Abstract
Oxytocin (OXT) is well known for its ability to the milk ejection reflex and uterine contraction. It is also involved in several other behaviors, such as anti-nociception, anxiety, feeding, social recognition, and stress responses. OXT is synthesized in the magnocellular neurosecretory cells (MNCs) in the hypothalamic paraventricular (PVN) and the supraoptic nuclei (SON) that terminate their axons in the posterior pituitary (PP). We generated transgenic rats that express the OXT and fluorescent protein fusion gene in order to visualize OXT in the hypothalamo-neurohypophysial system (HNS). In these transgenic rats, fluorescent proteins were observed in the MNCs and axon terminals in the PP. This transgenic rat is a new tool to study the physiological role of OXT in the HNS.
Keywords: CCK, c-fos, double transgenic rat, mRFP1, paraventricular nucleus, posterior pituitary, supraoptic nucleus
Introduction
Oxytocin (OXT), a nine amino acid neuropeptide, was discovered in 1906 as an extract with uterus-contracting effects from the pituitary (Dale, 1906). In 1953, OXT was the first peptide hormone to be sequenced and synthesized (du Vigneaud et al., 1953a,b, 1954). OXT is synthesized primarily in magnocellular neurosecretory cells (MNC) in the hypothalamic paraventricular (PVN) and the supraoptic nuclei (SON), which cells project their axon terminals into the posterior pituitary (PP), where it is released into the systemic circulation, in the same way as arginine vasopressin (AVP). OXT is well known for its roles in reproduction, especially during and after childbirth. Many previous studies have shown that OXT is involved in several physiological functions, such as antinociception, anxiety, feeding, social recognition, and stress responses (Carmichael et al., 1987, 1994; Stock and Uvnäs-Moberg, 1988; Uvnäs-Moberg et al., 1993; Leckman et al., 1994; Russell and Leng, 1998).
We recently have reported the generation and characterization of rats which faithfully express an AVP-enhanced green fluorescent protein (eGFP) fusion transgene (Ueta et al., 2005; Fujio et al., 2006; Shibata et al., 2007; Suzuki et al., 2009; Maruyama et al., 2010; Todoroki et al., 2010; Iwanaga et al., 2011; Ohno et al., 2012). Previous studies that used animals to examine OXT dynamics by fluorescent visualization reported about OXT-enhanced cyan fluorescent protein (eCFP) transgenic mice (Young et al., 1999; Zhang et al., 2002). Although we first generated an OXT-eCFP transgenic rat, the expression of the transgene was unstable for unknown reasons (Katoh et al., 2010). Monomeric red fluorescent protein (mRFP) was developed from DsRed, which is the red fluorescent protein from Discosoma (Campbell et al., 2002; Long et al., 2005), and we succeeded in generating transgenic rats bearing an OXT-mRFP1 fusion gene (Katoh et al., 2011).
In this review, we focus on (1) the distribution of OXT, (2) the regulation of synthesis and release of OXT, and (3) recent research about the visualization of OXT in OXT transgenic animals using a fluorescent protein, which is a new tool to study the physiological role of OXT in the hypothalamo-neurohypophysial system (HNS).
Distribution of oxytocin and oxytocin receptor
In MNC in the PVN and the SON, OXT neurons project their axon terminals into the PP. In parvocellular neurosecretory cells (PNC) in the PVN, OXT neurons project their axon terminals to the spinal code, including the intermediolateral nucleus and gelatinous substance, where OXT have some role to modify pains and sympathetic nervous system (Sofroniew, 1980). OXT cells also project to the ambiguus nucleus, the nuclei of solitary tract (NTS), the dorsal motor nucleus of vagus, the Edinger–Westphal nucleus, circularis nucleus (CN), the parabrachial nucleus, the hippocampus, the amygdaloid nucleus, and the septulum (Reaves and Hayward, 1979; Nilaver et al., 1980; Sofroniew, 1980; Sofroniew et al., 1981; Hatton and Tweedle, 1982; Sawchenko and Swanson, 1982). Parvocellular OXT cells are found in the preoptic area and the lateral hypothalamus, whereas accessory magnocellular OXT cells are found scattered across the hypothalamus.
The central effects of OXT are mediated by OTRs distributed widely in the brain. OTR mRNAs are distributed in the ventromedial nucleus of the hypothalamus (VMH) and the PVN, which are involved in steroid-sensitive reproductive behaviors; in the substantia nigra and ventral tegmental area, which is involved in maternal behaviors; in the hippocampus, which is involved in learning and memory; and in the lateral septum, caudate putamen, amygdaloid nuclei, olfactory tubercle and cingulate, perirhinal, and frontal cortices, all of which are involved in reinforcement (Ostrowski, 1998).
