Abstract
Background
We investigated the potential of galangal rhizomes to induce cytotoxic and apoptotic effects in the cultured human breast carcinoma cell line, (MCF-7) in compare with the non-malignant (MRC-5) cells.
Methods
Both cells were cultured in DMEM medium and treated with galangal rhizomes for three consecutive days. The percentage of apoptotic cells was determined by flow cytometry using Annexin-V fluorescein isothiocyanate.
Results
The results showed that the ethanolic extract of galangal rhizomes decreased cell viability in the malignant cells as a concentration- and time- dependent manner. The IC50 values against MCF-7 were determined at 400.0 ± 11.7 and 170.0 ± 5.9 μg/ml after 48 and 72 h respectively. The morphology of MCF-7 cells treated with the ethanolic extract confirmed the cell proliferation assay results. Alpinia galanga induced apoptosis in MCF-7 cells, as determined by flow cytometry.
Conclusions
We concluded that the extract of Alpinia galanga exerts pro-apoptotic effects in a breast cancer-derived cell line and could be considered as a potential chemotherapeutic agent in breast cancer.
Keywords: Alpinia galanga L, Cytotoxicity, MCF-7, MRC-5, MTT
Background
Breast cancer is the second leading cause of cancer deaths among woman. Unfortunately, the development of resistance to chemotherapeutic agents is a common obstacle in the treatment of different types of cancers including breast cancer [1]. Several important drugs with different structures and mechanisms of anti-tumor activities fail to be effective due to the drug resistance [2] and also the failure of the conventional chemotherapy to affect a major reduction in mortality indicates that the new approaches are critically needed [3]. It has been recognized that a large groups of therapeutic agents can stop cancer cells proliferation by inducing apoptosis. The induction of apoptosis has been emphasized in anticancer strategies [4]. Apoptosis is a gene regulated phenomenon which is induced by many chemotherapeutic agents in cancer treatment [5]. It is characterized by a series of typical morphological features, such as nuclear and cellular convolution, chromatin condensation and the final disintegration of the cell into membrane-bound apoptotic bodies which are phagocytosed by neighboring cells [6]. Most normal cells can die by apoptosis but tumor cells very often have some defects in the apoptotic pathway, leading not only to the increase of tumor mass but also to tumor resistance to chemotherapy. Since chemotherapy and irradiation act primarily by inducing apoptosis, defects in the apoptotic pathway make the therapy less efficient [7]. Increasing evidences suggest that the related processes of neoplastic transformation involve alteration of the normal apoptotic pathway [8]. The major focus of the research in chemotherapy for cancer in recent times is the use of naturally occurring compounds with the chemopreventive and chemotherapeutic properties in the treatment of cancers [9,10]. Epidemiological studies suggest that a diet rich in antioxidants may help to prevent the development of breast carcinoma [11]. Excess generation of oxygen free radicals can cause oxidative damage to bimolecular resulting in lipid peroxidation, mutagenesis and carcinogenesis. All cells are exposed to oxidative stress, and thus oxidation and free radicals may be important in carcinogenesis at multiple tumor sites [12]. The antioxidant activity may be the result of the specific scavenging of reactive free radicals, scavenging of oxygen containing compounds such as hydrogen peroxide and chelating metals [13,14]. Phytochemical and dietary antioxidants may decrease the risk of much chronic disease such as cancer. Antioxidants scavenge free radicals, and consequently are a very special group of nutrition supplements [15]. Plants have played an important role as a source of effective anticancer agents, and it is significant that 60% of currently used anticancer agents are derived from natural sources including plants, marine organisms, and microorganisms [16]. Alpina galanga (galangal) is a well-known plant in the Southeast Asia. The rhizomes of Zingiberaceae family are widely used in many ancient countries in traditional medicine which is found to be effective in the treatment of diseases [17]. Their function have been broadly discussed and accepted in many traditional recipes. Alpinia galangal has bben studied by various researchers and a number of active constituents from the plant have been isolated and reported. Phenolic compounds such as flavonoids and phenolic acids are found abundantly in this plant [18]. The dominant components isolated from the rhizomes were galangoisoflavonoid [19], β-sitosterol diglucosyl caprate [20], methyleugenol, p-coumaryl diacetate, 1′-acetoxyeugenol acetate, trans-p-acetoxycinnamyl alcohol, trans-3,4-dimethoxycinnamyl alcohol, p-hydroxybenzaldehyde, p-hydroxycinnamaldehyde, trans-p-coumaryl alcohol, galangin, trans-p-coumaric acid, and galanganol B [21]. The major phytoconstituents which have been isolated from the rhizomes are acetoxychavicol acetate (ACA) and hydroxychavicol acetate (HCA) [22]. Rhizomes are lowest in fat but richest in carbohydrate [23]. The chemical investigation of A. galanga has led to the isolation of β-caryophyllene (17.95%) and β-selinene (10.56%), terpinen-4-ol [24], 4-allylphenyl acetate and β-bisabolene, 5-hydroxymethyl furfural (59.9%), benzyl alcohol (57.6%), methylcinnamate (9.4%), 3-phenyl-2-butanone (8.5%) and 1,2-benzenedicarboxylic acid (8.9%) [25]. A new phenylpropanoid, 4,4′[(2E, 2′E)-bis(prop-2-ene)-1,1′-oxy]-diphenyl-7,7′-diacetata [26], as well as p-coumaryl alcohol-γ-O-methyl ether (CAME) and p-coumaryl diacetate (CDA), has also been isolated from the plant [27,28]. Volatile oil of plant contained zerumbone (44.9%), β-farnesene, myrcene and 1,8-cineole, respectively [29,30]. Bicyclo (4.2.0) oct-1-ene, 7-exoethenyl (58.46%), trans-caryophyllene (7.05%), α-pinene (14.94%) with camphene (2.15%), germacrene (1.78%) and citronellyl acetate (1.41%) were reported in A.galanga as major components [31]. Several authors have studied the anti-inflammatory and analgesic effects of A. galanga in a variety of rheumatological conditions [26,27,32,33]. The extracts of A. galanga showed acetylcholinesterase-inhibitory [34], platelet-activating factor (PAF)-inhibitory [35], antimicrobial [36], antibacterial [37], anti-amoebic [38], antifungal [39], antioxidant [28] and apoptosis [40] activities. It has been reported that Alpinia plants possess antioxidant [41], anti-inflammatory [42], immunostimulating [43], antinociceptive [44], hepatoprotective [45] and anticancer [46] activities. However our information about anticancer activates of galangal is very few. To prevent initiation and promotion carcinogenesis, administration of nationally accruing agent is being increasingly appreciated. In this study, the anticancer effects of the ethanolic extract of galangal on the cultured human breast carcinoma, MCF-7 cells, was investigated.
