Abstract
Objectives
Apurinic/apyrimidinic-endonuclease 1 (APE1) heterozygous mice have chronically elevated blood pressure. Renin of the renin–angiotensin (ANG) system for blood pressure maintenance regulates production of ANG II, a vasoactive hormone. Renin expression and secretion from kidney juxtaglomerular cells are regulated by intracellular calcium. Our objective in this study is to investigate APE1’s regulatory role in renin expression.
Methods
Effect of APE1 on calcium-mediated modulation of renin expression was examined by real-time reverse transcriptase-PCR, Western analysis and renin promoter-dependent luciferase activity in APE1-knockdown, APE1-overexpressing or control mouse kidney As4.1 cells. Furthermore, coimmunoprecipitation and chromatin immunoprecipitation assays were utilized to examine the association of APE1 with histone deacetylase (HDAC)1 corepressor complex and their recruitment to renin enhancer. Finally, kidney renin mRNA level and plasma– renin activity were measured in wild-type and APE1-heterozygous mice.
Results
Here we show that APE1 is involved in calcium mediated repression of renin gene. Our results further indicate that APE1 is a component of HDAC1 corepressor complex bound to renin-enhancer region. Increase in intracellular calcium ion concentration enhances the association of APE1 with HDAC1 corepressor complex and their recruitment to the enhancer region. Furthermore, APE1’s N-terminal region is critical for formation and recruitment of the enhancer-bound corepressor complex. Increased renin expression in kidneys and higher plasma–renin activity in APE1 heterozygous mice further supports APE1’s corepressor role in vivo.
Conclusion
This study uncovers APE1’s function as a novel negative regulator of renin expression, and thereby in blood pressure maintenance.
Keywords: apurinic/apyrimidinic-endonuclease 1 (APE1/Ref-1), calcium, hypertension, renin, transcriptional repression
INTRODUCTION
Hypertension is a major cardiovascular disorder that affects more than 65 million Americans and 75% of the population above the age of 65 [1]. Blood pressure is precisely regulated via integration of information from the central nervous system, kidneys, vasculature, endocrine systems and the heart. The renin–angiotensin (ANG) system (RAS) plays a major role in regulating both blood pressure and fluid/electrolyte homeostasis [2–9]. Renin is an aspartic acid protease secreted by the juxtaglomerular cells of the kidneys, the principle source of circulating renin [2–9]. Renin cleaves angiotensinogen to produce ANG I, a decapeptide. ANG I is further cleaved by ANG-converting enzyme to produce an octapeptide ANG II, a vasoconstrictor. Thus, renin plays a rate-limiting role in RAS. Several studies have established that intracellular calcium ion concentration ([Ca2+]i) regulates renin synthesis and its secretion from the juxtaglomerular cells [10–14]. Furthermore, transcriptional repression of the renin gene was also observed in juxtaglomerular cells due to increase in [Ca2+]i [15–17]. Moreover, ANGII and endothelin, known to increase [Ca2+]i,]i, inhibit renin transcription via activation of phospholipase C and protein kinase C pathways, and thus explains the negative feedback regulation of renin expression [17–20].
The multifunctional mammalian apurinic/apyrimidinic-endonuclease 1 (APE1/Ref-1) plays a central role in the repair of both endogenous and environmentally induced oxidative and alkylation DNA damage in the genome via base excision repair pathway [21–23]. Besides its repair function, the mammalian APE1 has two unique and apparently distinct transcriptional regulatory activities, which require its nonconserved N-terminal domain [24]. It was independently identified as a reductive activator of c-Jun and several other transcription factors including p53, HIF1-α, PAX5, PAX8 and NF-κB and also named Ref-1 [24–26]. A second, distinct function of APE1 as a transacting factor was discovered during investigation of Ca2+-dependent repression of human parathyroid hormone (PTH) gene [27]. An increase in calcium concentration in parathyroid cells triggers downregulation of PTH gene expression [27]. This is mediated by binding of cognate transacting factors to negative calcium response elements (nCaRE) identified in the PTH promoter [27]. Okazaki’s group first identified two such elements (nCaRE-A and nCaRE-B) in PTH promoter, and showed APE1 as one of the regulatory proteins that binds to these elements [27]. We subsequently showed the presence of nCaREs in the regulatory region of the APE1 gene itself suggesting its autoregulation [28]. We later showed that acetylation of APE1 at Lys6 and Lys7 by histone acetyl transferase p300 enhances its binding to nCaRE-B leading to repression of the PTH promoter [29]. In a recent study, acetylation of APE1 has also been found to be regulated by histone deacetylase Sirtuin 1 and that modulates its repair function [30].