Regulation of synthesis and release of oxytocin
OXT is produced in the MNC of the PVN and the SON, and is released into the systemic circulation from axon terminals in the neurohypophysis, particularly during parturition, lactation and in response to osmotic challenges (Burbach et al., 2006). The structure of the OXT gene was elucidated in 1984 (Ivell and Richter, 1984). Expression of the OXT gene is stimulated during pregnancy and lactation (Van Tol et al., 1988; Zingg and Lefebvre, 1988). Interestingly, although estrogen or progesterone alone does not increase OXT synthesis expression of the OXT gene in the PVN and the SON was increased by the prolonged administration of estrogen and progesterone, followed by progesterone withdrawal (Thomas and Amico, 1996). By contrast, OXT gene expression in the uterine was highly stimulated by the combined application of estrogen and progesterone (Lefebvre et al., 1994).
OXT is well known for its roles in reproduction, especially during and after childbirth. The pulsatile OXT release into the circulation is stimulated by vaginocervical stimulation associated with labor and the stimulus of suckling on the nipple. The uterine muscle increases its OXT receptors (OTRs) and sensitivity to OXT during the latter few months of pregnancy. That level of OXT release from the neurohypophysis is considerably increased at the time of labor. In lactation, OXT causes milk to be expressed from the alveoli into the ducts of the breast so that the baby can obtain it by suckling. The suckling stimulus on the nipple of the breast causes signals to be transmitted through sensory nerves to the OXT, secreting neurons in the MNC in the PVN and the SON. OXT in plasma is carried to the breast, where it causes contraction of myoepithelial cells that lie outside of and form a latticework surrounding the alveoli of the mammary glands. In less than a minute after a baby starts suckling, milk begins to flow.
The sequence of the OTR was reported in 1992 (Kimura et al., 1992; Kubota et al., 1996). Gonadal steroids play an important role in mediating the regulation of OTR expression. Most peripheral OXT-binding sites, including the pituitary, renal, and uterine, are upregulated by estrogens (Fuchs et al., 1983; Soloff et al., 1983; Maggi et al., 1992). The upregulation is accompanied by OTR mRNA expression, suggesting that the upregulation is a consequence of a genomic estrogen effect on the OTR gene transcription (Breton et al., 1995; Larcher et al., 1995). Behavioral studies have clearly shown that a necessary potential of OXT to elicit maternal or sexual behavior is priming with estrogen alone or with both estrogen and progesterone (Pedersen et al., 1982; Fahrbach et al., 1985). This evidence suggests that OTRs are under the control of gonadal steroids in the central nervous system (CNS).
OTR gene expression increases during pregnancy and/or at parturition in the olfactory bulb, medial preoptic area, bed nucleus of the stria terminalis (BNST), the SON, and in the medial amygdala in rat (Young et al., 1997; Meddle et al., 2007). Studies have shown that OTR-binding sites increase in the medial preoptic area, the BNST, VMH, and the ventral tegmental area on postpartum day 1 (Insel, 1990; Pedersen et al., 1994; Young et al., 1997). These changes suggest that OXT and OTR receptors play a role in both lactation and the regulation of maternal behavior.
OXT is also recognized as having endocrine and paracrine roles in male reproduction. OXT is synthesized within the mammalian testis, epididymis and prostate, and OTRs in the reproductive tract support a local action for OXT (Ivell et al., 1990, 1997; Foo et al., 1991; Nicholson and Hardy, 1992; Frayne and Nicholson, 1995, 1998; Harris et al., 1996; Filippi et al., 2002; Whittington et al., 2004). In ejaculation, a burst of OXT is released from the neurohypophysis into the systemic circulation and stimulates contractions of the reproductive tract for sperm release (Ogawa et al., 1980; Carmichael et al., 1987; Murphy et al., 1987). OXT plays a paracrine role in stimulating contractility of the seminiferous tubules, epididymis and the prostate gland.
Interestingly, OXT is also released from soma and dendrites during parturition and lactation (Ludwig and Leng, 2006). Although OXT released from the soma and dendrites of the MNC in the SON and the PVN may act in a paracrine to activate distant receptors (Ludwig and Leng, 2006), OXT-like immunoreactivity (LI) fibers can be found throughout the brain, including the nucleus accumbens (NAcc), lateral septum, amygdala, and some areas in the hindbrain, brainstem, and spinal cord (Sofroniew, 1980; Castel and Morris, 1988). A notable reduction of OXT-LI fibers was observed throughout the brain by the lesioning of the PVN (De Vries and Buijs, 1983). Although little is known about the regulation of OXT release from these forebrain projections, they might contribute significantly to the regulation of behavior.
Transgenic animal of oxytocin
OXT deficient mice
Previous studies have generated mice carrying a deletion of the OXT-coding region using homologous recombination in embryonic stem cells (Nishimori et al., 1996; Young et al., 1996). Mice lacking OXT are both viable and fertile. Males do not have any reproduction behavioral or functional defects in the absence of OXT. Similarly, females have no obvious deficits in fertility or reproduction, including gestation and parturition. Although OXT-deficient females demonstrated normal maternal behavior, all their offspring died of starvation shortly after birth, because OXT-deficient mothers were unable to nurse. After injections of OXT to OXT-deficient mothers, milk ejection was induced and the offspring survived. OXT-deficient male mice fail to develop social memory (Ferguson et al., 2000). A measurement of both olfactory foraging and olfactory habituation tasks has indicated that olfactory detection of non-social stimuli is intact in OXT-deficient male mice, and treatment with OXT reinstates social memory in those mice. These data indicate that OXT is necessary for the normal development of social memory in mice and support the hypothesis that social memory has a neural basis distinct from other forms of memory.