Methods
Chemicals and reagents
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Amerso (USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco BRL (Grand Island, NY, USA). Annexin V/fluorescein isothiocyanate (FITC) was obtained from Invitrogen Corporation (Camarillo, CA, USA). Fetal bovine serum was purchased from PAA Laboratories GmbH, Austria.
Preparation of the galangal extract
Protocols of current study were approved by the Ethical Committee of Mashhad Medical University. The Fresh rhizomes of alpinia galanga used in this study was collected from a private garden in the flowering period in india and identified by botanists in the herbarium of Ferdowsi University of Mashhad and also has been deposited in the herbarium. Voucher specimen is deposited in the specially maintained herbarium, Department of Botany, Ferdowsi University of Mashhad. The cleaned fresh rhizomes were cut into small pieces and air-dried. The dried powder (50 g) was mixed with ethanol (97%) in a balloon; the balloon was shaken for 3 days at room temperature. The preparation was then filtered off through a Gauze mesh and the solvent was dried by evaporation under reduced pressure at 45°C. The final product yielded 12% w/w dried extract; it was stored in a refrigerator until the experiment.
Cell culture
The human breast adenocarcinoma cell line (MCF-7) and the human fetal lung fibroblast cell line (MRC-5) were obtained from Pasteur Institute (Tehran, Iran). The cells were grown either in 96-well tissue (TC) plate (NUNC, Wiesbaden, Germany) or in 25-cm2 TC flasks (NUNC, Wiesbaden, Germany), cells were cultured in CO2 incubator MCO-17AI (Sanyo Electric Co., Ltd, Japan) at 37°C in 95% humidified atmosphere enriched by 5% CO2 and subcultured every 3 to 4 days. The malignant (MCF-7) and nonmalignant cells (MRC-5) were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (Gibco–Invitrogen), 100 U/ml of penicillin (Gibco–Invitrogen), and 100 μg/ml streptomycin (Gibco–Invitrogen).
Cell viability assay
Cell viability was measured using the MTT assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenises [6]. Briefly, both cell lines were plated at a density of (1 × 103 cells/ml) in 96-well plates. Cells were seeded overnight, and then incubated with various concentrations of galangal rhizomes extract (0, 125, 250, 500, and 750 μg/ml) for 24, 48 h and 72 h. For MTT assay, after treatment with the galangal extract for 24 and 48 and 72 h, 10 μl MTT was added into each well. After removing the medium, the cells were then labeled with MTT solution) 5 mg/ml in PBS) for 4 h and the resulting Formosan was solubilized in 100 μl dimethyl sulfoxide (DMSO). Absorbance was measured at 570 nm using an automated microplate reader (Bio-Rad 550). The absorption was measured at 570 nm (620 nm as a reference) in an ELISA reader. The cytotoxic effects of the galangal extract on the cell lines (MCF-7 and MRC-5) were expressed as the IC50 value (the drug concentration reducing the absorbance of treated cells by 50% with respect to untreated cells). All experiments were carried out in triplicate
Morphological studies of cell lines using the normal inverted microscope
Morphological studies using the normal inverted microscope were carried out to observe the morphological changes of cell death in MCF-7 and MRC-5 cell lines elicited by the ethanolic extract of galangal. Different concentrations of galangal extract (0, 125, 250, 500, and 750 μg/ml) for 24, 48 h and 72 h were used for the morphological studies. The untreated cells served as the negative control. The morphological changes of the cells were observed under the normal inverted microscope after 24 and 48 h post-treatment.
Annexin V/PI staining and flow cytometry analysis
Apoptotic cell death was measured using a flouresin isothiocynate (FITC)-conjugated Annexin V/PI assay kit by flow cytometry. Briefly, MCF-7 cells (1 × 106 cells) treated with the galangal extract at concentrations of 250 and 500 μg/ml for 48 h were harvested and washed twice with cold PBS (phosphate-buffered saline) resuspended in 100 μl binding buffer, and stained with 5 μl of FITC- conjugated Annexin V (10 mg/ml) and 10 μl of propidium iodide (PI) (50 mg/ml). The cells were incubated for 15 min at room temperature in the dark, 400 μl of binding buffer was added, and the cells were analyzed (FACScan, Becton- Dickinson, USA). The MCF-7 cells were gated separately according to their granularity and size on forward scatter (FSC) versus Side Scatter (SSC) plots. The early and late apoptosis were evaluated on fluorescence 2 (FL2 for PI) versus fluorescence 1 (FL1 for Annexin) plots. Cells stained with only annexin V were evaluated as being in the early apoptosis; cells stained with both annexin V and PI were evaluated as being in the late apoptosis or in the necrotic stage [7,47].