APE1 is essential for cell survival and APE1-null mice showed early embryonic lethality [31–33]. Although APE1-heterozygous (APE1+/−) mice are viable and apparently normal, they have markedly elevated blood pressure [34], which implicates APE1’s role in blood pressure homeostasis. APE1 was identified as a component in the nCaRE complex in human-renin promoter analogous to that in PTH, and Ca2+-induced translocation of APE1 to the nucleus was observed [35]. However, it is not clear if APE1 is involved in Ca2+-dependent downregulation of mouse-renin expression. In this study, we showed that APE1 inhibits renin expression in mouse kidney As4.1 line constitutively expressing renin. We also established that Ca2+-mediated repression is caused by recruiting histone deacetylase (HDAC) 1 corepressor complex to the reninenhancer region. We extrapolated these results to in-vivo observation in the mouse by showing higher kidney renin mRNA level and plasma–renin activity in APE1+/− mice.
METHODS
Cell culture, plasmids, transfection and treatment
The renin expressing mouse kidney As4.1 line [36] was obtained from American Type Culture Collection (CRL-2193) and maintained in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (Sigma, St Louis, Missouri, USA) and penicillin-streptomycin (Gibco-BRL, Life Technologies, Grand Island, New York, USA). All cells were transfected using LipofectAMINE 2000 (Invitrogen, Life Technologies, Grand Island, New York, USA) according to manufacturer’s protocol. Nontransfected or transfected cells were treated with 0.1µmol/l thapsigargin (Calbiochem, Merck KGaA, Darmstadt, Germany) for 2 h and subsequently harvested at various times. This dose of treatment did not show any cytotoxic effects (data not shown). PCMV 5.1 FLAG expression plasmids for wild-type (WT) APE1 and its 1–33 amino acid deletion mutant (NΔ33) were described elsewhere [37,38].
Downregulation of apurinic/apyrimidinic-ndonuclease 1 by siRNA
For APE1 downregulation, 80 nmol/l ON-Target Plus APE1 siRNAs or a nontarget control (Dharmacon, Lafayette, Colorado, USA) were used for LipfectAMINE 2000-mediated transfection according to manufacturer’s protocol. APE1 downregulation was confirmed in extracts of cells harvested after 48 and 72 h byWestern analysis with rabbit α-APE1 antibody [39] and with mouse α-β-actin antibody (clone AC15; Sigma) or α-α-Tubulin antibody (clone DM1A; Sigma) for loading control. Renin protein level was examined by Western analysis using α-Renin 1 antibody (R & D Systems, Minneapolis, Minnesota, USA).
Coimmunoprecipitation and Western analysis
Nuclear extracts from FLAG-tagged expression plasmid transfected As4.1 cells were immunoprecipitated with mouse α-FLAG M2 antibody-conjugated agarose beads (Sigma) as described elsewhere [29,37,40,41]. The coimmunoprecipitated proteins were resolved by SDS-PAGE and identified by Western analysis with the following antibodies: mouse α-HDAC1 (clone 2E10; Upstate, Billerica, Massachusetts, USA), mouse α-HDAC2 (clone 3F3, Upstate), rabbit α-mSin3A (clone AK-11, Santa Cruz Biotechnology, Santa Cruz, California, USA) and α-FLAG (clone M2, Sigma).
Luciferase assay
As4.1 cells were cotransfected with mouse renin-1c promoter (4.2 kb fragment 5′ of the transcription start site)-luciferase reporter plasmid (a kind gift from K.W. Gross, Roswell Park Cancer Institute, Buffalo, New York, USA) and expression plasmids for wild-type APE1, NΔ33 APE1 or its empty vector. Forty hours after transfection, the cells were harvested and processed for luciferase activity measurement in a luminometer (AutoLumat LB 953, Berthold, Oak Ridge, Tennessee, USA), using the luciferase assay kit (Promega, Madison, Wisconsin, USA) as per manufacturer’s protocol. In other experiments, APE1-downregulated or control cells were transfected with the luciferase reporter plasmid and harvested for luciferase assay after 30 h. Luciferase measurement after thapsigargin treatment was similarly carried out. The luciferase activity was normalized with respect to total protein content of the lysates.
RNA isolation and real-time reverse transcriptase PCR
Total RNA was isolated from cells with Qiagen RNeasy mini kit followed by DNase 1 (New England BioLabs, Ipswich, Massachusetts, USA) treatment and cDNA synthesis using Superscript III first-strand synthesis kit (Invitrogen) according to manufacturer’s protocol. Renin mRNA in the samples were quantitated via SYBR GREEN-based real-time PCR (7000 Sequence Detection System or 7000 Real-Time PCR System; Applied Biosystems, Carlsbad, California, USA) using SYBR Premix Ex Taq (TaKaRa Bio Inc., Otsu, Shiga, Japan) and renin mRNA-specific primers (forward: 5′-CCTCTACCTTGCTTGTGGGATT-3′ and reverse: 5′-CTGGCTGAGGAAACCTTTGACT-3), or of 18 s rRNA (forward: 5′-GTAACCCGTTGAACCCCATT-3′ and reverse: 5′-CCATCCAATCGGTAGTAGCG-3′) as internal control.