OXTR deficient mice
OXTR-deficient mice were viable and had no obvious deficits in fertility or reproductive behavior, the same as OXT-deficient mice (Takayanagi et al., 2005). OXTR-deficient dams mice exhibited normal parturition but demonstrated defects in lactation and maternal nurturing. Infant OXTR-deficient males emitted fewer ultrasonic vocalizations than their wild-type littermates in response to social isolation. Adult OXTR-deficient males also showed deficits in social discrimination, and demonstrated increased aggressive behavior. OXT-deficient males from OXT-deficient but not from heterozygote dams showed high levels of aggression. These data suggest a developmental role for the OXT/OXTR system in shaping adult aggressive behavior.
Animals bearing fluorescent fusion transgenes
Previous studies have shown the placement of the eGFP coding sequence (Young et al., 1999; Zhang et al., 2002) or chloramphenicol acetyltransferase (CAT) reporters at various locations within an OXT transgene (Jeong et al., 2001) (Table 1). We generated rats bearing an OXT-eCFP fusion transgene designed from a murine construct previously shown to be faithfully expressed in transgenic mice (Katoh et al., 2010) (Table 1). However, the expression of the transgene was unstable for unknown reasons.
Table 1.
Oxytocin transgenes.
| Transgenesis | Transgene | Reporter gene | Specificity expression in HNS | Ectopic expression | References | |
|---|---|---|---|---|---|---|
| From | To | |||||
| Mouse | Mouse | AI-02 | eGFP | None | None | Young et al., 1999 |
| Mouse | Mouse | AI-01 | eGFP | Few | None | Young et al., 1999 |
| Mouse | Mouse | AI-03 | eGFP | + | None | Young et al., 1999 |
| Mouse | Mouse | JL-01 | IRES-eGFP | + | None | Young et al., 1999 |
| Mouse | Mouse | OT-3-CAT-3.5 | CAT | + | None | Jeong et al., 2001 |
| Mouse | Rat | AI-03 | eCFP | + | None | Katoh et al., 2010 |
| Rat | Rat | OXT-mRFP1 | mRFP1 | + | None | Katoh et al., 2011, 2014 |
HNS, hypothalamo-neurohypophysial system.
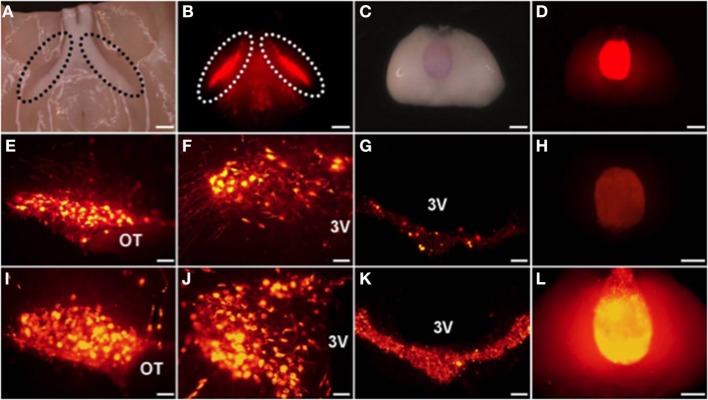
The mRFP was developed from DsRed, which is the red fluorescent protein from Discosoma (Campbell et al., 2002; Long et al., 2005). We have succeeded in generating transgenic rats bearing an OXT-mRFP1 fusion gene (Katoh et al., 2011) (Table 1) (Figure 1). Interestingly, when the brains of these rats were mounted on a slide, the mRFP1 fluorescence was visible in the ventral part of the SON and in the PP without cutting. We could observe the mRFP1 fluorescence throughout the SON, especially in the dorsal parts. We could observe abundant mRFP1 fluorescence in the magnocellular division of the PVN and scattered mRFP1 fluorescence in the parvocellular division of the PVN. We also observed mRFP1 fluorescence in the internal layer of the median eminence (ME) and in the PP. In situ hybridization histochemistry showed mRFP1 mRNA localized in the SON and in the magnocellular and parvocellular divisions of the PVN. In comparing male and female transgenic rats under normal conditions, there were no differences in the expression of mRFP1 mRNA in the SON and the PVN. In comparing nontransgenic and transgenic rats under normal conditions, there were no differences between them in plasma osmorality, sodium, OXT, AVP, and the expression of the endogenous OXT gene and AVP gene in the SON and the PVN.
Figure 1.
