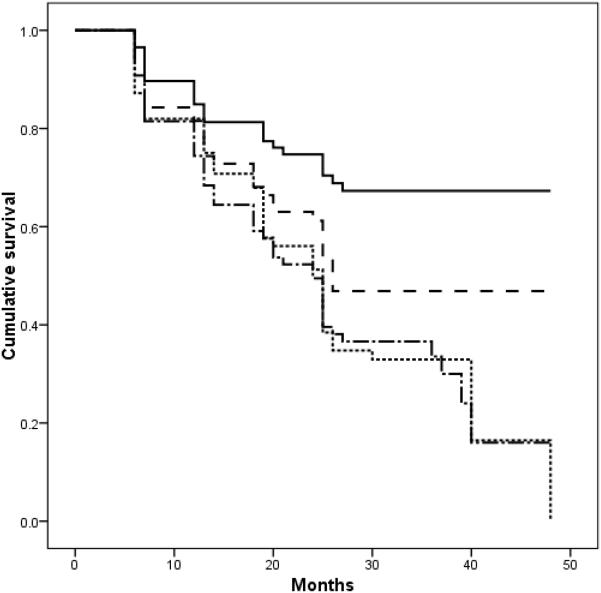
The Wilcoxon-Breslow survival analysis was significant (p=0.001) for the four subgroups identified by dichotomized ECV, stratified by WMH severity; individuals with high ECV and low WMH appeared to be at particularly low likelihood of declining rapidly (Figure 2).
FIGURE 2.
Cumulative survival of individuals in the with high ECV and low WMH (solid line), high ECV and high WMH (dashed line), low ECV and low WMH (dotted line), and low ECV and high WMH (dash-dotted line). Groups were defined by median split.
Subjects experiencing MMSE points drop of ≥ 3 on 6month or ≥ 6 on 12 months were more likely to have converted to AD by the end of the follow-up period ( X2=82, p<0.001).
DISCUSSION
By focusing on clinically-meaningful definitions that utilize the MMSE, one of the most widely used clinical instruments, we demonstrated that the severity of WMH predicts the likelihood that individuals with MCI have an aggressive clinical course. We also confirmed that diminished ECV, a marker of neurodegeneration associated with AD, predicts aggressive course with similar magnitude. Importantly, the two biological markers interact such that individuals with large ECV and small amounts of WMH burden appear to have synergistically-diminished risk for decline. This latter finding suggests a mechanistic interaction between the two pathologic markers on clinical course.
A diagnosis of MCI increases individuals’ risk for future development of AD, but it is not synonymous with a diagnosis of early AD. Indeed, several individuals with MCI do not ultimately “convert” to AD or have a precipitous clinical decline 27. However, the design of the ADNI study included individuals with “late MCI,” thought to be at a high risk to develop clinical AD. The question of what factors have prognostic utility in determining which individuals diagnosed with MCI have a precipitous clinical event through their follow-up is critical to both clinicians making the MCI diagnosis and to the individuals receiving the diagnosis. Here, we showed that the burden of WMH is a reliable predictor of which patients, diagnosed with MCI at baseline, will decline with an aggressive clinical course. We purposefully focused on a psychometrically-defined criterion for aggressive course rather than, say, “conversion” to AD, to parallel explicitly outcomes that are common in clinical settings where research diagnostic procedures (e.g., amyloid imaging, cerebrospinal studies) are less available. Nonetheless, we recognize that “aggressive course” in MCI overlaps to a certain extent with “conversion” to AD and demonstrated that those who had a more precipitous decline were indeed more likely to carry an AD diagnosis on follow-up examination.
To capture important prognostic information, we applied an operational cut-off that defines an “aggressive” drop in cognitive performance during a narrow observational window in subjects at risk or in very early stages of the disease. Previous research has applied a similar approach to a wide range of AD severity. For example, Doody and colleagues 28 showed that estimated progression rates prior to enrollment in the study, computed with the formula ((30 - baseline MMSE)/years since symptoms onset), predicted future changes in cognition and activities of daily living, and mortality. The baseline measure identified a slow, intermediate, and fast-progressing group and reliable differences among the three groups persisted or increased even on a long-term follow-up observation. The MMSE has a well-known “floor effect” among patients with prevalent AD 29; thus, its utility in detecting dementia progression is somewhat limited among more impaired patients. By examining MCI patients in the current study, we avoided the problem of floor effects.
Our observations add to a growing body of literature that implicates WMH in the clinical course and, possibly, pathogenesis of AD. In community-based studies, we previously showed that WMH volume is elevated in individuals with MCI2 and AD30, predicts future incident AD among non-demented older adults3, progresses over time in individuals with in incident AD, and predicts rate of cognitive decline among individuals with prevalent AD 31. Our previous efforts in ADNI showed that among individuals with evidence of amyloidosis, those with elevated WMH were more likely to meet clinical criteria for AD than those with lower amounts of WMH 4. We also showed that WMH volume is correlated with degree of atrophy in the entorhinal cortex among individuals with MCI 32. Here, we extend those findings and suggest that medial temporal lobe atrophy, reflecting neurodegeneration, and WMH may interact mechanistically or result from a common upstream driver. Whether the relationship between WMH and neurodegenerative changes is fundamental or epiphenomenological may still be up for debate, but what is clear is that WMH at least contribute additively to clinical course in the context of other AD biological markers.
In conclusion, our findings demonstrate that both WMH and entorhinal cortex volume predict rapid progression in early stages of the disease and interact synergistically. The findings may be useful for prognosis of outcomes especially in clinical settings but also contribute to a growing body of work that implicates small vessel cerebrovascular disease in AD pathogenesis and clinical expression. Further, because many of the risk factors for WMH have been established and are modifiable through lifestyle or pharmacological intervention, our findings suggest avenues for prevention or treatment of rapid progressing course among patients with MCI.
