Abstract
Detectable human immunodeficiency virus (HIV) RNA in the cerebrospinal fluid (CSF) is associated with central nervous system (CNS) complications. We developed the CSF HIV risk score through prediction modeling to estimate the risk of detectable CSF HIV RNA (threshold >50 copies/mL) to help identify persons who might benefit most from CSF monitoring. We used baseline data from 1,053 participants receiving combination antiretroviral therapy who were enrolled in the 6-center, US-based CNS HIV Antiretroviral Therapy Effects Research (CHARTER) prospective cohort in 2004–2007. Plasma HIV RNA, CNS penetration effectiveness, duration of combination antiretroviral therapy, medication adherence, race, and depression status were retained correlates of CSF HIV RNA, displaying good discrimination (C statistic = 0.90, 95% confidence interval (CI): 0.87, 0.93) and calibration (Hosmer-Lemeshow P = 0.85). The CSF HIV risk score ranges from 0 to 42 points, with a mean of 15.4 (standard deviation, 7.3) points. At risk scores greater than 25, the probability of detecting CSF HIV RNA was at least 42.9% (95% CI: 36.6, 49.6). For each 1-point increase, the odds of detecting CSF HIV RNA increased by 26% (odds ratio = 1.26, 95% CI: 1.21, 1.31; P < 0.01). The risk score correlates with detection of CSF HIV RNA. It represents an advance in HIV management and monitoring of CNS effects, providing a potentially useful tool for clinicians.
Keywords: central nervous system, cerebrospinal fluid, cerebrospinal fluid human immunodeficiency virus risk score, human immunodeficiency virus, prediction model
The introduction of combination antiretroviral therapy (cART) has improved survival and reduced opportunistic infections in persons infected with human immunodeficiency virus (HIV) (1). cART has also been associated with a greatly reduced incidence and phenotypical severity of human immunodeficiency virus–associated neurocognitive disorder (HAND), although milder forms of HAND have increased in prevalence (2–4). Even though cART use improves cognition, a significant percentage of patients develop new cognitive impairment or depression during treatment (5). This is important because neurocognitive impairment and depression are both associated with poor HIV outcomes and substantially worse survival rates (6–8).
HIV infection in the central nervous system (CNS) may be associated with glial and endothelial activation resulting in inflammatory and degenerative insults that lead to neuronal injury and neurocognitive impairment (9–11). HIV viral replication in the cerebrospinal fluid (CSF) is hypothesized to be clinically relevant to neurological and psychiatric complications of HIV. Residual CSF viral replication in patients with undetectable plasma HIV RNA or CSF HIV RNA concentration ≥1 log higher than plasma HIV RNA concentration, called CSF viral escape, is estimated to occur in more than 10% of patients and is considered clinically relevant by several groups across United States and Europe (10, 12, 13). CSF viral escape has been associated with HAND, depression, and neurological deficits in HIV patients (9, 10, 14).
Recent findings suggest CSF testing to examine residual viral activity, immune activation, and neural damage in managing HAND (15). The 2013 European Acquired Immune Deficiency Syndrome (AIDS) Clinical Society also provides guidelines for measuring CSF HIV RNA levels as a component of their clinical strategy for managing HAND (16). The MIND Exchange Program, in a recent consensus report, recommends CSF testing for HIV RNA in persons with suspected or demonstrated neurocognitive impairment (17).
Some antiretroviral drugs are hypothesized to be more neurologically active than others on the basis of their ability to penetrate the CNS and effectively suppress virus replication as reflected by reductions in CSF HIV RNA (18, 19). Improvement in cognition with higher CNS-penetrating drugs and suppression of CSF HIV RNA are hypothesized to be primary modulators of favorable neurological response in HIV patients (20, 21). CSF analysis is useful to assess the activity of HIV in the CNS as reflected by detectable HIV RNA (15). Poor cART penetration of the blood-brain barrier and active efflux systems that reduce the parenchymal concentration of cART may allow HIV replication to continue in the CNS despite peripheral suppression (22).
Because lumbar punctures may pose a resource utilization challenge in HIV clinics worldwide and are not without risks, we developed an algorithm to determine correlates (referred to herein as “predictors”) of detectable CSF HIV RNA during HIV treatment using readily attainable clinical and demographic data that would be useful for clinical management of HIV. We accomplished this by using data from the Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) Study cohort.
METHODS
Study design and patients
The CHARTER Study is a 6-center, US-based, prospective observational study that started in 2004 and is designed to comprehensively assess a representative US clinical population of persons who are HIV seropositive (23). There were no general exclusion criteria except the inability to consent to participation in study assessments. The CHARTER Study aimed to evaluate the changing presentation of neurological complications of HIV in the context of cART. Study sites include Johns Hopkins University (Baltimore, Maryland), Icahn School of Medicine at Mt. Sinai (New York, New York), University of California (San Diego, California), University of Texas (Galveston, Texas), University of Washington (Seattle, Washington), and Washington University (St. Louis, Missouri). The institutional review board at each study site or the Western Institutional Review Board (Puyallup, Washington) approved the study.
We analyzed data from 1,053 of 1,561 CHARTER Study participants who were currently receiving cART at study entry between 2004 and 2007. Because we sought to examine correlates of the presence of CSF HIV RNA in persons receiving cART, we excluded all participants who were not receiving cART at study entry (n = 508). A total of 1,053 persons receiving cART were included in these analyses. Participants underwent extensive evaluation, including HIV and treatment history verified by medical records, physical examination, lumbar puncture, and venipuncture.