The mRFP1 fluorescence was clearly observed in ventral parts of the supraoptic nucleus (SON) (A,B) and in the PP (C,D) without cutting. Endogenous florescence of mRFP1 in the SON (E), the paraventricular nucleus (PVN) (F), the median eminence (ME) (G), and the posterior pituitary (PP) (H). Effects of salt loading for 5 days on the mRFP1 fluorescence of the SON (I), the PVN (J), the ME (K), and the PP (L). Under light (A,C) and fluorescent (B,D–L). Scale bars, 1 mm (A–D,H,L) and 0.1 mm (E–G,I–K). OT, Optic tract; 3V, third ventricle. Modified with permission from Figure 1 in Katoh et al. (2011).
Previous studies have reported that OXT transcripts significantly increased in the rat hypothalamus after chronic osmotic stimuli, such as salt loading (Lightman and Young, 1987; McCabe et al., 1990; Yue et al., 2008). In our OXT-mRFP1 transgenic rats, the fluorescence of mRFP1 was remarkably increased by 5 to 7-fold throughout the SON and in the PVN, ME, and PP after salt loading for 5 days (Katoh et al., 2010) (Figure 1). In situ hybridization histochemistry showed dramatically increased the expression of the mRFP1 mRNA in the SON and the PVN after salt loading. Comparing nontransgenic and transgenic rats after salt loading, there were no differences in plasma osmorality, sodium, OXT, AVP, and the expression of the endogenous OXT gene and AVP gene in the SON and the PVN.
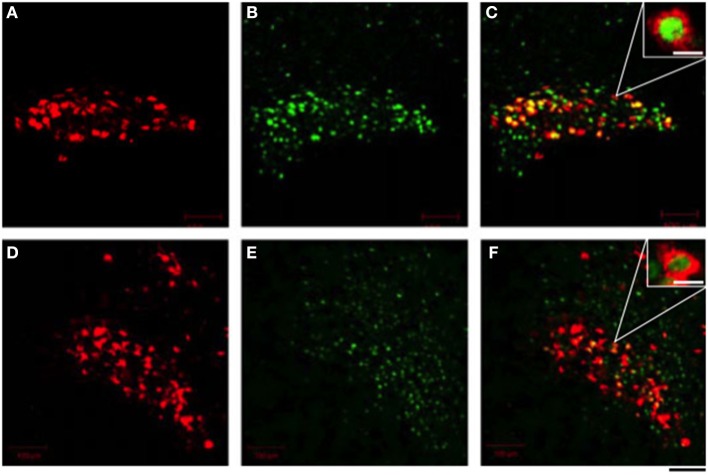
The peripheral administration of cholecystokinin (CCK) -8 stimulated secretion of OXT but not AVP (Verbalis et al., 1986; Ueta et al., 2000; Hashimoto et al., 2005), and excited OXT-secreting magnocellular neurons in the SON and the PVN in rats (Hamamura et al., 1991; Ueta et al., 1993, 2000; Hashimoto et al., 2005). CCK-8 stimulates gastric vagal afferents and activated noradrenergic neurons in the nucleus of the tractus solitarius (Luckman, 1992). It is postulated that these noradrenergic inputs activate OXT-secreting neurons in the SON and the PVN and cause the secretion of OXT into the systemic circulation in rats (Hamamura et al., 1991). Recently, we have developed a novel transgenic rat that enables the trivial visualization of c-fos expression using an eGFP tag (Katoh et al., 2014). These rats express a transgene consisting of c-fos gene regulatory sequences that drive the expression of a c-fos-eGFP fusion protein. Moreover, we generated a double transgenic rat that expresses both the c-fos-eGFP and an OXT-mRFP1 fusion gene. In these double transgenic rats, nuclear eGFP fluorescence appeared in OXT-mRFP1 neurons in the SON and the PVN 90 min after i.p. administration of CCK-8 (Figure 2). Three-dimensional reconstruction imaging enables the visualization of nuclear eGFP in the cytoplasm of OXT neurons illuminated and identified by virtue of their expression of mRFP1. In these neurons, abundant OXT granules in the cytoplasm are clearly visible by a plane image obtained from a higher magnification by confocal laser microscopy (Katoh et al., 2014).
Figure 2.
Effects of i.p. administration of cholecystokinin-8 on the endogenous fluorescence of monomeric red fluorescent protein 1 (mRFP1) (A,D) and nuclear enhanced green fluorescent protein (eGFP) (B,E) in the supraoptic nucleus (A–C) and the paraventricular nucleus (D–F). The merged view of fluorescence of mRFP1 and eGFP was seen as a yellow color (C,F). Scale bars shown in white represent 10 μm in (C,F). The scale bar shown in black = 40 μm. Modified with permission from Figure 3 in Katoh et al. (2014).