Statistical analysis
All results were expressed as mean ± SEM. The significance of difference was evaluated with ANOVA and Bonfrroni’s test. A probability level of P < 0.05 was considered statistically significant.
Results
Morphological evaluation
To discriminate between the early and late effects of galangal rhizomes action, the malignant (MCF7) and non-malignant control (MRC-5) cells were exposed to increasing the galangal rhizomes concentrations for 24, 48 and 72 h (Figure 1).After 48 h of incubation with the ethanolic extract of galangal rhizomes (250, 500, and 750 μg/ml) morphologic changes were observed in MCF-7 cells versus MRC-5 cells which is consisting of reduction in the number of living cells, volume and rounding until the nucleus constituted the majority of the cellular volume. No morphological changes were detected in MRC-5 cells. Our data showed the reduction of MCF-7 cells compared with MRC-5 cells. Our MTT result showed that this cytotoxicity was increased at higher concentrations (Figure 1), so that, the galangal rhizomes treated MCF-7 (125 μg/ml) were damaged but there were no morphological changes in galangal rhizomes treated MRC-5 cells at the same concentration, this effect again became obvious at the other concentration (250 μg/ml). Although the morphological features were not dramatically changed in the MCF-7 versus MRC-5 cells after 24 h at lower concentration(data was not shown), however after 72 h, at concentration (125, 250 μg/ml), there was observationally sever difference between galangal rhizomes - treated MCF-7 and MRC-5 cells at morphological level (Figure 2).
Figure 1.
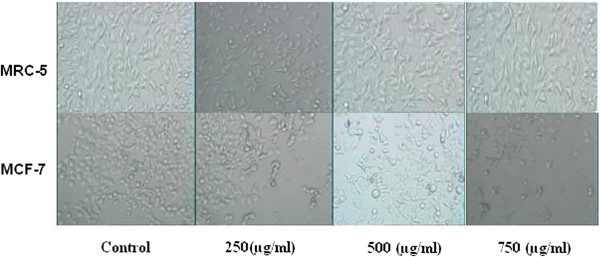
Effect of galangal extract on morphological evaluation in MCF-7 and MRC-5 cells after 48 h treatment.
Figure 2.
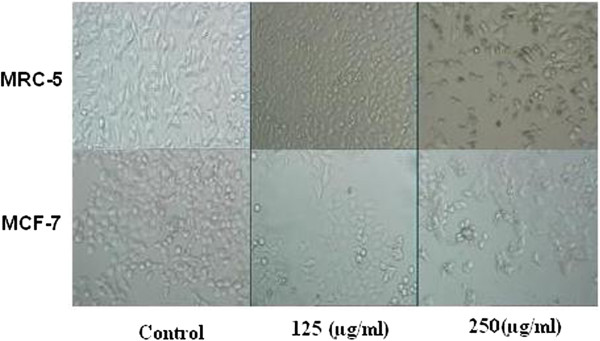
Effect of galangal extract on morphological evaluation in MCF-7 and MRC-5 cells after 72 h treatment.
Effects of galangal on cell viability
In order to evaluate the effect of the ethanolic extract of galangal rhizomes on the growth of MRC-5 and MCF-7 human breast cancer cells, the cells were treated with different concentrations of galangal rhizomes for 3 consecutive days and their growth inhibitory effects were compared. The impact of the galangal rhizomes extract on cell viability was quantitated by the MTT assay. The ethanolic galangal rhizomes extract showed significantly a high growth inhibitory effects on MCF-7 cell line in dose-dependent manner (p < 0.001) compared with MRC-5 cells. The ethanolic extract (250, 500, 750 μg/ml) decreased the cell viability in the malignant cells but not in non-malignant cells after 24 h (p < 0.01) (Figure 3A). This toxicity was consistent with the morphologic changes. However, the extract at the different concentrations (125 μg/ml) could not decrease the cell viability in compare with the MRC-5 cells. After 48 h, the higher concentrations of ethanolic extract (500, 750 μg/ml) dramatically decreased the cell viability in the MCF-7 cells (p < 0.001) (Figure 3B). After 72 h, again the ethanolic extract statistically deceased cell viability in MCF-7 cells versus MRC-5 cells even at the lower concentration (125 μg/ml) (Figure 3C). The dose inducing 50% cell growth inhibition (IC50) against MCF-7 cells was determined as 400.0 ± 11.7 and 170.0 ± 5.9 μg/ml after 28 and 48 h respectively (Table 1).
Figure 3.
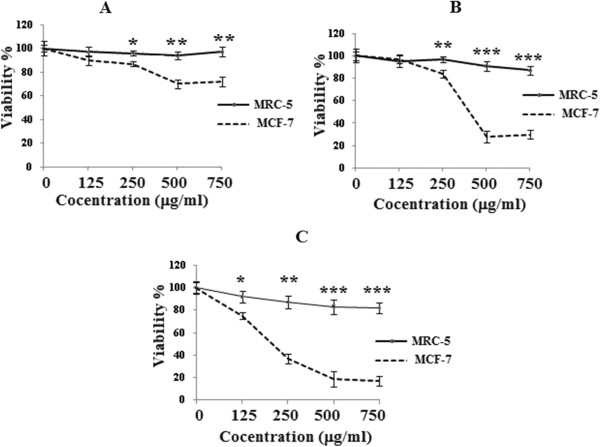
Comparison cytotoxic effect of galangal extract on cell viability of MCF-7 and MRC-5 cells. Cells were treated with different concentrations of the extract for 24 h (A), 48 h (B) and 72 h (C), results are mean ± SEM(n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control (MRC-5).