Diagnostic predictors of CSF HIV
Potential predictors included patient demographic characteristics and HIV treatment–related data, including current and nadir CD4+ T-cell counts, plasma and CSF HIV RNA levels, duration of HIV seropositivity, duration and type of cART regimens (i.e., protease inhibitor– vs. nonnucleoside reverse transcriptase inhibitor–based regimens), medication adherence assessed by the AIDS Clinical Trials Group 4-Day Adherence Questionnaire (24), number of drugs, and central nervous system penetration effectiveness (CPE) scores of current and past cART regimens, which are an estimate of the CNS penetration of cART (10). The CPE score ranks individual antiretroviral medications from 1–4, with higher scores reflecting greater CNS penetration. Derivation of the class of penetration is based on 1) chemical properties suggesting the extent of penetration; 2) concentration of the drug in the CSF based on human and animal studies or comparison of the CSF concentration of the drug compared with half the maximum inhibitory concentration, a measure of the extent to which the anti-HIV drug is effective in inhibiting the virus; and 3) demonstrated effectiveness in reducing CSF viral load or improving cognition in clinical studies (25). The concept of CPE scores circumvents the direct measurement of CSF drug concentrations, but may not accurately correlate with the CNS concentrations of anti-HIV drugs. Appendix Table 1 shows the CPE scores of frequently used antiretroviral drugs (10).
Other potential predictors included the Global Deficit Score, a validated measure that is derived from the standardized, comprehensive neurocognitive assessment and summarizes overall functioning across 7 cognitive abilities (26); major depressive episode within last 30 days assessed by Diagnostic Statistical Manual, Fourth Edition, (DSM-IV) criteria (27); Beck Depression Inventory score (28); history of opportunistic infection; and hepatitis C virus seropositivity. Information on illicit substance use and abuse was obtained by using the Composite International Diagnostic Interview of the DSM-IV.
Outcome
The outcome was the presence of CSF HIV RNA at a threshold of 50 copies/mL. Both CSF and plasma HIV RNA levels were determined by commercial ultrasensitive reverse transcriptase-polymerase chain reaction (AMPLICOR, Roche Diagnostics, Indianapolis, Indiana).
Statistical analysis
The population characteristics were examined by using summary statistics. We fit a stepwise backwards multivariable logistic regression model (29), the outcome being detectable CSF HIV RNA. Variables associated with detectable CSF HIV were retained at a significance level of 0.157, which equates to the Akaike information criterion for selection of a single predictor (30). Selection at this level rather than conventional significance levels reduces selection bias, thus preventing overestimated regression coefficients, and diminishes optimism from overfitting of the data, which results in poor prediction in independent data.
Transformed continuous variables were compared with linear forms to determine the best-fitting functional forms to be included in the final model (29, 31). Continuous forms of all variables were preferentially fitted in regression models because of the advantage of creating robust regression models compared with using relatively arbitrary cut-points of categorization (30).
The accuracy of the retained model was assessed and compared by forcing in specific variables and reducing the selected model. Discrimination was assessed by the receiver operator curve C statistic (32), a measure of chance that the prediction model will assign a higher probability to a patient with detectable CSF HIV RNA compared with a patient without detectable CSF HIV RNA. We assessed model calibration by the Hosmer-Lemeshow test (33), which compares the observed and predicted probabilities of detectable CSF HIV RNA in various risk classes in the study population.
Internal validation was performed by 5-fold cross-validation and bootstrapping techniques (29). To prevent our model from being overly optimistic in future applications, we calculated shrunken estimates by applying the calculated Van Houwelingen and Le Cessie heuristic shrinkage estimator (34). To enhance the future application of our findings in clinical settings, we used the risk score approach to apply points to the final prediction model (31). We calculated the predicted probability estimates of detectable CSF HIV RNA for the logistic regression model provided by (31).
We examined the distribution of CSF viral load by risk score category and fit logistic regression models to examine the odds of detectable CSF HIV RNA per 1-, 5-, and 10-point increase in risk score. Missing data were handled by multivariate imputation using chained equations by linear and augmented logistic regression (35) imputing the median of 50 values generated from each observation (36). We performed model checking, diagnostic procedures, and sensitivity analyses (the latter are included in Appendix 2). We examined interactions between cART adherence and CPE of current cART, as well as cART adherence and months on cART, and we evaluated discriminatory performance by receiver operator curve comparisons to assess whether the difference in performance of the various models was 0. To evaluate the utility of the retained potential predictors in persons with plasma HIV RNA less than 10,000 copies/mL or less than 50 copies/mL, we examined discriminatory performance in these subpopulations.
Because the CPE score is an estimate of the extent to which cART penetrates the CNS, we also examined its discriminatory and calibration performance to potentially predict detectable CSF HIV RNA. E.R.H. performed the statistical analysis with Stata, version 12, statistical software (StataCorp LP, College Station, Texas).
RESULTS
Demographic and clinical characteristics of participants included in our analysis are displayed in Table 1. The mean age was 43.3 years, and 78% of participants were male. A total of 39.2% of participants were white, 49.7% were black, and 12.8% were Hispanic. The mean duration of cART usage was 20.6 (standard deviation (SD), 23.0) months, and the mean duration of HIV seropositivity was 130.1 (SD, 72.6) months. Prior diagnosis of opportunistic infection was reported in 14.3% of subjects, and 13.4% of subjects met DSM-IV criteria for current depression. Overall, CSF HIV RNA was detectable (at 50 copies/mL) in 15.7% of subjects (127 of 811).
Table 1.