Conclusions
We did not observe any fluorescence of mRFP1 in the ectopic area of OXT in the OXT-mRFP1 transgenic rats. The OXT neuron has the same proper response to physiological stimulation in the OXT-mRFP1 transgenic rats as in nontransgenic rats. Using OXT-mRFP1 rats, we can identify the OXT neuron easily and see changes in the neuron's activity and release of OXT in realtime. Moreover, we can see smaller changes that we had not been able to see before, because OXT-mRFP1 transcription is more sensitive than endogenous OXT transcription to the same stimulation. The OXT-mRFP1 transgenic rats are a useful animal model to study dynamic changes in OXT in the HNS.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank to Endocrine Society and British Society for Neuroendocrinology for permission to use figures of journals, respectively, Endocrinology and Journal of Neuroendocrinology. This study was supported by Grant-in-Aid for Scientific Research (C) No. 25460324 (Hirofumi Hashimoto) and Grant-in-Aid for Scientific Research on Innovative Areas No. 25113721 (Yoichi Ueta) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
References
- Breton C., Pechoux C., Morel G., Zingg H. H. (1995). Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology 136, 2928–2936 [DOI] [PubMed] [Google Scholar]
- Burbach P., Young L. J., Russell J. (2006). Oxytocin: synthesis, secretion and reproductive functions, in Knobil and Neill's Physiology of Reproduction, ed Neill J. D. (Amsterdam: Elsevier; ), 3055–3127 [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., et al. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. U.S.A. 99, 7877–7882 10.1073/pnas.082243699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael M. S., Humbert R., Dixen J., Palmisano G., Greenleaf W., Davidson J. M. (1987). Plasma oxytocin increases in the human sexual response. J. Clin. Endocrinol. Metab. 64, 27–31 10.1210/jcem-64-1-27 [DOI] [PubMed] [Google Scholar]
- Carmichael M. S., Warburton V. L., Dixen J., Davidson J. M. (1994). Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch. Sex. Behav. 23, 59–79 10.1007/BF01541618 [DOI] [PubMed] [Google Scholar]
- Castel M., Morris J. (1988). The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience 24, 937–966 10.1016/0306-4522(88)90078-4 [DOI] [PubMed] [Google Scholar]
- Dale H. H. (1906). On some physiological actions of ergot. J. Physiol. 34, 163–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries G. J., Buijs R. M. (1983). The origin of vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 273, 307–317 10.1016/0006-8993(83)90855-7 [DOI] [PubMed] [Google Scholar]
- du Vigneaud V., Ressler C., Swan J., Roberts C. (1954). The synthesis of oxytocin. J. Am. Chem. Soc. 76, 3115–3121 10.1021/ja01641a004 [DOI] [Google Scholar]
- du Vigneaud V., Ressler C., Swan J., Roberts C., Katsoyannis P., Gordon S. (1953b). The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 75, 4879–4880 10.1021/ja01115a55311134819 [DOI] [Google Scholar]
- du Vigneaud V., Ressler C., Trippett S. (1953a). The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J. Biol. Chem. 205, 949–957 [PubMed] [Google Scholar]
- Fahrbach S. E., Morrell J. I., Pfaff D. W. (1985). Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology 40, 526–532 10.1159/000124125 [DOI] [PubMed] [Google Scholar]
- Ferguson J. N., Young L. J., Hearn E. F., Matzuk M. M., Insel T. R., Winslow J. T. (2000). Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288 10.1038/77040 [DOI] [PubMed] [Google Scholar]
- Filippi S., Vannelli G. B., Granchi S., Luconi M., Crescioli C., Mancina R., et al. (2002). Identification, localisation and functional activity of oxytocin receptors in epididymis. Mol. Cell. Endocrinol. 193, 89–100 10.1016/S0303-7207(02)00101-6 [DOI] [PubMed] [Google Scholar]
- Foo N. C., Carter D., Murphy D., Ivell R. (1991). Vasopressin and oxytocin gene expression in rat testis. Endocrinology 128, 2118–2128 10.1210/endo-128-4-2118 [DOI] [PubMed] [Google Scholar]
- Frayne J., Nicholson H. D. (1995). Effect of oxytocin on testosterone production by isolated rat Leydig cells is mediated via a specific oxytocin receptor. Biol. Reprod. 52, 1268–1273 10.1095/biolreprod52.6.1268 [DOI] [PubMed] [Google Scholar]
- Frayne J., Nicholson H. D. (1998). Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol. Hum. Reprod. 4, 527–532 10.1093/molehr/4.6.527 [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Periyasamy S., Alexandrova M., Soloff M. S. (1983). Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology 113, 742–749 10.1210/endo-113-2-742 [DOI] [PubMed] [Google Scholar]
- Fujio T., Fujihara H., Shibata M., Yamada S., Onaka T., Tanaka K., et al. (2006). Exaggerated response of arginine vasopressin-enhanced green fluorescent protein fusion gene to salt loading without disturbance of body fluid homeostasis in rats. J. Neuroendocrinol. 18, 776–785 10.1111/j.1365-2826.2006.01476.x [DOI] [PubMed] [Google Scholar]
