Abstract
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. It is unclear why human neurons infected in vitro with VZV at low multiplicity of infection do not exhibit a cytopathic effect (CPE) even though all VZV genes are transcribed, VZV proteins from all kinetic classes are translated and minimal infectious virus is produced. Here, we show that the lack of VZV-induced CPE correlates with the low abundance of viral DNA.
Keywords: VZV DNA, human neurons, tissue culture
Introduction
Varicella zoster virus (VZV), an exclusively human alphaherpesvirus, becomes latent in ganglionic neurons along the entire neuraxis after primary infection. Decades later, virus can reactivate to produce zoster and additional serious neurological and ocular complications (Gilden et al., 2011; Nagel and Gilden, 2013). VZV can infect neurons in vitro from multiple sources, including those derived from human embryonic stem cells [hESC; (Markus et al., 2011; Sloutskin et al., 2013)], human neural stem cells [hNSC; (Pugazhenthi et al., 2011)] and human induced pluripotent stem cells [iPSC; (Baird et al., 2014; Grose et al., 2013; Yu et al., 2013)]. Although human neurons die after VZV infection at high multiplicity of infection (MOI; >1X 10−3) (Markus et al., 2011; Sloutskin et al., 2013), no CPE develops within 2 weeks after infection at low MOI (<1 × 10−3) (Baird et al., 2014; Grose et al., 2013; Yu et al., 2013). While the absence of a CPE in neurons 5 days after infection at low MOI has been attributed to non-productive infection based on the absence of detectable VZV proteins (Sloutskin et al., 2013), we have shown that VZV transcripts and proteins as well as infectious virus are produced in neurons 14 days after low MOI infection, and ultrastructural analysis revealed numerous empty capsids in the nuclei of VZV-infected neurons (Grose et al., 2013; Yu et al., 2013). Furthermore, Next Generation RNA sequencing (NextGen RNAseq) showed that every annotated VZV open reading frame (ORF) is transcribed in human neurons 14 days post-infection (dpi), and that the abundance of VZV transcripts does not differ significantly from that found in VZV-infected human fibroblasts with extensive CPE (Baird et al., 2014). Thus, we examined VZV DNA abundance in VZV-infected human neurons at multiple times over a 2-week period.
Results
Virus production is both delayed and reduced in human neurons compared to fibroblasts
No virus was produced in VZV-infected neurons at 1, 3 and 7 dpi; at 14 dpi, 31 PFU/culture were detected (Fig. 1). The few PFU detected in infected human neurons 14 dpi were not due to residual inoculum since no infectious plaques were seen at earlier times. In contrast, VZV-infected fibroblasts began producing infectious virus 1 dpi, which peaked at 2.1 X 104 PFU/culture 7 dpi then decreased slightly 10 dpi concomitant with cell death.
Fig. 1. VZV production is both delayed and reduced in human neurons.
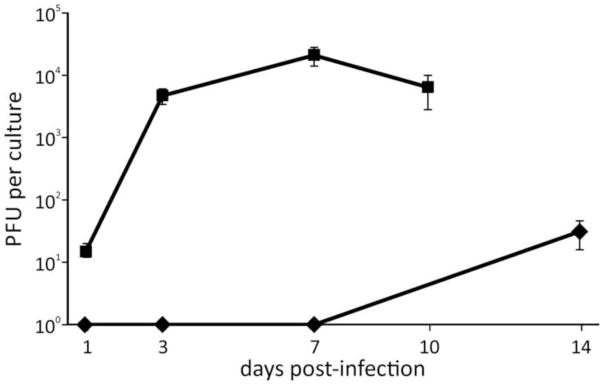
Cells of duplicate cultures of human neurons (diamonds) and fibroblasts (squares) infected with VZV at a low MOI were harvested at four time points with trypsin-EDTA, washed with PBS, and analyzed in plaque assays by co-cultivation with uninfected fibroblasts. The inoculum was removed 3 h after co-cultivation and cell cultures were fixed, stained and plaques counted 7 days later.