Characteristics of Individuals Receiving Combination HIV Therapy at Study Entry in the CHARTER Study Cohort, 2004–2007
| Characteristic | Total No. | Distribution of Participant Characteristics |
|||
|---|---|---|---|---|---|
| Group No. | % | Mean (SD) | Median (IQR) | ||
| Age, years | 1,053 | 44.3 (8.0) | |||
| Male sex | 1,053 | 822 | 78.1 | ||
| Race | 1,053 | ||||
| White | 414 | 39.2 | |||
| Black | 504 | 49.7 | |||
| Hispanic/other | 135 | 12.8 | |||
| Education, years | 1,053 | 12.6 (2.5) | |||
| CD4 T-cell nadir, cells/mm3 | 1,053 | 150.6 (147.1) | 120.0 (29.0–218.0) | ||
| Current CD4 T-cell count, cells/mm3 | 1,042 | 458.0 (290.7) | 418.5 (248.0–610.0) | ||
| Log plasma HIV RNA, copies/mL | 1,040 | 2.35 (1.04) | 1.70 (1.70–2.70) | ||
| Log CSF HIV RNA, copies/mL | 811 | 1.86 (0.49) | 1.70 (1.70–1.70) | ||
| CSF HIV virus present | 811 | 127 | 15.7 | ||
| HIV regimen type | 1,053 | ||||
| NNRTI based | 362 | 34.4 | |||
| PI based | 599 | 56.9 | |||
| PI/NNRTI based | 54 | 5.1 | |||
| Other | 36 | 3.6 | |||
| No. of HIV drugs | 1,053 | ||||
| 3 | 794 | 75.4 | |||
| >3 | 259 | 24.6 | |||
| CPE score | 1,053 | 7.3 (1.7) | |||
| HIV medication adherence, % | 1,045 | ||||
| ≥95 | 915 | 87.6 | |||
| 85–94 | 46 | 4.4 | |||
| <85 | 84 | 8.0 | |||
| HCV positive | 1,034 | 275 | 26.6 | ||
| Total Beck Depression Inventory II score (28) |
1,044 | 13.9 (10.8) | |||
| Current depressiona diagnosis within last 30 days |
1,049 | 140 | 13.4 | ||
| Beck Depression Inventory II score >14 | 1,044 | 459 | 44.0 | ||
| Lifetime depression | 1,049 | 527 | 50.2 | ||
| Duration of current treatment, months | 1,041 | 20.6 (23.0) | |||
| Duration of HIV infection, months | 1,050 | 130.1 (72.6) | |||
| Any opportunistic infection | 1,053 | 150 | 14.3 | ||
Abbreviations: CHARTER, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research; CPE, central nervous system penetration effectiveness; CSF, cerebrospinal fluid; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
a Depression assessed by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (27).
In multivariable logistic regression analysis, CPE, race, depression, plasma HIV RNA, duration of cART, and cART adherence were retained as correlates of detectable CSF HIV RNA. There were no interactions between cART adherence and CPE of current cART or of cART adherence and months on cART. Five-fold cross-validation displayed very good discrimination (C statistic = 0.90, 95% confidence interval (CI): 0.87, 0.93 for the full model) (Figure 1). The calibration plot for the full model demonstrates that predicted probabilities were similar to observed probabilities (Hosmer-Lemeshow P = 0.85) (Figure 2). Further internal validation by bootstrapping methods correcting for model optimism (e.g., overfitting of data) displayed good discrimination (C statistic = 0.84, 95% CI: 0.81, 0.87). Average optimism was 0.074. The mean variance inflation factor, a measure of how predictor variables are correlated, was 1.03. When we examined the performance of the CPE score alone in potentially predicting detectable CSF HIV RNA, we observed a lower discrimination and calibration performance (C statistic = 0.55, 95% CI: 0.5, 0.61; Hosmer-Lemeshow P = 0.008). The discriminatory ability, C statistic, of retained correlates of CSF HIV RNA among person with plasma HIV RNA levels less than 10,000 copies/mL was 0.84 (95% CI: 0.79, 0.89; Hosmer-Lemeshow P = 0.69). Among persons with plasma HIV RNA of less than 50 copies/mL, the discriminatory ability was 0.70 (95% CI: 0.55, 0.85; Hosmer-Lemeshow P = 0.08). The discriminatory ability of detectable plasma HIV RNA alone was lower compared with the full retained model (C statistic = 0.72, 95% CI: 0.64, 0.80). A calibration plot of plasma HIV RNA alone revealed that predicted probabilities were significantly different from observed probabilities, indicating poor model fit (Hosmer-Lemeshow P = 0.009).
Figure 1.
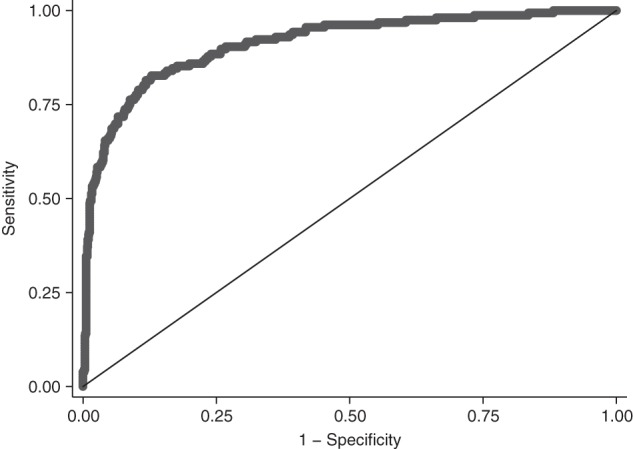
Discrimination plot of the cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) risk score, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) cohort at study entry, 2004–2007. The discrimination plot shows the plot of sensitivity versus 1 − specificity of the CSF HIV risk score indicated by the curved line. The diagonal line shows the noninformative line where sensitivity is equal to specificity. The area under the curve = 0.90 (95% confidence interval: 0.87, 0.93) and represents the discriminative ability of the test to correctly differentiate between 2 individuals, 1 with detectable CSF HIV RNA and the other without detectable CSF HIV RNA.