- Hamamura M., Leng G., Emson P. C., Kiyama H. (1991). Electrical activation and c-fos mRNA expression in rat neurosecretory neurones after systemic administration of cholecystokinin. J. Physiol. 444, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C., Frayne J., Nicholson H. D. (1996). Epididymal oxytocin in the rat; its origin and regulation. Int. J. Androl. 19, 278–286 10.1111/j.1365-2605.1996.tb00476.x [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Onaka T., Kawasaki M., Chen L., Mera T., Soya A., et al. (2005). Effects of cholecystokinin (CCK)-8 on hypothalamic oxytocin-secreting neurons in rats lacking CCK-A receptor. Auton. Neurosci. 121, 16–25 10.1016/j.autneu.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Tweedle C. D. (1982). Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res. Bull. 8, 197–204 10.1016/0361-9230(82)90046-6 [DOI] [PubMed] [Google Scholar]
- Insel T. R. (1990). Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behaviour. J. Neuroendocrinol. 2, 539–545 10.1111/j.1365-2826.1990.tb00445.x [DOI] [PubMed] [Google Scholar]
- Ivell R., Balvers M., Rust W., Bathgate R., Einspanier A. (1997). Oxytocin and male reproductive function. Adv. Exp. Med. Biol. 424, 253–264 10.1007/978-1-4615-5913-9_47 [DOI] [PubMed] [Google Scholar]
- Ivell R., Furuya K., Brackmann B., Dawood Y., Khan-Dawood F. (1990). Expression of the oxytocin and vasopressin genes in human and baboon gonadal tissues. Endocrinology 127, 2990–2996 10.1210/endo-127-6-2990 [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. (1984). Structure and comparison of the oxytocin and vasopressin genes from rat. Proc. Natl. Acad. Sci. U.S.A. 81, 2006–2010 10.1073/pnas.81.7.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Ohno M., Katoh A., Ohbuchi T., Ishikura T., Fujihara H., et al. (2011). Upregulation of arginine vasopressin synthesis in the rat hypothalamus after kainic acid-induced seizures. Brain Res. 1424, 1–8 10.1016/j.brainres.2011.09.030 [DOI] [PubMed] [Google Scholar]
- Jeong S. W., Castel M., Zhang B. J., Fields R. L., Paras P., Arnheiter H., et al. (2001). Cell-specific expression and subcellular localization of neurophysin-CAT-fusion proteins expressed from oxytocin and vasopressin gene promoter-driven constructs in transgenic mice. Exp. Neurol. 171, 255–271 10.1006/exnr.2001.7785 [DOI] [PubMed] [Google Scholar]
- Katoh A., Fujihara H., Ohbuchi T., Onaka T., Hashimoto T., Kawata M., et al. (2011). Highly visible expression of an oxytocin-monomeric red fluorescent protein 1 fusion gene in the hypothalamus and posterior pituitary of transgenic rats. Endocrinology 152, 2768–2774 10.1210/en.2011-0006 [DOI] [PubMed] [Google Scholar]
- Katoh A., Fujihara H., Ohbuchi T., Onaka T., Young W. S., 3rd., Dayanithi G., et al. (2010). Specific expression of an oxytocin-enhanced cyan fluorescent protein fusion transgene in the rat hypothalamus and posterior pituitary. J. Endocrinol. 204, 275–285 10.1677/JOE-09-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh A., Shoguchi K., Matsuoka H., Yoshimura M., Ohkubo J. I., Matsuura T., et al. (2014). Fluorescent visualisation of the hypothalamic oxytocin neurones activated by cholecystokinin-8 in rats expressing c-fos-enhanced green fluorescent protein and oxytocin-monomeric red fluorescent protein 1 fusion transgenes. J. Neuroendocrinol. 26, 341–347 10.1111/jne.12150 [DOI] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. (1992). Structure and expression of a human oxytocin receptor. Nature 356, 526–529 10.1038/356526a0 [DOI] [PubMed] [Google Scholar]
- Kubota Y., Kimura T., Hashimoto K., Tokugawa Y., Nobunaga K., Azuma C., et al. (1996). Structure and expression of the mouse oxytocin receptor gene. Mol. Cell. Endocrinol. 124, 25–32 10.1016/S0303-7207(96)03923-8 [DOI] [PubMed] [Google Scholar]
- Larcher A., Neculcea J., Breton C., Arslan A., Rozen F., Russo C., et al. (1995). Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136, 5350–5356 [DOI] [PubMed] [Google Scholar]
- Leckman J. F., Goodman W. K., North W. G., Chappell P. B., Price L. H., Pauls D. L., et al. (1994). The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 19, 723–749 10.1016/0306-4530(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Lefebvre D. L., Farookhi R., Giaid A., Neculcea J., Zingg H. H. (1994). Uterine oxytocin gene expression. II. Induction by exogenous steroid administration. Endocrinology 134, 2562–2566 [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd. (1987). Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J. Physiol. 394, 23–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. Z., Lackan C. S., Hadjantonakis A. K. (2005). Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein. BMC Biotechnol. 5:20 10.1186/1472-6750-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckman S. M. (1992). Fos-like immunoreactivity in the brainstem of the rat following peripheral administration of cholecystokinin. J. Neuroendocrinol. 4, 149–152 10.1111/j.1365-2826.1992.tb00152.x [DOI] [PubMed] [Google Scholar]