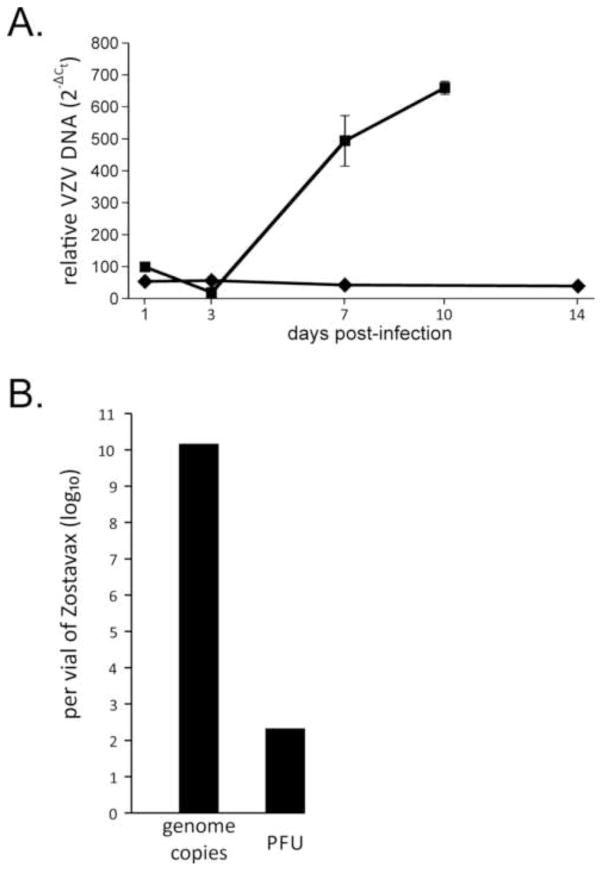
VZV-infected human neurons do not accumulate viral DNA
To determine whether reduced and delayed VZV production was due to limited numbers of VZV genomes available for packaging, virus DNA abundance was monitored in infected human neurons. The abundance of VZV DNA in neurons infected with a low MOI remained constant over the 2-week study period (Fig. 2A). Importantly, viral genomes detected at 1 dpi were likely from virus DNA in the inoculum because a vial of Zostavax contains 1.4 × 1010 genomes (±0.1 ×1010; n=2) and 200 PFU (±100 PFU; n=4) (Fig. 2B), resulting in 7×107 VZV genomes per PFU. At 1 dpi, the virus-to-cell DNA ratio was equivalent in infected fibroblasts and neurons. By 10 dpi, the virus-to-cell DNA ratio had increased 6.7-fold in fibroblasts.
Fig. 2. VZV DNA does not accumulate in VZV-infected neurons.
(A) Duplicate cultures of human neurons (diamonds) and human fetal lung fibroblasts (squares) were infected with cell-free VZV at low MOI as in Fig. 1 and total DNA was extracted at four time points post-infection. Viral and cellular DNA was detected by qPCR using primers for VZV ORF 29 and GAPdH, respectively. Relative quantities of VZV DNA, normalized to cell DNA, were determined as detailed in the text. Error bars represent standard deviation. (B) Viral genome copy number and PFU were determined. DNA extractions of two separate vials of Zostavax were analyzed by qPCR for VZV genomes; plaque assay was performed from 4 separate vials of Zostavax.
Discussion
Overall, VZV DNA did not accumulate in productively infected human neurons that lacked a CPE, but did accumulate in fibroblasts with extensive CPE. The lack of VZV-induced CPE in human neurons is likely due to defective viral DNA replication or rapid degradation of VZV DNA. An absolute block in viral DNA replication in neurons is unlikely since late viral transcripts and their proteins, for which viral DNA replication is required, were detected (Azarkh et al., 2011; Baird et al., 2014; Grose et al., 2013; Yu et al., 2013). VZV DNA replication was ultimately sufficient to produce 31 PFU/culture, a level that would not have been detected above the ~109 input genomes. Importantly, human fibroblasts infected with the same inoculum replicated VZV genomes above input virus DNA. A similar inhibition of HSV-1 growth and lack of CPE in neurons obtained from explanted fetal human brain, spinal cord or ganglia has been reported, although in these ex vivo cultures, HSV-1 replicates and a CPE develops in non-neuronal cells (Kennedy et al., 1983).