Figure 2.
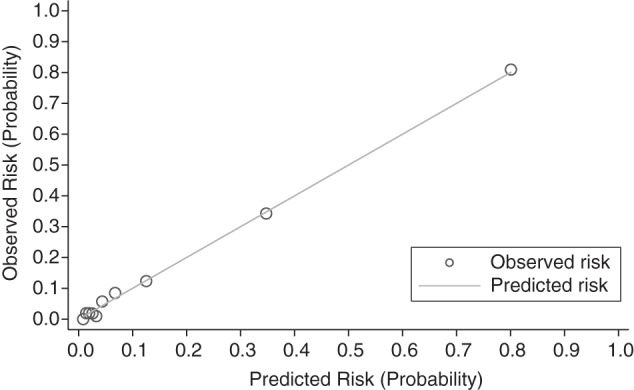
Calibration plot of the cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) risk score, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) cohort at study entry, 2004–2007. The x-axis shows the risk (probability) of detectable CSF HIV RNA as predicted by the CSF HIV risk score. The y-axis shows the observed risk in the CHARTER cohort. Circles represent a risk class with corresponding predicted and observed risk. The solid line represents perfect agreement (Hosmer-Lemeshow P = 0.85) and tests whether predicted and observed risks differ significantly across classes.
Table 2 summarizes the development of the CSF HIV risk score. The observed predictor variable coefficients were multiplied by the calculated shrinkage factor 0.937 to prevent overfitting if this model should be applied to another population. Model fit due to noise was 6.3%.
Table 2.
Regression Coefficients, Odds Ratios and Development of the CSF HIV Risk Scorea in the CHARTER Study Cohort at Study Entry, 2004–2007
| Variable | Regression Coefficient | Odds Ratio | 95% CI | P Value | Shrunkenb Regression Coefficient βi | Reference Value Wij (Midpoint) | βi (Wij-WiRef) | Risk Score (βi × [(Wij-WiRef)]/Bc) |
|---|---|---|---|---|---|---|---|---|
| CPE score | −0.266d | 0.77 | 0.67, 0.88 | <0.001 | −0.249 | |||
| ≥10 | 12 (W1Ref) | 0 | 0 | |||||
| 5–9 | 7 | 1.245 | 6 | |||||
| <5 | 4 | 1.992 | 9 | |||||
| Race | ||||||||
| White | 1.00 | Referent | 0 (W2Ref) | 0 | 0 | |||
| Black | 0.593 | 1.81 | 1.06, 3.09 | 0.02 | 0.556 | 1 | 0.556 | 3 |
| Hispanic/other | 0.875 | 2.39 | 1.16, 4.95 | 0.02 | 0.820 | 1 | 0.820 | 4 |
| Current depression | ||||||||
| No | 1.00 | Referent | 0 (W3Ref) | 0 | 0 | |||
| Yes | 0.808 | 2.25 | 1.18, 4.28 | 0.01 | 0.757 | 1 | 0.757 | 4 |
| HIV medication adherence, % | ||||||||
| ≥95 | 1.00 | Referent | 97.5 (W4Ref) | 0 | 0 | |||
| 85–94 | 0.584 | 1.79 | 0.67, 4.79 | 0.23 | 0.547 | 89.5 | 0.547 | 3 |
| <85 | 0.599 | 1.82 | 0.90, 3.68 | 0.10 | 0.561 | 80.0 | 0.561 | 3 |
| Log plasma RNA, copies/mL | 1.584d | 4.88 | 3.91, 6.09 | <0.001 | 1.486 | |||
| <1.699 | 1.699 (W5Ref) | 0 | 0 | |||||
| 1.699–2.299 | 1.999 | 0.446 | 2 | |||||
| 2.301–3.999 | 3.150 | 2.156 | 10 | |||||
| >4.0 | 5.627 | 3.928 | 18 | |||||
| Current cART, months | −0.011d | 0.99 | 0.98, 1.00 | 0.07 | −0.010 | |||
| ≥36 | 75 (W6Ref) | 0 | 0 | |||||
| 25–35 | 30 | 0.450 | 2 | |||||
| 13–24 | 18 | 0.570 | 3 | |||||
| 7–12 | 9 | 0.660 | 3 | |||||
| ≤6 | 3 | 0.720 | 4 | |||||
Abbreviations: cART, combination antiretroviral therapy; CHARTER, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research; CI, confidence interval; CNS, central nervous system; CPE, central nervous system penetration effectiveness; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus.
a Under the points system, meaningful categories of predictors were created, and the distance from a chosen reference category was determined in regression units. Integer points were assigned to each level of predictor, and the risk estimate was determined from total points assigned by a reference table (30). Points were rounded to the next integer.
b Regression coefficients were multiplied by a shrinkage factor of 0.937.
c B represents the constant for the point system or the number of regression units that will correspond to 1 point. The constant reflects the risk of detecting CSF HIV RNA associated with the study population's mean duration on cART, 20.6 months (B = 20.6 × 0.011 = 0.227).
d Regression coefficient for continuous form of variable.