- Ludwig M., Leng G. (2006). Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- Maggi M., Magini A., Fiscella A., Giannini S., Fantoni G., Toffoletti F., et al. (1992). Sex steroid modulation of neurohypophysial hormone receptors in human nonpregnant myometrium. J. Clin. Endocrinol. Metab. 74, 385–392 [DOI] [PubMed] [Google Scholar]
- Maruyama T., Ohbuchi T., Fujihara H., Shibata M., Mori K., Murphy D., et al. (2010). Diurnal changes of arginine vasopressin-enhanced green fluorescent protein fusion transgene expression in the rat suprachiasmatic nucleus. Peptides 31, 2089–2093 10.1016/j.peptides.2010.08.010 [DOI] [PubMed] [Google Scholar]
- McCabe J. T., Kawata M., Sano Y., Pfaff D. W., Desharnais R. A. (1990). Quantitative in situ hybridization to measure single-cell changes in vasopressin and oxytocin mRNA levels after osmotic stimulation. Cell. Mol. Neurobiol. 10, 59–71 10.1007/BF00733636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle S. L., Bishop V. R., Gkoumassi E., van Leeuwen F. W., Douglas A. J. (2007). Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology 148, 5095–5104 10.1210/en.2007-0615 [DOI] [PubMed] [Google Scholar]
- Murphy M. R., Seckl J. R., Burton S., Checkley S. A., Lightman S. L. (1987). Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab. 65, 738–741 10.1210/jcem-65-4-738 [DOI] [PubMed] [Google Scholar]
- Nicholson H. D., Hardy M. P. (1992). Luteinising hormone differentially regulates the secretion of testicular oxytocin and testosterone by purified rat Leydig cells in vitro. Endocrinology 130, 671–677 [DOI] [PubMed] [Google Scholar]
- Nilaver G., Zimmerman E. A., Wilkins J., Michaels J., Hoffman D., Silverman A. J. (1980). Magnocellular hypothalamic projections to the lower brain stem and spinal cord of the rat. Immunocytochemical evidence for predominance of the oxytocin-neurophysin system compared to the vasopressin-neurophysin system. Neuroendocrinology 30, 150–158 10.1159/000122991 [DOI] [PubMed] [Google Scholar]
- Nishimori K., Young L. J., Guo Q., Wang Z., Insel T. R., Matzuk M. M. (1996). Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699–11704 10.1073/pnas.93.21.11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Kudo S., Kitsunai Y., Fukuchi S. (1980). Increase in oxytocin secretion at ejaculation in male. Clin. Endocrinol. (Oxf). 13, 95–97 10.1111/j.1365-2265.1980.tb01027.x [DOI] [PubMed] [Google Scholar]
- Ohno M., Fujihara H., Iwanaga M., Todoroki M., Katoh A., Ohbuchi T., et al. (2012). Induction of arginine vasopressin-enhanced green fluorescent protein expression in the locus coeruleus following kainic acid-induced seizures in rats. Stress 15, 435–442 10.3109/10253890.2011.637185 [DOI] [PubMed] [Google Scholar]
- Ostrowski N. L. (1998). Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology 23, 989–1004 10.1016/S0306-4530(98)00070-5 [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Ascher J. A., Monroe Y. L., Prange A. J., Jr. (1982). Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650 10.1126/science.7071605 [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Caldwell J. D., Walker C., Ayers G., Mason G. A. (1994). Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci. 108, 1163–1171 10.1037/0735-7044.108.6.1163 [DOI] [PubMed] [Google Scholar]
- Reaves T. A., Jr., Hayward J. N. (1979). Immunocytochemical identification of vasopressinergic and oxytocinergic neurons in the hypothalamus of the cat. Cell Tissue Res. 196, 117–122 10.1007/BF00236352 [DOI] [PubMed] [Google Scholar]
- Russell J. A., Leng G. (1998). Sex, parturition and motherhood without oxytocin? J. Endocrinol. 157, 342–359 10.1677/joe.0.1570343 [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. (1982). Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 205, 260–272 10.1002/cne.902050306 [DOI] [PubMed] [Google Scholar]
- Shibata M., Fujihara H., Suzuki H., Ozawa H., Kawata M., Dayanithi G., et al. (2007). Physiological studies of stress responses in the hypothalamus of vasopressin-enhanced green fluorescent protein transgenic rat. J. Neuroendocrinol. 19, 285–292 10.1111/j.1365-2826.2007.01532.x [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. (1980). Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J. Histochem. Cytochem. 28, 475–478 10.1177/28.5.7381192 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Weindl A., Schrell U., Wetzstein R. (1981). Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta Histochem. Suppl. 24, 79–95 [PubMed] [Google Scholar]