Table 1.
Doses induce 50% cell growth inhibition (IC 50 ) of ethanolic galangal extract against MCF-7 cell line
| IC50 |
48 h |
72 h |
| MCF7 | 500 (μg/ml) | 250 (μg/ml) |
Induction of apoptosis in MCF-7 cells by galangal
In order to assess the rate of cell death by apoptosis or necrosis, the galangal-treated MCF-7 cells were assayed by Annexin V-FITC/PI dual staining (Figure 4). As it can be seen in Figure 4, the malignant cells were treated with concentrations of 250 and 500 μg/ml galangal for 48 h, then the cells were harvested, and apoptosis was examined by flow cytometry. The cells were treated with 250 and 500 μg/ml galangal for 48 h(symbol П, ПI) or media (control symbol I), and apoptosis was examined with flow-cytometry after Annexin V-PI double staining. The necrotic cells lost cell membrane integrity that permits PI entry. The viable cells exhibit Annexin V (-)/PI (-); early apoptotic cells exhibit Annexin (+)/ PI (-); late apoptotic cells or necrotic cells exhibit Annexin V (+)/PI (+). To study roles of galangal in apoptosis, the ethanolic extract of galangal was used to setup apoptosis system on the MCF-7 cells. Quantitative analysis using annexin V/PI assay further showed that the proportion of the early-stage apoptotic cells (annexin V+/PI-) increased significantly from 9.5% to 31.5%, while proportion of the late stage apoptotic cell (annexin V+/PI+) increased significantly from 13.5% to 39.5% in the cells were treated with 250 and 500 μg/ml galangal extract for 48 h respectively. Apoptosis induced by 250 and 500 μg/ml of galangal was statistically higher than control, and the percentage of early and late apoptotic cells significantly increased by increasing the galangal concentrations (p < 0.05). At 48 h, the total early apoptotic cells were significantly elevated from 2% (control) to 9.5 and 31.5%.at 250 and 500 μg/ml respectively. The percentage of the late apoptotic/necrotic cells increased from 6% (control) to 13.5 and 39.5% at 250 and 500 μg/ml respectively (Figure 5). Although no significant difference was detected between the percentage of early and late apoptotic cells at concentration of 250 μg/ml, however, the number of late apoptotic cells versus to early apoptotic cells at concentration of 500 μg/ml galangal treated cells was statically significant (p < 0.001); thus, the percentage of late apoptotic cells increased significantly versus the percentage of early apoptotic cells.
Figure 4.
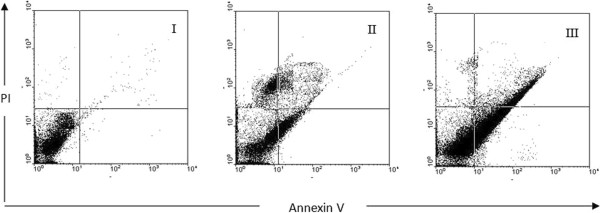
Assessment of apoptosis by Annexin V/PI staining on the human breast adenocarcinoma cell line (MCF-7). The cells were treated with 250 and 500 μg/ml galangal extract for 48 h (symbol II, III) or media (control symbol I), and apoptosis was examined with flow-cytometry after Annexin V-PI double staining. The necrotic cells lost cell membrane integrity that permits PI entry .Viable cells exhibit Annexin V (-)/PI (-); early apoptotic cells exhibit Annexin (+)/PI (-); late apoptotic cells or necrotic cells exhibit Annexin V (+)/PI (+).
Figure 5.
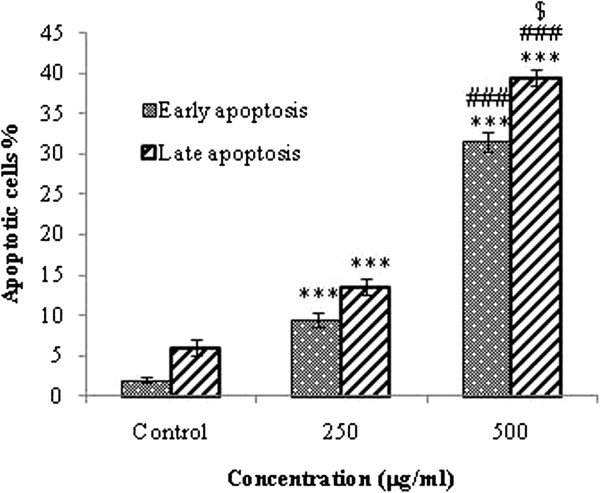
Assessment of apoptosis by Annexin V/PI on the human breast adenocarcinoma cell line (MCF-7) after 48 h. Percentage of cell death based on the assessment of apoptosis by Annexin V/PI. ***, ### p < 0.001 compared with control and the other dose respectively. $ p < 0.05 compared with early apoptosis.
Discussion
Several studies have shown that a number of herbal medicine or mixtures have anticancer potential in vitro and in vivo. They might be good candidate for the development of anti-cancer drugs [48]. Most chemo-preventive agents suppress the various stages of signaling events of carcinogen-induced transformation of normal cells, with striking inhibition of diverse cellular events related to cancer development. Inhibition of cancer cell proliferation, growth factor signaling, induction of apoptosis in cells with malignant potential, etc., contributes for chemo-preventive properties. Increasing evidences suggest that most chemo-preventive agents are capable of inducing apoptosis in the target cancer cells either with mitochondrial or by an uncharacterized mechanism in certain cases [49,50]. Search for novel agents from plants with promising features of apoptosis induction in cancer cells is an attractive strategy.