Materials and Methods
Cells and virus
Human iPSCs (iCell neurons; Cellular Dynamics International, Madison, WI) and human fetal lung fibroblasts were cultured and infected with cell-free VZV (Zostavax; Merck, Whitehouse Station, NJ) at an MOI < 1 × 10−3 as described (Grose et al., 2013; Yu et al., 2013).
Plaque assay
To quantify infectious virus production, both VZV-infected human neurons and fibroblasts were harvested with trypsin-EDTA at multiple dpi, washed with PBS, diluted and co-cultivated with uninfected fibroblasts; after 3 h at 37°C, cells were washed, medium was replenished and plaques were visualized by crystal violet staining and counted 7 days later.
qPCR
VZV-infected human neurons and fibroblasts were harvested on multiple dpi and at each time point, DNA was extracted (GenElute; Sigma, St. Louis, MO) and quantified by TaqMan-based quantitative PCR (qPCR) using 10 ng of total DNA and primer/probe sets specific for both cell (GAPdH) and virus (VZV ORF 29) DNA as described (Yu et al., 2013). The amount of VZV DNA compared to cell DNA was determined using the formula 2−ΔCt, where ΔCt= Ct(VZV ORF29) - Ct(GAPdH).
DNA was isolated from Zostavax (GenElute; Sigma) and quantified by TaqMan-based qPCR. Standards to determine copy number were prepared from purified VZV bacterial artificial chromosome (BAC) DNA (Tischer et al., 2007).
Highlights.
Human neurons infected with low MOI VZV do not develop CPE 2 weeks post infection.
Virus production is delayed and reduced in human neurons compared to fibroblasts.
VZV-infected human neurons do not accumulate viral DNA.
Acknowledgments
This work was supported by Public Health Service grants AG006127 (D.G.), AG032958 (D.G. and R.J.C.) and NS082228 (R.J.C.) from the National Institutes of Health. Dr. Baird was supported by Training Grant NS007321 to Dr. Gilden from the National Institutes of Health. Dr. Jonjić was supported by the Instrument for Pre-Accession Assistance (IPA), component III C, Science and Innovation Investment Fund (SIIF), phase; grant number IPA2007/HR/16IPO/001-040517. We thank Marina Hoffman for editorial review and Lori DePriest for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azarkh Y, Dolken L, Nagel M, Gilden D, Cohrs RJ. Synthesis and decay of varicella zoster virus transcripts. J Neurovirol. 2011;17:281–287. doi: 10.1007/s13365-011-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NL, Bowlin JL, Cohrs RJ, Gilden D, Jones KL. Comparison of VZV RNA sequences in human neurons and fibroblasts. J Virol Epub March. 2014;5:2014. doi: 10.1128/JVI.00476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. Review: The neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441–463. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C, Yu X, Cohrs RJ, Carpenter JE, Bowlin JL, Gilden D. Aberrant Virion Assembly and Limited Glycoprotein C Production in Varicella-Zoster Virus-Infected Neurons. J Virol. 2013;87:9643–9648. doi: 10.1128/JVI.01506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Clements GB, Brown SM. Differential susceptibility of human neural cell types in culture to infection with herpes simplex virus. Brain. 1983;106 (Pt 1):101–119. doi: 10.1093/brain/106.1.101. [DOI] [PubMed] [Google Scholar]
- Markus A, Grigoryan S, Sloutskin A, Yee MB, Zhu H, Yang IH, Thakor NV, Sarid R, Kinchington PR, Goldstein RS. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J Virol. 2011;85:6220–6233. doi: 10.1128/JVI.02396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol. 2013;15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugazhenthi S, Nair S, Velmurugan K, Liang Q, Mahalingam R, Cohrs RJ, Nagel MA, Gilden D. Varicella-zoster virus infection of differentiated human neural stem cells. J Virol. 2011;85:6678–6686. doi: 10.1128/JVI.00445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloutskin A, Kinchington PR, Goldstein RS. Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose. Virology. 2013;443:285–293. doi: 10.1016/j.virol.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer BK, Kaufer BB, Sommer M, Wussow F, Arvin AM, Osterrieder N. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol. 2007;81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Seitz S, Pointon T, Bowlin JL, Cohrs RJ, Jonjic S, Haas J, Wellish M, Gilden D. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J Neurovirol. 2013;19:75–81. doi: 10.1007/s13365-012-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]