- Soloff M. S., Fernstrom M. A., Periyasamy S., Soloff S., Baldwin S., Wieder M. (1983). Regulation of oxytocin receptor concentration in rat uterine explants by estrogen and progesterone. Can. J. Biochem. Cell Biol. 61, 625–630 10.1139/o83-078 [DOI] [PubMed] [Google Scholar]
- Stock S., Uvnäs-Moberg K. (1988). Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol. Scand. 132, 29–34 10.1111/j.1748-1716.1988.tb08294.x [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kawasaki M., Ohnishi H., Otsubo H., Ohbuchi T., Katoh A., et al. (2009). Exaggerated response of a vasopressin-enhanced green fluorescent protein transgene to nociceptive stimulation in the rat. J. Neurosci. 29, 13182–13189 10.1523/JNEUROSCI.2624-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y., Yoshida M., Bielsky I. F., Ross H. E., Kawamata M., Onaka T., et al. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 16096–16101 10.1073/pnas.0505312102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Amico J. A. (1996). Sequential estrogen and progesterone (P) followed by P withdrawal increases the level of oxytocin messenger ribonucleic acid in the hypothalamic paraventricular nucleus of the male rat. Life Sci. 58, 1615–1620 10.1016/0024-3205(96)00136-1 [DOI] [PubMed] [Google Scholar]
- Todoroki M., Ueta Y., Fujihara H., Otsubo H., Shibata M., Hashimoto H., et al. (2010). Induction of the arginine vasopressin-enhanced green fluorescent protein fusion transgene in the rat locus coeruleus. Stress 13, 281–291 10.3109/10253890903383406 [DOI] [PubMed] [Google Scholar]
- Ueta Y., Fujihara H., Serino R., Dayanithi G., Ozawa H., Matsuda K., et al. (2005). Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology 146, 406–413 10.1210/en.2004-0830 [DOI] [PubMed] [Google Scholar]
- Ueta Y., Kannan H., Higuchi T., Negoro H., Yamaguchi K., Yamashita H. (2000). Activation of gastric afferents increases noradrenaline release n the paraventricular nucleus and plasma oxytocin level. J. Auton. Nerv. Syst. 78, 69–76 10.1016/S0165-1838(99)00049-1 [DOI] [PubMed] [Google Scholar]
- Ueta Y., Kannan H., Higuchi T., Negoro H., Yamashita H. (1993). CCK-8 excites oxytocin-secreting neurons in the paraventricular nucleus in rats—possible involvement of noradrenergic pathway. Brain Res. Bull. 32, 453–459 10.1016/0361-9230(93)90290-R [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K., Bruzelius G., Alster P., Lundeberg T. (1993). The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol. Scand. 149, 199–204 10.1111/j.1748-1716.1993.tb09612.x [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bolwerk E. L., Liu B., Burbach J. P. (1988). Oxytocin and vasopressin gene expression in the hypothalamo–neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology 122, 945–951 10.1210/endo-122-3-945 [DOI] [PubMed] [Google Scholar]
- Verbalis J. G., McCann M. J., McHale C. M., Stricker E. M. (1986). Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science 232, 1417–1419 10.1126/science.3715453 [DOI] [PubMed] [Google Scholar]
- Whittington K., Assinder S., Gould M., Nicholson H. (2004). Oxytocin, oxytocin associated neurophysin and the oxytocin receptor in human prostate. Cell Tissue Res. 318, 375–382 10.1007/s00441-004-0968-5 [DOI] [PubMed] [Google Scholar]
- Young L. J., Winslow J. T., Wang Z., Gingrich B., Guo Q., Matzuk M. M., et al. (1997). Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm. Behav. 31, 221–231 10.1006/hbeh.1997.1377 [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd., Iacangelo A., Luo X. Z., King C., Duncan K., Ginns E. I. (1999). Transgenic expression of green fluorescent protein in mouse oxytocin neurones. J. Neuroendocrinol. 11, 935–939 10.1046/j.1365-2826.1999.00410.x [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd., Shepard E., Amico J., Hennighausen L., Wagner K. U., LaMarca M. E., et al. (1996). Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 8, 847–853 10.1046/j.1365-2826.1996.05266.x [DOI] [PubMed] [Google Scholar]
- Yue C., Mutsuga N., Sugimura Y., Verbalis J., Gainer H. (2008). Differential kinetics of oxytocin and vasopressin heteronuclear RNA expression in the rat supraoptic nucleus in response to chronic salt loading in vivo. J. Neuroendocrinol. 20, 227–232 10.1111/j.1365-2826.2007.01640.x [DOI] [PubMed] [Google Scholar]
- Zhang B. J., Kusano K., Zerfas P., Iacangelo A., Young W. S., 3rd., Gainer H. (2002). Targeting of green fluorescent protein to secretory granules in oxytocin magnocellular neurons and its secretion from neurohypophysial nerve terminals in transgenic mice. Endocrinology 143, 1036–1046 10.1210/endo.143.3.8700 [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L. (1988). Oxytocin and vasopressin gene expression during gestation and lactation. Brain Res. 464, 1–6 10.1016/0169-328X(88)90011-3 [DOI] [PubMed] [Google Scholar]