Alpinia galangal is known to possess flavanoids such as kaempferol, kaempferide, and galangin as well as the terpenoids 10-acetoxy chavicol acetate, 10-acetoxy eugenol, galanal A, galanal B, and galanolactone [47]. We are reporting for the first time that the ethanolic extract of galangal rhizome-induced cytotoxicity and apoptosis on MCF-7 cells versus MRC-5 cells. Much effort has been directed toward the effect of the galangal extract on apoptosis and understanding their mechanisms of action. To study whether the result of MTT assay was due to apoptosis, cells were stained with Annexin V-FITC and PI. The apoptosis evoked by the extract was confirmed by the annexin V–FITC (Figure 3). In the present study, the galangal-induced apoptosis was involved in cell death. Apoptosis is characterized by distinct morphological features including the chromatic condensation, cell and nuclear shrinkage, membrane blabbing, and oligonucleosomal DNA fragmentation [51]. As shown in Figures 3 and 4, the Alpina extract at 250 and 500 μg/ml induced significant cell toxicity in MCF-7 cells in a dose-dependent manner. Therefore, this anti-proliferative effect was due to the induction of apoptosis as shown by the annexin-V-flow cytometric approach. But, apoptosis only partially contributed in this toxicity, and it might be conducted that nonapoptotic cell death can also be involved in the galangal-induced toxicity in these cells. Although the significant of nonapoptotic cell death in chemotherapy remains largely unclear, it is believed that the nonapoptotic cell death is important under conditions in which apoptosis is inhibited [52,53]. Overall, this study showed that the alpina extarct may contain bioactive compounds that inhibit the proliferation of breast cancer cell lines (MCF-7) with the involvements of apoptosis or programmed cell death. Further studies are needed to fully recognize the mechanism involved in cell death, alpina extract could be considered as promising chemotherapeutic agent in lung cancer treatment.
The fresh rhizomes of galangal has been assessed for free radical scavenging activity against 1, 1- diphenyl-2-picrylhydrazyl (DPPH) radical and cytotoxic activity. It has been reported that Alpinia plants possess antioxidant [33]. In vitro cell proliferation inhibition test using MTT viability assay confirmed that the ethanolic extract at 24, 48 and 72 h, has strongly cytotoxic activity against MCF-7 cell line but not in nonmalignant cells (MRC-5) (Figure 3). The present results were consistent with previous studies indicating that MeOH and CH2Cl2 extractable of Alpinia possess significant cytotoxicity against CORL23 human large-cell carcinoma with IC50 values of 13.3 and 5.4 μg/ml respectively [48]. xPresent data were also consistent with previous studies indicating that galangal rhizomes and its ingredients possess cytotoxcity and free radical scavenging activities [54,55]. Phytochemical studies showed that of the many chemical constituents isolated from Alpinia, diaryheptanods, are among the characteristic compounds [56]. Curcumin, a well-known diarylheptanoid, has been postulated to be of potential use not only in cancer chemo-prevention but also in chemotherapy [57]. Two new diarylheptanoids 1, 2 together with two known analogs 3, 4 were isolated from the rhizomes of Alpinia officinarum. The new compounds were elucidated to be (5S)-5-hydroxy-7-(3, 4-dihydroxyphenyl)-1-phenyl-3-heptanone 1 and (5R)-5-hydroxy-7-(3-methoxy-4, 5-dihydroxyphenyl)-1-phenyl-3-heptanone 2 based on their spectral analysis. Compound 4 showed moderate cytotoxicity against human tumor cell lines; HepG2 and SF-268, while no significant effect were found for compounds 1–3 [58]. Ning and Pan tested compounds 1–4 for their cytotoxic activity against human cancer cell lines. They showed that compound 4 even at low concentration (6–10 μg/ml) exhibited potent cytotoxicity against all cell lines [59,60]. Galangal rhizomes also contains pinocembrin (5, 7-dihdroxyflavanone) that shows cytotoxicity against some cancer cells including normal lung fibroblasts with relative nontoxicity to human umbilical cord endothelial cells. Pinocembrin induced loss of mitochondrial membrane potential with subsequent release of cytochrome c and processing of caspase-9 and -3 colon cancer cell line HCT116 [50]. Therefore, it seems likely that potential compounds responsible for the inhibitory effect of galangal on tumor cell growth are its ingredients. Our data showed that galangal extract inhibits significantly proliferation of the human breast adenocarcinoma cells in a concentration- and time-dependent manner versus the normal human fetal lung fibroblast. The morphological features also confirmed these results. We also showed that apoptosis induced by the galangal extract was significantly higher than control, and the percentage of the both early and late apoptotic cells statistically increased by increasing galangal concentration. On the other hand, although no significant difference was detected between the percentage of early and late apoptotic cells at lower concentration, however, the number of the late apoptotic cells versus the early apoptotic cells at higher concentrations of the galangal treated cells was statically significant.