Chromatin immunoprecipitation assay
Chromatin Immunoprecipitation (ChIP) assay was performed using Magna ChIP assay kit (Upstate) according to manufacturer’s protocol and as described elsewhere [38] using the following antibodies; α-APE1 (Novus Biologicals, Littleton, Colorado, USA), α-HDAC1 (2E10; Upstate), α-HDAC2 (3F3, Upstate), α-mSin3A (AK-11, Santa Cruz), α-FLAG (M2; Sigma) or control immunoglobulin G (IgG; Santa Cruz). The immunoprecipitated purified DNA was subjected to SYBR GREEN-based real-time PCR with primers (forward: 5′-ATGACCTTGGCCTCTAGCCCTGT-3′ and reverse: 5′-ACAGCCAGGTCACCATCTGCGT-3′) for renin-enhancer sequence (−2857 to −2666). For re-ChIP assay, the second immunoprecipitation was performed on the elutes of the first immunoprecipitation (with α-APE1, α-FLAG antibody or control IgG) with α-HDAC1, α-HDAC2 or α-mSin3A antibody.
istone deacetylase activity in apurinic/apyrimidinic-endonuclease 1 immunocomplex
A peptide corresponding to two to 24 amino acid in histone H4 (HDAC Assay Kit, Upstate) was acetylated with recombinant p300 HAT domain polypeptide in the presence of 1 mmol/l [3H]acetyl CoA, as described previously [29,42]. Wild-type or NΔ33 APE1 was immunoprecipitated from FLAG-tagged wild-type or NΔ33 APE1 transfected cells, respectively, by α-FLAG antibody conjugated beads and were incubated with [3H]acetate-labeled H4 peptide in histone deacetylation reaction (HDAC Assay Kit, Upstate). [3H] in the supernatant was measured in a scintillation counter.
Kidney renin mRNA and plasma–renin activity measurement
Animal care and experimentation were approved and carried out in accordance with institutional guidelines following Institutional Animal Care and Use Committee (IACUC)-approved protocol. C57BL/6 wild type and APE1+/− 31,43] male mice (16–20 weeks old) of the same litter were genotyped by PCR ([33]; data not shown). Total RNA was isolated from one kidney per euthanized mouse using RNeasy mini kit for real-time reverse transcriptase (RT)-PCR to measure APE1 and renin mRNA levels. Primers for mouse APE1 mRNA were obtained from http://www.RealTimePrimers.com. The plasma was isolated after centrifugation of EDTA-containing blood obtained from the heart of these anesthetized mice prior to euthanization. Plasma–renin activity was measured by radioimmunoassay (RIA) of generated ANG I from each sample using Gammacoat [125I] Plasma–Renin Activity RIA Kit (DiaSorin, Stillwater, Minnesota, USA) according to manufacturer’s protocol.
Statistics
Data are expressed as mean±standard deviation of triplicates. The significance of differences between different groups was determined utilizing Student’s t-test, and P less than 0.05 was considered significant.