Table 2.
Cox regression model results.
| ADNI MCIs (n= 332) | ||||||||
|---|---|---|---|---|---|---|---|---|
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | |||||
| HR (CI) | p | HR (CI) | p | HR (CI) | p | HR (CI) | p | |
| SEX | 1.2 (.9-1.64) | ns | 0.92 (0.66-1.28) | ns | 1.19 (.87-1.64) | ns | .94 (.68-1.32) | ns |
| AGE | .99 (.97-1.01) | ns | 0.98 (0.96-1) | ns | 1 (.98-1.02) | ns | .98 (.96-1.01) | ns |
| EDUCATION | .98 (.92-1.02) | ns | 0.97 (0.92-1.02) | ns | .98 (.93-1.03) | ns | .97 (.92-1.02) | ns |
| ApoE4 | 1.49 (1.09-2.05) | .013 | 1.41 (1.03-1.93) | .03 | ||||
| ECV | 0.64 (0.54-0.76) | <.001 | .66 (.55-, 79) | <.001 | ||||
| WMH | 1.23 (1.05-1.43) | .01 | 1.2 (1.02-1.4) | .03 | ||||
“HR”: hazard ratio. “C.I.”: confidence interval, “p”: p-value
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
REFERENCES
- 1.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet neurology. 2013 Feb;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Brickman AM, Reitz C, et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009 Aug 11;73(6):450–456. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Archives of neurology. 2012 Dec;69(12):1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provenzano FA, Muraskin J, Tosto G, et al. White Matter Hyperintensities and Cerebral Amyloidosis: Necessary and Sufficient for Clinical Expression of Alzheimer Disease? JAMA Neurol. 2013 Feb 18;:1–7. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2010 May;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of neurology. 2008 Apr;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Carcaillon L, Peres K, Pere JJ, Helmer C, Orgogozo JM, Dartigues JF. Fast cognitive decline at the time of dementia diagnosis: a major prognostic factor for survival in the community. Dementia and geriatric cognitive disorders. 2007;23(6):439–445. doi: 10.1159/000102017. [DOI] [PubMed] [Google Scholar]
- 10.Ballard C, O'Brien J, Morris CM, et al. The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer's disease. International journal of geriatric psychiatry. 2001 May;16(5):499–503. doi: 10.1002/gps.381. [DOI] [PubMed] [Google Scholar]
- 11.Soto ME, Andrieu S, Cantet C, et al. Predictive value of rapid decline in mini mental state examination in clinical practice for prognosis in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2008;26(2):109–116. doi: 10.1159/000144073. [DOI] [PubMed] [Google Scholar]
- 12.Buccione I, Perri R, Carlesimo GA, et al. Cognitive and behavioural predictors of progression rates in Alzheimer's disease. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2007 Apr;14(4):440–446. doi: 10.1111/j.1468-1331.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava D, Weiner MF, Hynan LS, Diaz-Arrastia R, Lipton AM. Vascular disease and risk factors, rate of progression, and survival in Alzheimer's disease. Journal of geriatric psychiatry and neurology. 2006 Jun;19(2):78–82. doi: 10.1177/0891988706286505. [DOI] [PubMed] [Google Scholar]
- 14.Josephs KA, Ahlskog JE, Parisi JE, et al. Rapidly progressive neurodegenerative dementias. Archives of neurology. 2009 Feb;66(2):201–207. doi: 10.1001/archneurol.2008.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt C, Wolff M, Weitz M, Bartlau T, Korth C, Zerr I. Rapidly progressive Alzheimer disease. Archives of neurology. 2011 Sep;68(9):1124–1130. doi: 10.1001/archneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet neurology. 2010 Jan;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachinski VC, Iliff LD, Zilhka E, et al. CErebral blood flow in dementia. Archives of neurology. 1975;32(9):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010 Jan 19;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Jr., Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008 Apr;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmichael O, Xie J, Fletcher E, Singh B, DeCarli C. Localized hippocampus measures are associated with Alzheimer pathology and cognition independent of total hippocampal volume. Neurobiol Aging. 2012 Jun;33(6):1124 e1131–1141. doi: 10.1016/j.neurobiolaging.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging. 2009;21:239–251. doi: 10.1007/978-3-642-02498-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999 Aug;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 24.Wolz R, Julkunen V, Koikkalainen J, et al. Multi-method analysis of MRI images in early diagnostics of Alzheimer's disease. PLoS One. 2011;6(10):e25446. doi: 10.1371/journal.pone.0025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996 May-Jun;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 26.Wyman BT, Harvey DJ, Crawford K, et al. Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement. 2013 May;9(3):332–337. doi: 10.1016/j.jalz.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer disease. Archives of neurology. 2001 Mar;58(3):449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 29.Franco-Marina F, Garcia-Gonzalez JJ, Wagner-Echeagaray F, et al. The Mini-mental State Examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. International psychogeriatrics / IPA. 2010 Feb;22(1):72–81. doi: 10.1017/S1041610209990822. [DOI] [PubMed] [Google Scholar]
- 30.Meier IB, Manly JJ, Provenzano FA, et al. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012 May;18(3):414–427. doi: 10.1017/S1355617712000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brickman AM, Honig LS, Scarmeas N, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Archives of neurology. 2008 Sep;65(9):1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman VA, Carmichael OT, Schwarz C, Tosto G, Zimmerman ME, Brickman AM. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimer's & Dementia. 2013;9(5, Supplement):S124–S131. doi: 10.1016/j.jalz.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]