The CSF HIV risk score ranges from 0 to 42 points. In our study population, the risk score ranged from 0 to 39 points with a mean of 15.4 (SD, 7.3) points. The largest potential predictor of detectable CSF HIV was plasma HIV RNA at levels greater than 10,000 copies/mL, contributing 42.9% (18 points) of the maximum possible total risk score.
The predicted probabilities of detectable CSF HIV RNA in persons receiving cART for various levels of the CSF HIV risk score are displayed in Table 3 and Figure 3. For example, a black patient (3 points) with plasma HIV RNA of 300 copies/mL (10 points), who is presently depressed (4 points), and is fully adherent to the cART regimen (0 points), whose regimen has a total CPE of 9 (6 points) for a duration of 10 months (4 points) has a total CSF HIV RNA score of 27 points and a 54.3% (95% CI: 46.5, 62.0) probability of having detectable CSF HIV RNA. At risk scores greater than 25, the observed probability of detecting CSF HIV RNA is at least 42.9% (95% CI: 36.6, 49.6).
Table 3.
Predicted Probabilities of Detectable CSF HIV RNA (at >50 copies/mL) by the CSF HIV Risk Score in 811 Persons Receiving Combination Antiretroviral Therapy at Study Entry, CHARTER Study Cohort, 2004–2007
| CSF HIV Risk Score | Predicted Probability, % | 95% CI |
|---|---|---|
| 0 | 0.24 | 0.11, 0.53 |
| 5 | 0.75 | 0.40, 1.4 |
| 10 | 2.32 | 1.5, 3.7 |
| 15 | 7.0 | 5.1, 9.5 |
| 20 | 19.2 | 15.7, 23.2 |
| 25 | 42.9 | 34.6, 49.6 |
| 30 | 70.3 | 61.5, 77.9 |
| 35 | 88.2 | 81.2, 92.9 |
| 39 | 95.0 | 90.5, 97.4 |
Abbreviations: CHARTER, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research; CI, confidence interval; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus.
Figure 3.
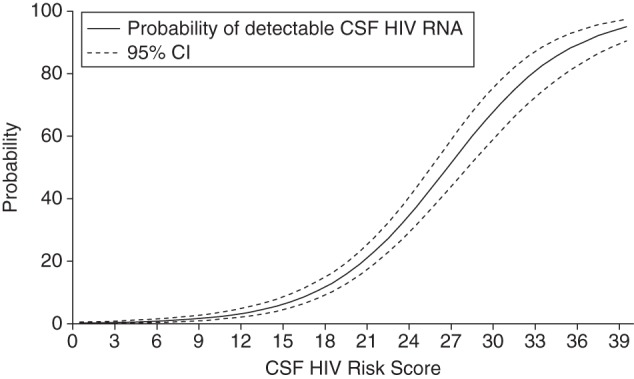
Predicted probabilities and 95% confidence intervals (CIs) of detectable cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) RNA by the CSF HIV risk score, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) cohort at study entry, 2004–2007.
We observed a dose-response association between increasing CSF HIV risk score category and increasing CSF viral load (Table 4, Figure 4). For each 1-point increase on the risk score, the odds of detecting CSF HIV RNA increased by 26% (odds ratio = 1.26, 95% CI: 1.21, 1.31; P < 0.001) (Figure 5). Likewise, increases in risk score of 5 and 10 points were associated with a 3-fold increase in odds (odds ratio = 3.16, 95% CI: 2.64, 3.79; P < 0.001) and a 9-fold increase in odds (odds ratio = 9.99, 95% CI: 6.95, 14.1; P < 0.001), respectively.
Table 4.
Distribution of CSF Viral Load by CSF HIV Risk Score Category in 811 Persons With HIV at Study Entry, CHARTER Study Cohort, 2004–2007
| Category | Risk Score | No. of Subjects | Log10 CSF Viral Load, mean (SD) |
P Value for Comparison of CSF HIV Risk Score Categories |
|||
|---|---|---|---|---|---|---|---|
| Versus Category 1 | Versus Category 2 | Versus Category 3 | Versus Category 4 | ||||
| 1 | 0–10 | 217 | 0.1099 (0.4146) | 0.080 | <0.001 | <0.001 | |
| 2 | 11–20 | 404 | 0.2716 (0.6800) | 0.080 | <0.001 | <0.001 | |
| 3 | 21–30 | 145 | 1.4934 (1.1495) | <0.001 | <0.001 | <0.001 | |
| 4 | 31–39 | 45 | 2.7313 (1.2672) | <0.001 | <0.001 | <0.001 | |
Abbreviations: CHARTER, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; SD, standard deviation.
Figure 4.
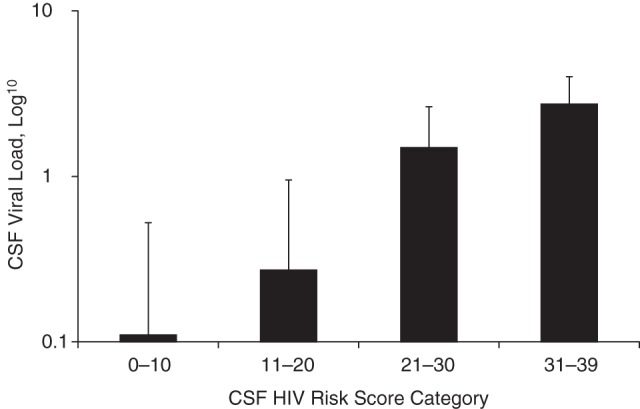
Distribution of cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) RNA viral load by category of CSF HIV risk score, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) cohort at study entry, 2004–2007, (n = 811). P < 0.001 comparing CSF HIV risk score category 2 versus categories 3 and 4; P < 0.001 comparing CSF risk score category 3 versus categories 1, 2, and 4; and P < 0.001 comparing CSF risk score category 4 versus 1, 2, and 3. Participants per risk score category: category 1 = 217; category 2 = 404; category 3 = 145; and category 4 = 45.