RESULTS
Apurinic/apyrimidinic-endonuclease 1-dependent modulation of mouse-renin gene expression and promoter activity
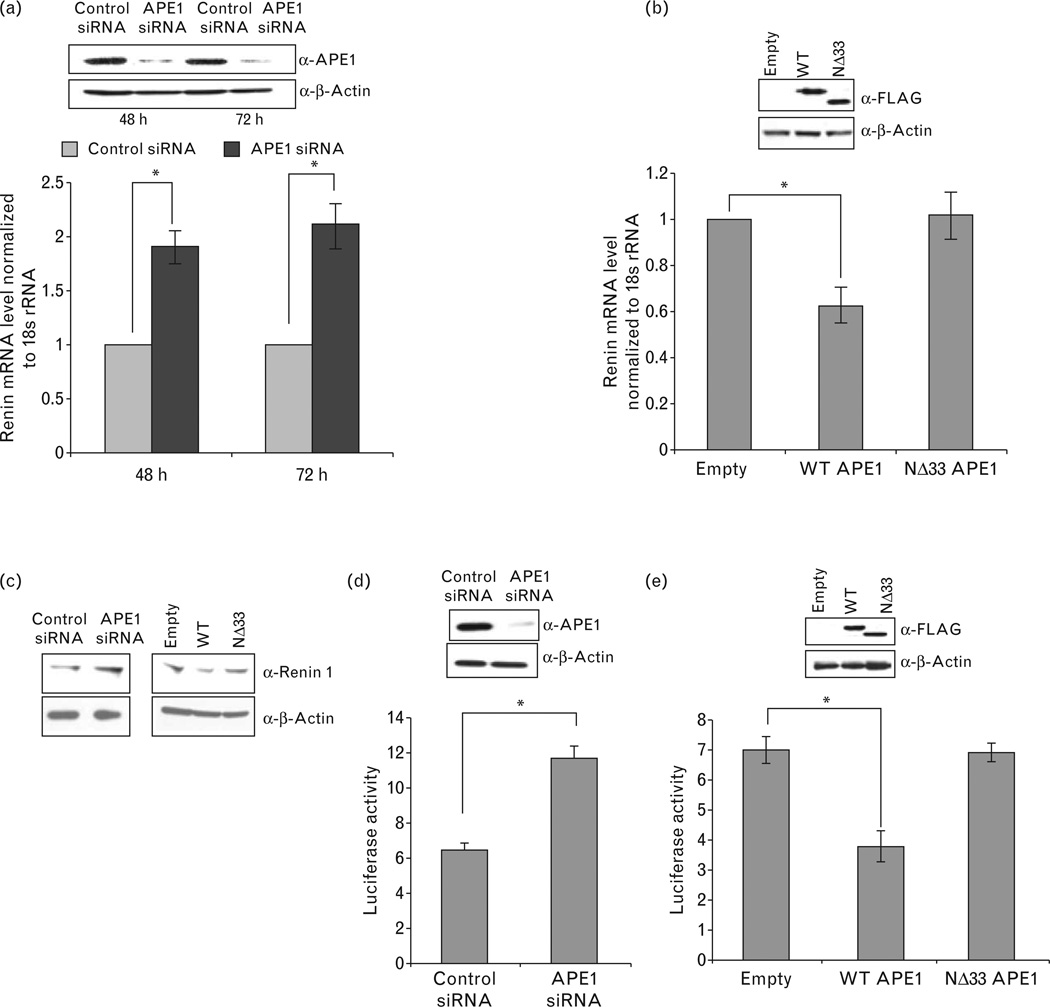
To examine the role of APE1 in endogenous renin expression in As4.1 cell line, we used quantitative real-time RT-PCR analysis and showed significant increase in renin mRNA level upon APE1-downregulation via transient siRNA transfection (Fig. 1a). To further confirm APE1’s role as a negative regulator of renin expression, we overexpressed wild-type APE1 or NΔ33 mutant APE1. Overexpression of wild-type but not of the NΔ33 mutant significantly downregulated renin mRNA levels (Fig. 1b). Western analysis of As4.1 cell extracts also showed that APE1 knockdown enhanced renin expression and overexpression of wild-type but not NΔ33 APE1 decreased renin expression (Fig. 1c). To confirm that APE1-mediated downregulation of renin occurs at the transcription level, we examined the effect of APE1 on renin promoter-dependent luciferase reporter activity. Significant increase in luciferase activity in APE1 downregulated cells as compared to control siRNA transfected cells was observed (Fig. 1d). Complementarily, ectopic expression of wild-type APE1 but not of the NΔ33 mutant significantly reduced the promoter activity relative to the vector control (Fig. 1e). Together, these data support the role of APE1 as a negative regulator of renin expression.
Figure 1.
AP-endonuclease 1 (APE1) regulates renin expression. (a) After APE1 downregulation in As4.1 cells by siRNA, the renin mRNA levels were quantitated by real-time RT-PCR and normalized to 18 s rRNA. Western analysis of APE1 (inset) confirms its knockdown relative to the control siRNA (β-actin was used as an internal control) at 48 and 72 h of transfection. (b) Renin mRNA level normalized to 18 s rRNA in As4.1 cells after transfection with wild-type (WT), NΔ33 FLAG-APE1 plasmid or empty vector. Inset shows ectopic FLAG-APE1 levels with β-Actin as internal control. (c) Western analysis for renin and β-Actin levels in (i) control or APE1-knockdown cells (by siRNA transfection; left panel) and (ii) cells transfected with empty vector, WT APE1 or NΔ33 APE1 (right panel). (d) Mouse promoter-dependent luciferase activity in control or APE1 knockdown As4.1 cells transfected with mouse renin-1c promoter (4.2 kb fragment upstream of the transcription start site)-luciferase plasmid. (e) Effect of WT vs. NΔ33 APE1 expression on mouse-renin promoter-dependent luciferase activity as in (d) other details are in methods.*, P<0.05.