Generally, apoptosis can occur via two fundamental pathways: (1) the mitochondrial or intrinsic pathway; and, (2) the death receptor or extrinsic pathway [61]. The intrinsic pathway is triggered by release of mitochondrial proteins, such as cytochrome c, which bind with Apaf-1 and procaspase-9 in a dATP-dependent manner to form the apoptosome [62]. The apoptosome can induce activation of caspase- 9, thereby initiating apoptotic caspase cascades [63,64]. Conversely, the extrinsic pathway is initiated by the interaction of ligands with their respective death receptors, sequentially leading to cleavage of initiator caspase-8. The active caspase-8 cleaves executioner caspase-3, resulting in apoptosis [65]. Activated caspase-9 and -8 further initiate activation of the caspase cascade, leading to biochemical and morphological changes associated with apoptosis [66,67]. Thus caspases have been shown to be activated during apoptosis in many cells and play critical roles in both initiation and execution of apoptosis [68]. The intrinsic pathway of apoptosis is regulated by the Bcl-2 family of proteins. Anti-apoptotic (e.g. Bcl-2 and Bcl-xL) and pro-apoptotic (e.g. Bad, Bax and Bak) are two of the major members in Bcl-2 family [69-71]. Anti-apoptotic Bcl-2 and Bcl-xL inhibit apoptosis by sequestering proforms of capsases or by preventing the release of mitochondrial apoptogenic factors [72,73]. Bad, Bax and Bak inhibit Bcl-2 activity and promote apoptosis [74]. These experimental findings suggest that A.galangal extract induced MCf-7 cells apoptosis however, further detailed investigations of this mechanism are warranted to obtain definite conclusions.
Conclusion
Overall, this study showed that the galangal extract may contain bioactive compounds that inhibit the proliferation of the human breast adenocarcinoma cell line (MCF-7) with the involvement of apoptosis or programmed cell death. Further research focuses on galangal gradients are needed to fully recognize the mechanisms involved in cell death. The ethanolic extract of galangal could be also considered as a promising chemotherapeutic agent in breast cancer treatment.
Competing interests
The authors confirm that this article content has no conflict of interest.
Authors’ contributions
SS: Supervisor, writing manuscript, design of the manuscrript. MH: Writing manuscript, Help to design the manuscript, doing MTT experiment. JTA: Writing manuscript, doing the morphological experiment. MH: Doing Anniexin /PI, Flocytometry experimetand and also analytical analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Saeed Samarghandian, Email: samarghandians@mums.ac.ir.
Mousa-Al-Reza Hadjzadeh, Email: hajzadehmr@mums.ac.ir.
Jalil Tavakkol Afshari, Email: tavakolaj@mums.ac.ir.
Mohadeseh Hosseini, Email: saeed1142@yahoo.com.
Acknowledgements
The authors would like to thank Research Affairs of Neyshabur University of Medical Sciences for financially supporting this work and Mr. Joharchi for his assistance (botanists) in a herbarium of Ferdosy University. We also thank Miss Aghaii for her assistance in making extracts.
References
- Li J, Xu LZ, He KL, Guo WJ, Zeng YH, Xia P, Chen Y. Reversal effects of nomegestrol acetate on multidrug resistance in adriamycin-resistant MCF-7 breast cancer cell line. Breast Cancer Res. 2001;3:253–263. doi: 10.1186/bcr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree IA, Knight L, Nicolantonio FDI, Sharma S, Guliford T. Chemosensitization of solid tumors by modulation of resistance mechanisms. Curr Opin Investig Drugs. 2002;3:634–640. [PubMed] [Google Scholar]
- Rajkapoor P, Sankari MI, Sumithra M, Anbu J, Harikrishnan N, Gobinath M, Suba V, Balaji R. Antitumor and cytotoxic effects of Phyllanthus polyphyllus on Ehrlich ascites carcinoma and human cancer cell lines. Bio Biotech Biochem. 2007;71:2177–2183. doi: 10.1271/bbb.70149. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Tavakkol Afshari J, Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl Biochem Biotechnol. 2011;164:238–247. doi: 10.1007/s12010-010-9130-x. [DOI] [PubMed] [Google Scholar]
- Adamsen BL, Kravik KL, Clausen OP, De Angelis PM. Apoptosis, cell cycle progression and gene expression in TP53-depleted HCT116 colon cancer cells in response to short-term 5-fluorouracil treatment. Int J Oncol. 2007;31:1491–1500. [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA, Elmore LW. Apoptosis as the predominant tumor cell response to chemotherapy and irradiation: a case of TUNEL vision? Curr Opin Investig Drugs. 2005;6:1199. [PubMed] [Google Scholar]
- Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6:133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S, Afshari JT, Davoodi S. Honey induces apoptosis in renal cell carcinoma. Pharmacogn Mag. 2011;7:46–52. doi: 10.4103/0973-1296.75901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BA. Breast cancer and the western diet: role of fatty acids and antioxidant vitamins. Eur J Cancer. 1998;34:1852–1856. doi: 10.1016/s0959-8049(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma S, Sultana S. Attenuation of potassium bromate-induced nephrotoxicity by coumarin (1,2-benzopyrone) in Wistar rats: chemoprevention against free radical-mediated renal oxidative stress and tumor promotion response. Redox Rep. 2004;9:19–28. doi: 10.1179/135100004225003860. [DOI] [PubMed] [Google Scholar]
- Priyadarsini KL. Free radical reactions of curcumin in membrane models. Free rad Bio Med. 1997;23:838–843. doi: 10.1016/s0891-5849(97)00026-9. [DOI] [PubMed] [Google Scholar]