Figure 5.
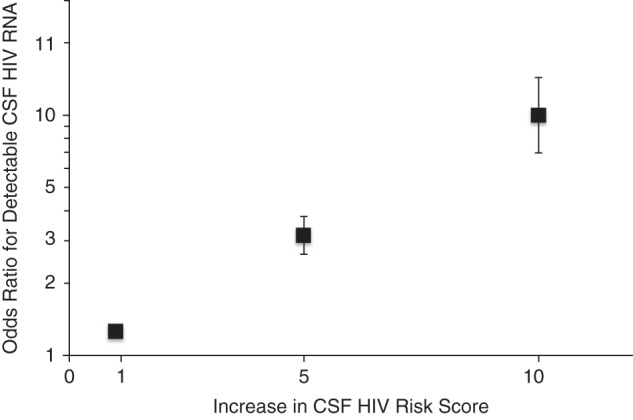
Odds ratios for the association between change in cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) risk score and detectable CSF HIV RNA, Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) cohort at study entry, 2004–2007, (n = 811). Odds ratios and 95% confidence intervals per 1-, 5-, and 10-point increments in the CSF HIV risk score.
DISCUSSION
We developed a CSF HIV risk score (Hammond score) to potentially predict the probability of identifying detectable CSF HIV RNA in persons receiving cART. This score may provide a practical means to estimate the probability of finding CSF HIV RNA, thus providing a meaningful tool for providers to use when discussing the utility of performing a lumbar puncture. Measures of predicting CSF HIV RNA have not been previously described, so our findings contribute substantially to the field and may allow meaningful inferences to be drawn in HIV management.
The CSF HIV risk score displayed high predictive accuracy with 2 robust internal validation techniques: cross-validation performance of 0.90 and bootstrapped performance with correction for optimism of 0.84. This can be interpreted as at least an 84% probability that the CSF HIV risk score will assign a higher probability of detecting CSF HIV RNA in a patient with positive CSF HIV than in a patient with undetectable CSF HIV RNA.
Our prediction tool holds promise for the clinical management of HIV. It relies on measurements that are readily available: plasma HIV RNA, CPE, duration of current cART regimen, cART adherence, race, and depression status. The mean variance inflation factor of 1.03 suggests no collinearity between predictor variables, an indication that each variable contributes sufficiently and independently toward potentially predicting detectable CSF HIV RNA. To the clinician, this may suggest that addressing modifiable factors may reduce the risk of persistent detectable CSF HIV RNA, which several studies have indicated to be harmful (10, 37).
A model with plasma HIV RNA alone as a predictor of detectable CSF HIV RNA was not well calibrated. Because of variability among patients, including clinical presentation, health behavior, and other health states, a single predictor variable is rarely of adequate prognostic value and may be misinforming even if it displays sufficient discrimination (29, 38, 39). The use of multivariable design and analyses is essential for prediction models with a goal to construct accurate and discriminating models. At plasma HIV RNA levels of less than 10,000 copies/mL, each potential predictor variable in the final regression model contributes substantially to assessing CSF HIV risk. Because retained predictors of detectable CSF HIV RNA showed good discriminatory performance at plasma HIV RNA levels of less than 10,000 copies/mL, and plasma HIV RNA alone did not yield good model fit, it may suggest that, below this threshold, routine monitoring of plasma HIV RNA alone may not be a sufficient surrogate for estimating CSF HIV activity, supporting previous findings of CSF viral escape in persons with undetectable plasma virus (13, 40). Consequently, it is likely that the risk score has utility even when plasma HIV RNA is not detectable.
Although routine plasma HIV RNA monitoring is recommended as standard of care (41), an increased CSF HIV risk score may suggest which patients will benefit from CSF HIV RNA monitoring. Application of this risk score may better focus resources to enable cost-effective monitoring of CNS disease.
When clinical deterioration is noticed in a previously stable patient, the risk score can be applied to provide insight into potential CNS HIV disease activity. Such assessments may necessitate an informed clinical decision to request CSF examination, including measurement of HIV RNA, immune activation, and viral resistance. The risk score can also be monitored during alteration of therapy. An assessment of the probability of detectable CSF HIV RNA may also facilitate measures to alter modifiable factors such as the CPE of the cART regimen, cART adherence, and depression.
Depression is the most common psychiatric disorder associated with HIV. In persons with HIV infection, depression has been associated with lower CD4+ cell counts, immune activation, and increased risk of death, whereas resolution of depression is associated with increased natural killer cell activity (7, 42). Our findings suggest that effectively treating depression may also reduce the probability of having a detectable level of CSF HIV RNA.
Race was identified as a nonmodifiable correlate of detectable CSF HIV RNA, with blacks and Hispanics displaying significantly higher risk. Higher risk of HIV/AIDS acquisition has been associated with being black or Hispanic (43). Reasons for racial HIV disparities are complex and include differences in access to care and utilization of cART, differences in clinical response to cART, and proposed genetic variations including the CCR5 (chemokine (C-C motif) receptor 5) and CCL3 (chemokine (C-C motif) ligand 3) genes (44–46). Application of knowledge about the overall risk of detectable CSF HIV RNA may help addresses these disparities.