Apurinic/apyrimidinic-endonuclease 1 is involved in calcium-mediated downregulation of renin expression
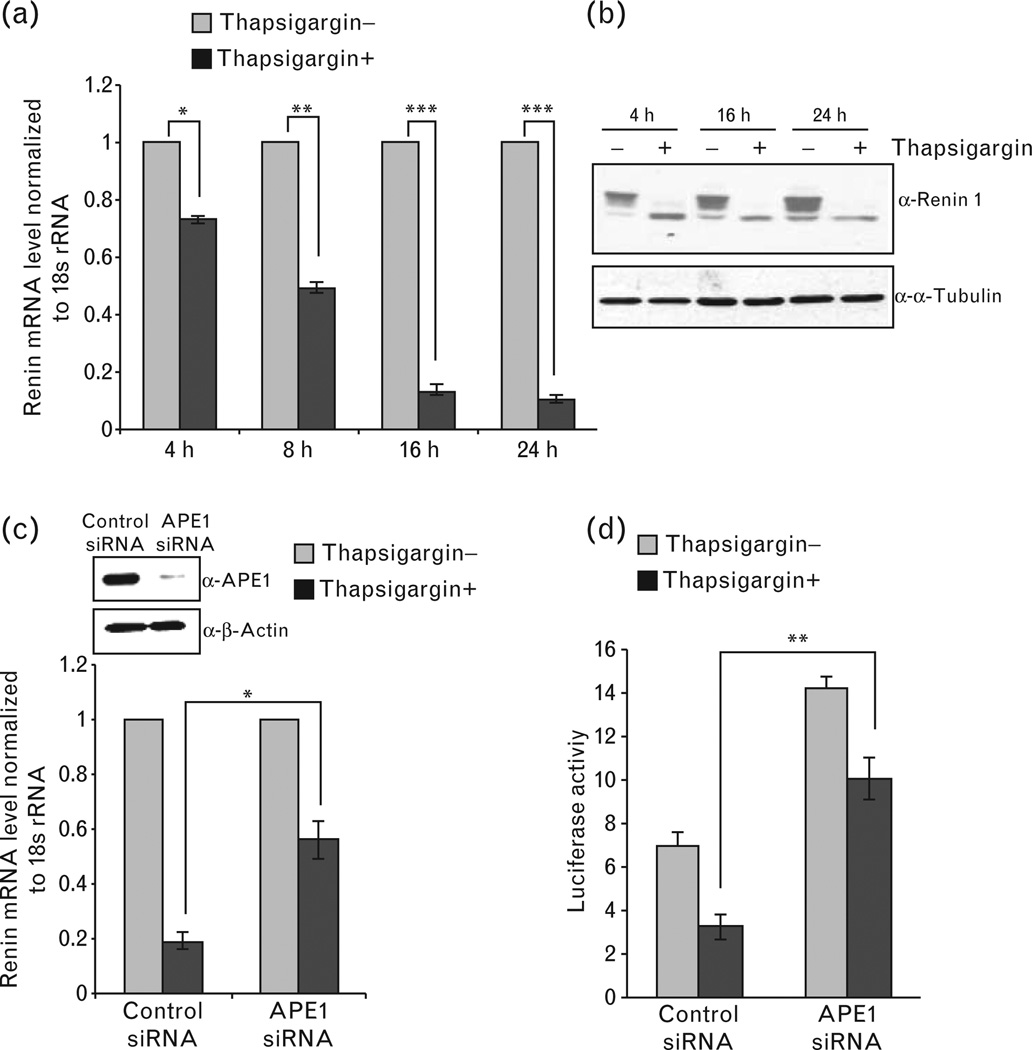
One earlier study has shown that an increase in [Ca2+]i decreased mouse renin mRNA levels by both transcriptional-dependent and independent mechanisms [16]. Although Fuchs et al. [35] identified a nCaRE-B in human renin gene promoter identical to that in PTH promoter and showed the presence of APE1 in the nCaRE-B-bound complex, such element was not identified in the 4.2 kb mouse renin 1c-promoter/enhancer sequence. We investigated whether even in the absence of nCaREs in the mouse renin gene, APE1 is still involved in [Ca2+]i-mediated downregulation of renin expression. Thapsigargin, an endoplasmic reticulum Ca2+-ATPase inhibitor has been extensively used by several groups to enhance [Ca2+]i [44,45] and that has significant effect in downregulating renin expression [16,46]. Consistent with this previous study [16], reduction of the renin mRNA level was observed after treatment with 0.1µmol/l thapsigargin (Fig. 2a). Thapsigargin-mediated decrease in renin expression was also evident from the renin protein level in cell extracts (Fig. 2b). Furthermore, knockdown of endogenous APE1 with siRNA significantly blocked Ca2+-mediated reduction of the renin mRNA level (Fig. 2c). Moreover, renin promoter-dependent luciferase activity was decreased by thapsigargin, which was also significantly relieved by APE1 knockdown (Fig. 2d). These results strongly suggest that APE1 is involved in [Ca2+]imediated repression of renin gene.
Figure 2.