- Chen X, He B. Oxygen free radical scavening activity and anti-lipid peroxidation of tea polyphenol. Zhong Yao Cai. 1998;21:141–144. [PubMed] [Google Scholar]
- Samarghandian S, Afshari JT, Davoodi S. Modulation of programmed cell death by honey bee in human prostate adenocarcinoma. J Med Plants Res. 2010;4:2551–2556. [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Chhabra SC, Mahunnah RL, Mshiu EN. Plants used in traditional medicine in eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae) J Ethnopharmacol. 1993;39:83–103. doi: 10.1016/0378-8741(93)90024-y. [DOI] [PubMed] [Google Scholar]
- Mayachiew P, Devahastin S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT Food Sci Technol. 2008;41:1153–1159. [Google Scholar]
- Jaju S, Indurwade N, Sakarkar D, Fuloria N, Ali M. Isolation of galangogalloside from rhizomes of Alpinia galanga. Int J Green Pharm. 2009;3:144–147. [Google Scholar]
- Jaju SB, Indurwade NH, Sakarkar DM, Fuloria NK, Ali MD, Basu SP. Isolation of β-sitosterol diglucosyl caprate from Alpinia galanga. Pharmacogn Res. 2010;2:264–266. doi: 10.4103/0974-8490.69129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Singh R, Dey CS, Sharma SS, Bhutani KK, Singh IP. Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd. Indian J Exp Biol. 2010;48:314–317. [PubMed] [Google Scholar]
- Min HJ, Nam JW, Yu ES, Hong JH, Seo EK, Hwang ES. Effect of naturally occurring hydroxychavicol acetate on the cytokine production in T helper cells. Int Immunopharmacol. 2009;9:448–454. doi: 10.1016/j.intimp.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Indrayan AK, Agrawal P, Rathi AK, Shatru A, Agrawal NK, Tyagi DK. Nutritive value of some indigenous plant rhizomes resembling Ginger. Nat Prod Rad. 2009;8:507–513. [Google Scholar]
- Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT Food Sci Technol. 2006;39:1214–1220. [Google Scholar]
- Rao K, Ch B, Narasu LM, Giri A. Antibacterial activity of Alpinia galanga (L) Willd crude extracts. Appl Biochem Biotechnol. 2010;162:871–884. doi: 10.1007/s12010-009-8900-9. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Yang MH, Luo JG, Huang XF, Kong LY. A new phenylpropanoid from Alpinia galangal. Chin J Nat Med. 2009;7:19–20. [Google Scholar]
- Yu ES, Min HJ, Lee K, Lee MS, Nam JW, Seo EK, Hong JH, Hwang ES. Anti-inflammatory activity of p-coumaryl alcohol-γ-O-methyl ether is mediated through modulation of interferon-γ production in Th cells. Br J Pharmacol. 2009;156:1107–1114. doi: 10.1111/j.1476-5381.2009.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaeoung S, Plubrukarn A, Keawpradub N. Cytotoxic and free radical scavenging activities of Zingiberaceous rhizomes. Songklanakarin J Sci Technol. 2005;27:799–812. [Google Scholar]
- Arambewela LSR, Arawwawala M, Owen NL, Jarvis B. Volatile oil of Alpinia galanga Willd of Sri Lanka. J Essent Oil Res. 2007;19:455–456. [Google Scholar]
- Pripdeevech P, Nuntawong N, Wongpornchai S. Composition of essential oils from the rhizomes of three Alpinia species grown in Thailand. Chem Nat Compd. 2009;45:562–564. [Google Scholar]
- Srivastava B, Singh P, Shukla R, Dubey NK. A novel combination of the essential oils of Cinnamomum camphora and Alpinia galanga in checking aflatoxin B1 production by a toxigenic strain of Aspergillus flavus. World J Microbiol Biotechnol. 2008;24:693–697. [Google Scholar]
- Pothacharoen P, Choocheep K, Pitak T, Pompimon W, Premanode B, Hardingham TE, Kongtawelert P. Effect of Alpinia galanga extract on cartilage degradation and on gene expression in human chondrocyte and synovial fibroblast metabolism. Cent Eur J Biol. 2006;1:430–450. [Google Scholar]
- Phitak T, Choocheep K, Pothacharoen P, Pompimon W, Premanode B, Kongtawelert P. The effects of p-hydroxycinnamaldehyde from Alpinia galanga extracts on human chondrocytes. Phytochemistry. 2009;70:237–243. doi: 10.1016/j.phytochem.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, Radhika S, Amit A, Venkateshwarlu K, Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Jantan I, Rafi IA, Jalil J. Platelet-activating factor (PAF) receptor-binding antagonist activity of Malaysian medicinal plants. Phytomedicine. 2005;12:88–92. doi: 10.1016/j.phymed.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Weerakkody NS, Caffin N, Turner MS, Dykes GA. In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control. 2010;21:1408–1414. [Google Scholar]
- Chan EWC, Lim YY, Omar M. Antioxidant and antibacterial activity of leaves of Etlingera species (Zingiberaceae) in Peninsular Malaysia. Food Chem. 2007;104:1586–1593. [Google Scholar]
- Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, Srisuwan N, Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol Res. 2006;98:588–592. doi: 10.1007/s00436-005-0119-2. [DOI] [PubMed] [Google Scholar]
- Nguyen VN, Nguyen DMC, Seo DJ, Park RD, Jung WJ. Antimycotic activities of Cinnamon-derived compounds against Rhizoctonia solani in vitro. Bio Control. 2009;54:697–707. [Google Scholar]
- Banjerdpongchai R, Punyati P, Nakrob A, Pompimon W, Kongtawelert P. 4′-Hydroxycinnamaldehyde from Alpinia galanga (Linn.) induces human leukemic cell apoptosis via mitochondrial and endoplasmic reticulum stress pathways. Asian Pac J Cancer Prev. 2011;12:593–598. [PubMed] [Google Scholar]