We present the CSF HIV risk score as a clinical tool to help improve the medical management of HIV. However, its utility may extend beyond the goal of suppressing HIV replication and apply to managing HAND. A recent algorithm for early detection of HAND identified age, current CD4 cell count, past CNS HIV-related diseases, and current cART duration as predictors (47). The CSF HIV risk score may allow research into how CSF HIV RNA, a proximal factor in the causal pathway to developing HAND, may affect HIV outcomes.
The CSF HIV risk score may offer insight into longitudinal cognitive changes when applied to already available and prospective epidemiologic data. It is conceivable that repeated monitoring of the CSF HIV risk score to ensure that low scores are maintained will lead to better neurocognitive and neuropsychiatric HIV outcomes. We caution, however, against the use of the risk score as a surrogate endpoint in clinical trials. This score may help identify patients for whom CSF examinations might be most informative.
A limitation to the use of this risk score is that depression was assessed by DSM-IV criteria. These criteria for depression may not be readily available to HIV care providers. In our population, persons who were depressed according to DSM-IV criteria had a mean Beck Depression Inventory II score of 25.0 (SD, 10.8). We did not evaluate the effect of current substance and alcohol use disorders because diagnoses of current DSM-IV substance and alcohol use disorders were infrequent, occurring in 0.9% and 0.8% of the population, respectively.
In this analysis, we were able to ascertain a temporal relationship between cART use and detectable CSF HIV. The type and duration of cART use was determined from patient medical records at the time of enrollment and were unlikely to be misclassified. Our study design also enhances the use of the risk score as a tool to make an assessment of current cART at the time of evaluation, which, in essence, is the utility of prognostic and diagnostic models. In the future, the risk predicted by the CSF HIV risk score needs to be compared with observed risks in another population for external validation. Its use in other populations may require calibration.
Strengths of this study include the large sample size of patients with CSF HIV RNA measurements. Approximately 15% of the study population had detectable CSF HIV RNA. The use of augmented multiple imputations allowed us to use data from the entire study cohort. The low ratio of retained potential predictors to number of outcomes, 1:26, ensured a good model fit with only 6.3% of fit due to noise, thereby reducing overoptimism in its application in other populations.
In conclusion, the CSF HIV risk score represents an advance in HIV management and monitoring of the CNS effects of HIV, providing a potentially useful tool for clinicians. Continuous CSF HIV risk score monitoring with the necessary physician actions, may help prevent HIV-related neurological and psychiatric complications and improve HIV outcomes.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Edward R. Hammond, Rosa M. Crum, Shruti H. Mehta, Justin C. McArthur); Department Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland (Edward R. Hammond, Rosa M. Crum, Glenn J. Treisman); Department of Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland (Glenn J. Treisman, Justin C. McArthur); Department of Neurology, University of Washington, Seattle, Washington (Christina M. Marra); Department of Neurology, Washington University, St. Louis, Missouri (David B. Clifford); Department of Pathology, Icahn School of Medicine at Mt. Sinai, New York, New York (Susan Morgello); Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, New York (David M. Simpson); Department of Pathology, University of Texas, Medical Branch, Galveston, Texas (Benjamin B. Gelman); Department of Neurosciences, University of California, San Diego, California (Ronald J. Ellis); Department of Psychiatry, University of California, San Diego, California (Igor Grant); Department of Medicine, University of California, San Diego, California (Scott L. Letendre); Department of Neurology, Johns Hopkins University, Baltimore, Maryland (Justin C. McArthur); and Department of Pathology, Johns Hopkins University, Baltimore, Maryland (Justin C. McArthur).
The Central Nervous System Human Immunodeficiency Virus Antiretroviral Therapy Effects Research (CHARTER) Study is supported by the National Institutes of Health (grants N01 MH22005, HHSN271201000027C, and HHSN271201000030C). The current analyses were funded by the National Institutes of Mental Health (grant R03 MH095640-02).
We thank Dr. Sanjay Rampal of the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, for his insightful contribution to the statistical analyses.
The study sponsor had no role in the design, analyses, interpretation, or reporting of the study. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Government.
Conflict of interest: D.B.C. has been a consultant to Biogen Idec, Inc.; Millennium Pharmaceuticals, Inc.; Bristol-Myers Squibb Co.; Pfizer, Inc.; Genzyme Corp.; Amgen, Inc.; Quintiles, Inc.; and Sun BioPharma, Inc. S.L.L. has research grants and grants pending from AbbVie, Inc.; GlaxoSmithKline, plc; and Merck & Co., Inc. C.M.M. has received payment for lectures from universities and societies, as well as royalties from LLW Consulting, Inc. and UpToDate, Inc. S.H.M. has received payment for lectures at a university. S.M. has lectured for Health Clear Strategies. D.M.S. has been a consultant for Astellas Pharma, Inc.; Merz Pharma GmbH & Co.; Ipsen; Acorda Therapeutics, Inc.; Depomed, Inc.; Syntaxin, Ltd.; Viromed Co., Ltd.; Biogen Idec, Inc.; and Allergan, Inc.; G.J.T. has received payment for lectures from universities.
APPENDIX 1
Appendix Table 1.