AP-endonuclease 1 (APE1) is involved in Ca2+-mediated renin repression. (a) As4.1 cells after treatment with 0.1µmol/l thapsigargin for 2 h were harvested at indicated times. Renin mRNA levels normalized to 18 s rRNA were quantitated by real-time (RT)-PCR relative to untreated controls. (b) Western analysis for renin and α-Tubulin (internal control) levels in cell extracts of thapsigargin-treated or control cells. (c) Effect of APE1 knockdown on thapsigargin-mediated inhibition of renin mRNA expression was examined in thapsigargin-treated or control APE1 knockdown cells as in (a). Other details are as in Fig. 1(a). (d) Effect of APE1 downregulation and thapsigargin treatment on renin promoter-dependent luciferase activity. Other experimental details are described in methods. *, P<0.05; **, P<0.005; ***, P<0.0005.
Apurinic/apyrimidinic-endonuclease 1 is bound to the renin-enhancer sequence in As4.1 cells
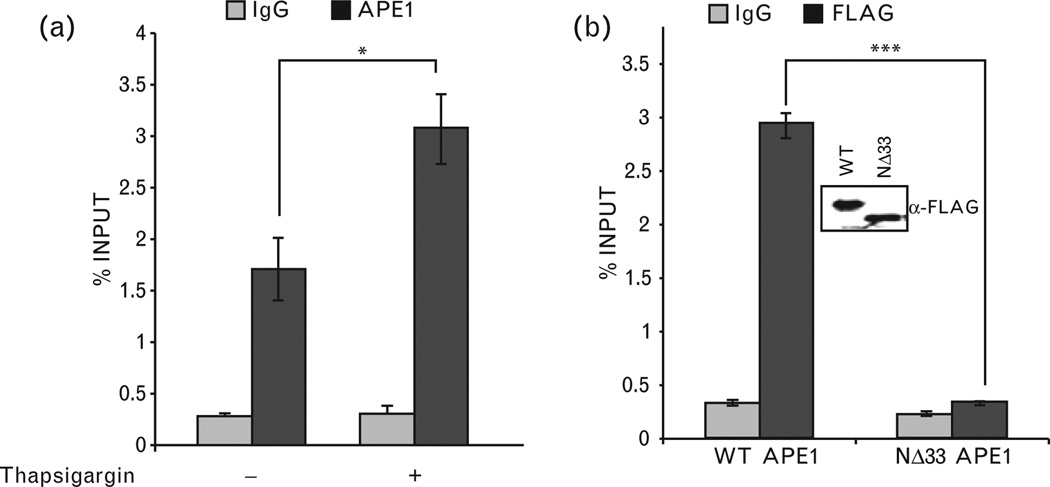
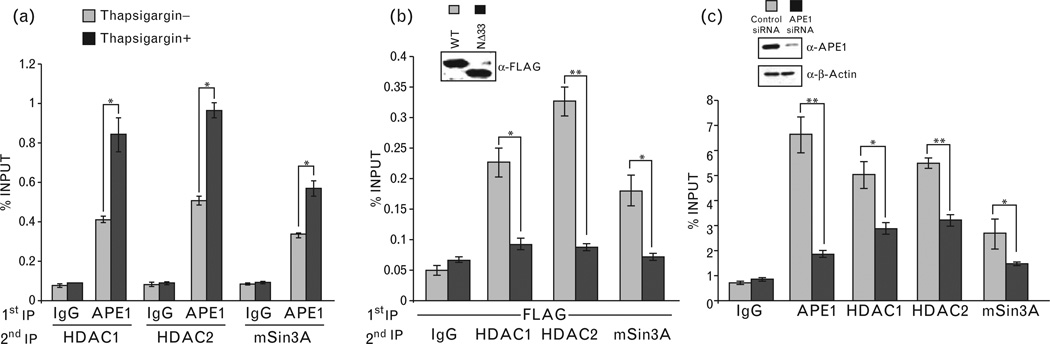
A proximal promoter region (−197 to −50) and a distal enhancer sequence (−2866 to −2625) in the mouse renin gene have been identified and both of them were shown to play critical roles in renin expression [17,47]. A recent study has shown that the regulatory elements involved in Ca2+-mediated inhibition of renin transcription are located in the enhancer region [16], which does not contain a nCaRE. Nevertheless, in order to be involved in renin repression, APE1 should be associated with one or more repressor complexes in this region. We examined APE1’s binding to this enhancer region using ChIP assay in As4.1 cells. The amount of renin-enhancer sequence in the APE1 immunoprecipitation was quantified using real time PCR. Figure 3a shows significant enrichment of this sequence in the APE1 immunoprecipitation, compared to that in control IgG. This indicates APE1’s association with this regulatory region that was furthermore significantly enhanced by thapsigargin treatment (Fig. 3a). Additionally, ChIP assay with α-FLAG antibody from cells ectopically expressing wild-type APE1-FLAG or NΔ33 APE1-FLAG showed significant strong association of wild-type APE1 but not NΔ33 mutant with the renin enhancer (Fig. 3b). These results indicate that APE1’s N-terminal region is necessary for its association with this regulatory region for its repressor activity and that increased [Ca2+]i-enhanced APE1’s recruitment.