- Yang HL, Chen SC, Chen CS, Wang SY, Hseu YC. Alpinia pricei rhizome extracts induce apoptosis of human carcinoma KB cells via a mitochondria-dependent apoptotic pathway. Food Chem Toxicol. 2008;46:3318–3324. doi: 10.1016/j.fct.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Shi GF, An LJ, Jiang B, Guan S, Bao YM. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci Let. 2006;403:206–210. doi: 10.1016/j.neulet.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells. Mol Immun. 2007;44:673–679. doi: 10.1016/j.molimm.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Bendjeddou D, Lalaoui K, Satta D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J Ethnopharmacol. 2003;88:155–160. doi: 10.1016/s0378-8741(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. Antinociceptive activities of aqueous and ethanolic extracts of Alpinia calcarata rhizomes in rats. J Ethnophrmacol. 2004;95:311–316. doi: 10.1016/j.jep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Kadota S, Tezuka Y, Prasain JK, Ali MS, Banskota AH. Novel diarylheptanoids of Alpinia blepharocalyx. Cur Top Med Chem. 2003;3:203–225. doi: 10.2174/1568026033392552. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics. 2011;66:1073–1079. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Del Bino G, Darzynkiewicz Z, Degraef C, Mosselmans R, Fokan D, Galand P. Comparison of methods based on annexin-V binding, DNA content or TUNEL for evaluating cell death in HL-60 and adherent MCF-7 cells. Cell Prolif. 1999;32:25–37. doi: 10.1046/j.1365-2184.1999.3210025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD, EL-Deirly WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- Samarghandian S, Shabestari MM. DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J Urol. 2013;29:177–183. doi: 10.4103/0970-1591.117278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JM, Owens TA, Barwe SP, Rajasekaran AK. Gramicidin A Induces Metabolic Dysfunction and Energy Depletion Leading to Cell Death in Renal Cell Carcinoma Cells. Mol Cancer Ther. 2013;12:2296–2307. doi: 10.1158/1535-7163.MCT-13-0445. [DOI] [PubMed] [Google Scholar]
- Dutta C, Day T, Kopp N, Van Bodegom D, Davis MS. BCL2 Suppresses PARP1 Function and Nonapoptotic Cell Death. Cancer Res. 2012;72:4193–41203. doi: 10.1158/0008-5472.CAN-11-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Rohr M, Jordan J. Identification of dihydrogalangal acetate in galangal [Alpinia galangal (L.) Swartz] extracts. J Agric Food Chem. 2009;22:3286–3290. doi: 10.1021/jf803387z. [DOI] [PubMed] [Google Scholar]
- Chan EWC, Ng VP, Tan VV, Low YY. Antioxidant and Antibacterial Properties of Alpinia galanga, Curcuma longa, and Etlingera elatior (Zingiberaceae) Pharmacogn J. 2011;3:54–61. [Google Scholar]
- Luo J, Rui W, Jiang M, Tian Q, Ji X, Feng Y. Separation and identification of diarylheptanoids in supercritical fluid extract of Alpinia officinarum by UPLC-MS-MS. J Chromatogr Sci. 2010;48:795–801. doi: 10.1093/chromsci/48.10.795. [DOI] [PubMed] [Google Scholar]
- Samini F, Samarghandian S, Borji A, Mohammadi G, Bakaian M. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 2013;110:238–244. doi: 10.1016/j.pbb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Stewrd WP, Gescher AJ. Curcumin in cancer management: recent results of analogue design and clinical studies and desirable future research. Mol Nutr Food Res. 2008;52:1005–1009. doi: 10.1002/mnfr.200700148. [DOI] [PubMed] [Google Scholar]
- An N, Zou ZM, Tian Z, Luo XZ, Yang SL, Xu LZ. Diarylheptanoids from the rhizomes of Alpinia officinarum and their anticancer activity. Fitoterapia. 2007;79:27–31. doi: 10.1016/j.fitote.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ning AL, Pan QQ. Studies on hematoporphyrin-photosensitized effects on human cancer cells in vitro: TEM and SEM observations. Adv Exp Med Biol. 1985;193:117–122. [PubMed] [Google Scholar]
- Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37:400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Borji A, Farahmand SK, Afshari R, Davoodi S. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. Biomed Res Int. 2013;2013:1–12. doi: 10.1155/2013/417928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun SY, Hail Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Nat Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. The EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmed M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Seldin MF, Mudryj M. Fas-mediated apoptosis of proliferating, transiently growth-arrested, and senescent normal human fibroblasts. Exp Cell Res. 2000;260:9–19. doi: 10.1006/excr.2000.4990. [DOI] [PubMed] [Google Scholar]
- Liu ZB, Hou YF, Di GH, Wu J, Shen ZZ, Shao ZM. PA-MSHA inhibits proliferation and induces apoptosis through the up-regulation and activation of caspases in the human breast cancer cell lines. J Cell Biochem. 2009;108:195–206. doi: 10.1002/jcb.22241. [DOI] [PubMed] [Google Scholar]
- Zhong LT, Sarafian T, Kane DJ, Charles AC, Mah SP, Edwards RH, Bredesen DE. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Begley JG, Fu W, Butterfield DA, Bredesen DE, Hutchins JB, Hensley K, Mattson MP. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem. 1998;70:31–39. doi: 10.1046/j.1471-4159.1998.70010031.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria. Genes to Cells. 1998;11:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Adams M, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Regulation of apoptosis by Bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]