Central Nervous System Penetration Effectiveness Rankings of Frequently Used Antiretroviral Regimen (10)
| Antiretroviral Class | Central Nervous System Penetration Effectiveness Rankinga |
|||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 | |
| Nucleoside analogue reverse transcriptase inhibitors | Zidovudine | Abacavir | Didanosine | Tenofovir |
| Emtricitabine | Lamivudine | Zalcitabine | ||
| Stavudine | ||||
| Nonnucleoside analogue reverse transcriptase inhibitors | Nevirapine | Delavirdine | Etravirine | |
| Efavirenz | ||||
| Protease inhibitors | Indinavir/ritonavir | Darunavir/ritonavir | Atazanavir | Nelfinavir |
| Fosamprenavir/ritonavir | Atazanavir/ritonavir | Ritonavir | ||
| Indinavir | Fosamprenavir | Saquinavir | ||
| Lopinavir/ritonavir | Saquinavir/ritonavir | |||
| Tipranavir/ritonavir | ||||
| Entry/fusion inhibitors | Maraviroc | Enfuvirtide | ||
| Integrase strand transfer inhibitors | Raltegravir | |||
a Higher rankings reflect better central nervous system penetration estimates.
APPENDIX 2
Sensitivity analyses
We conducted a sensitivity analysis by developing the cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) risk score using only the 790 participants with complete data (i.e., complete case analysis). The same set of potential predictors was retained as with the multiple imputation approach except “duration of current combination antiretroviral therapy (cART).” We forced “duration of current cART” in the final regression model under the assumption that therapeutic levels of cART need to be attained prior to objective assessment of effectiveness in reducing plasma and CSF HIV RNA. The observed associations and regression coefficients were similar for multiple imputations as with complete case analysis. In comparison, multiple imputation analysis yielded more precise estimates.
REFERENCES
- 1.Brodt HR, Kamps BS, Gute P, et al. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS. 1997;11(14):1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 3.Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(1-2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 6.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 7.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 8.Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005;21(8):706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- 9.Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letendre SL, Ellis RJ, Ances BM, et al. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Christo PP, Greco DB, Aleixo AW, et al. Factors influencing cerebrospinal fluid and plasma HIV-1 RNA detection rate in patients with and without opportunistic neurological disease during the HAART era. BMC Infect Dis. 2007;7:147. doi: 10.1186/1471-2334-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 13.Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvey LJ, Everitt A, Winston A, et al. Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. AIDS. 2009;23(11):1443–1444. doi: 10.1097/QAD.0b013e32832d077c. [DOI] [PubMed] [Google Scholar]
- 15.Schouten J, Cinque P, Gisslen M, et al. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25(5):561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- 16.European AIDS Clinical Society. Guidelines version 7.0. http://eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. Published October 2013. Accessed March 24, 2014.
- 17.Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the Mind Exchange Program. Clin Infect Dis. 2013;56(7):1004–1017. doi: 10.1093/cid/cis975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polis MA, Suzman DL, Yoder CP, et al. Suppression of cerebrospinal fluid HIV burden in antiretroviral naive patients on a potent four-drug antiretroviral regimen. AIDS. 2003;17(8):1167–1172. doi: 10.1097/00002030-200305230-00008. [DOI] [PubMed] [Google Scholar]
- 19.Price RW, Yiannoutsos CT, Clifford DB, et al. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS. 1999;13(13):1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 20.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59(6):923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 21.Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol. 2011;11:148. doi: 10.1186/1471-2377-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strazielle N, Khuth ST, Ghersi-Egea JF. Detoxification systems, passive and specific transport for drugs at the blood-CSF barrier in normal and pathological situations. Adv Drug Deliv Rev. 2004;56(12):1717–1740. doi: 10.1016/j.addr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 25.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Steer RA, Clark DA, Beck AT, et al. Common and specific dimensions of self-reported anxiety and depression: the BDI-II versus the BDI-IA. Behav Res Ther. 1999;37(2):183–190. doi: 10.1016/s0005-7967(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 29.Royston P, Moons KG, Altman DG, et al. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 30.Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–5528. doi: 10.1002/sim.3148. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 32.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: Wiley; 2000. [Google Scholar]
- 34.Van Houwelingen JC, Le Cessie S. Predictive value of statistical models. Stat Med. 1990;9(11):1303–1325. doi: 10.1002/sim.4780091109. [DOI] [PubMed] [Google Scholar]
- 35.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 36.Donders AR, van der Heijden GJ, Stijnen T, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moons KG, Royston P, Vergouwe Y, et al. Prognosis and prognostic research: What, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 39.Riley RD, Abrams KR, Sutton AJ, et al. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer. 2003;88(8):1191–1198. doi: 10.1038/sj.bjc.6600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Department of Health and Human Services; Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Panel on Antiretroviral Guidelines for Adults and Adolescents http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Updated February 12, 2013. Accessed March 24, 2014. [Google Scholar]
- 42.Cruess DG, Douglas SD, Petitto JM, et al. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am J Psychiatry. 2005;162(11):2125–2130. doi: 10.1176/appi.ajp.162.11.2125. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC) Racial/ethnic disparities in diagnoses of HIV/AIDS—33 states, 2001–2004. MMWR Morb Mortal Wkly Rep. 2006;55(5):121–125. [PubMed] [Google Scholar]
- 44.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382(6593):722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 45.Tedaldi EM, Absalon J, Thomas AJ, et al. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: results of CPCRA 058 (FIRST Study) J Acquir Immune Defic Syndr. 2008;47(4):441–448. doi: 10.1097/QAI.0b013e3181609da8. [DOI] [PubMed] [Google Scholar]
- 46.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 47.Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642–649. doi: 10.1111/j.1468-1293.2010.00834.x. [DOI] [PubMed] [Google Scholar]