Figure 3.
Chromatin immunoprecipitation (ChIP) analysis of APE1’s binding to renin-enhancer region and requirement of its N-terminal region. (a) The reninenhancer sequence was amplified from α-APE1 or immunoglobulin G (IgG) immunoprecipitate of crosslinked chromatin from thapsigargin-treated or control As4.1 cells, as described in methods. (b) Requirement of APE1’s N-terminal sequence for promoter binding. ChIP assay was performed in cells ectopically expressing FLAGtagged WT or NΔ33 APE1 with α-FLAG antibody or control IgG. Inset panel shows comparable expression levels of wild-type (WT) and mutant APE1. *, P<0.05; ***, P<0.0005.
Apurinic/apyrimidinic-endonuclease 1 participates in histone deacetylase 1 corepressor complex on the renin enhancer
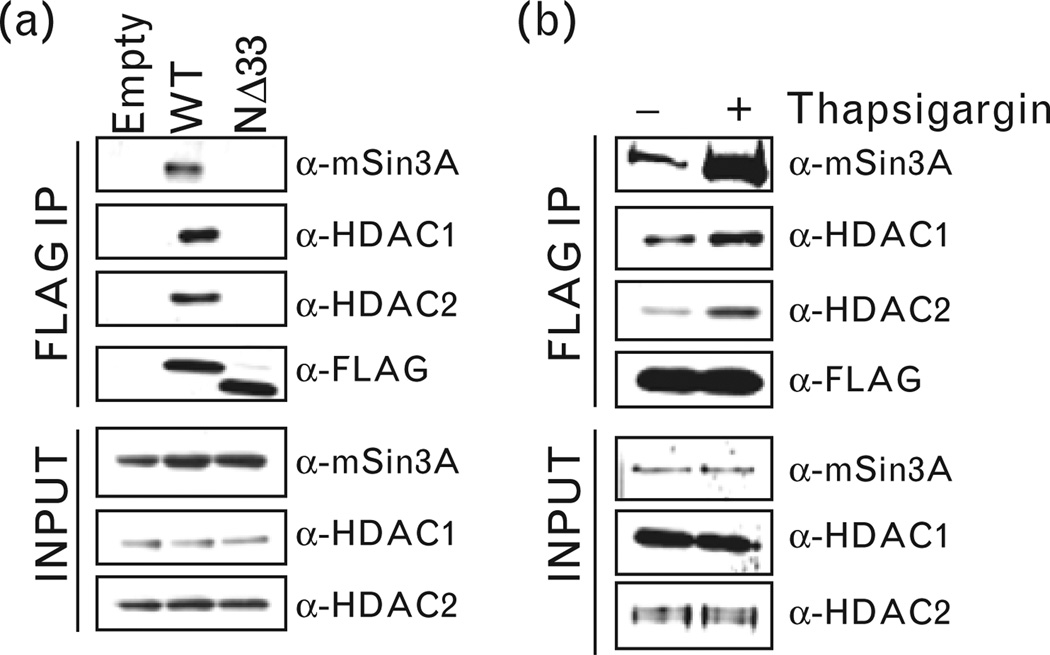
We identified components of the HDAC1 corepressor complex, namely, HDAC1, HDAC2 and mSin3A in FLAG immunoprecipitation isolated from extracts of cells expressing wild-type APE1-FLAG but not NΔ33 APE1-FLAG (Fig. 4a). Furthermore, APE1’s association with these proteins was enhanced upon treatment with thapsigargin (Fig. 4b). This indicates that increased [Ca2+]i promotes APE1’s association with the corepressor complex that requires APE1’s N-terminal region. To test that whether HDAC1/HDAC2/mSin3A are stably associated with APE1 in the renin-enhancer region, we carried out re-ChIP analysis (first immunoprecipitation with α-APE1 antibody and second immunoprecipitation from the elutes of the first immunoprecipitation with α-HDAC1, α-HDAC2 or α-mSin3A antibody). It is evident that APE1 and HDAC1/HDAC2/mSin3A were simultaneously and stably associated with the renin-enhancer region (Fig. 5a) that was significantly enhanced by thapsigargin (Fig. 5a). Furthermore, APE1’s N-terminal region was essential for its association with HDAC1, HDAC2 and mSin3A because similar re-ChIP assay showed negligible enhancer enrichment in HDAC1, HDAC2 and mSin3A immunoprecipitation when the NΔ33 APE1 mutant was ectopically expressed as compared to wild-type APE1 (Fig. 5b). We further confirmed APE1’s role in recruiting HDAC1 corepressor complex by ChIP analysis in cells after APE1 downregulation. We observed significant reduced recruitment of HDAC1, HDAC2, mSin3A and APE1 (Fig. 5c) in APE1-knockdown cells as compared to control cells.
Figure 4.
Association of APE1 with HDAC1, HDAC2 and mSin3A. (a) Nuclear lysates of As4.1 cells transfected with FLAG-tagged wild-type (WT, NΔ33 APE1 or empty vector was immunoprecipitated with α-FLAG antibody. Western analysis of the immunoprecipitates (IPs) and inputs with α-mSin3A, α-HDAC1, α-HDAC2 or α-FLAG antibody. (b) Nuclear lysates of FLAG-tagged WT APE1-transfected cells treated with thapsigargin (0.1µmol/l) or control cells were similarly immunoprecipitated and processed for Western analysis as in (a).
Figure 5.
Chromatin immunoprecipitation (ChIP)/Re-ChIP assays for recruitment and association of APE1 with HDAC1 corepressor complex on renin enhancer. (a) Effect of thapsigargin on APE1/HDAC1/HDAC2/mSin3A complex recruitment on the renin enhancer. α-APE1 or control immunoglobulin G (IgG) immunoprecipitates (IP) from crosslinked chromatin of thapsigargin-treated or control cells was eluted for second IP with α-HDAC1, α-HDAC2 or α-mSin3A antibody. ChIP analysis as in Fig. 3 was performed as described in methods. (b) Re-ChIP assay shows significant association of WT over NΔ33 APE1 with this corepressor complex. (c) Requirement of APE1 for corepressor complex binding to renin enhancer as shown by ChIP analysis. *, P<0.05; **, P<0.005.
Histone deacetylase activity in the apurinic/apyrimidinic-endonuclease 1 immunoprecipitation requires apurinic/apyrimidinic-endonuclease 1’s N-terminal domain
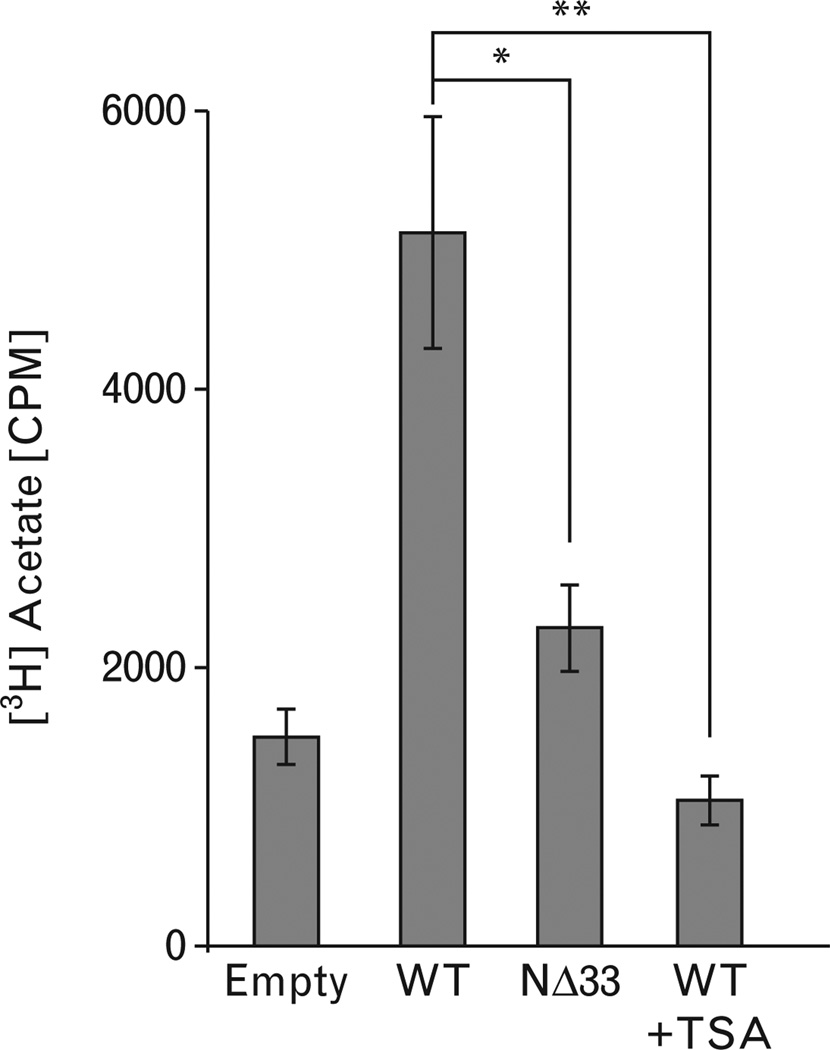
In view of the presence of HDAC1/HDAC2/mSin3A in APE1 immunoprecipitation, we tested HDAC activity in this immunoprecipitation. HDACs inhibit expression of target genes by deacetylating acetylated histones, and thus compacting the chromatin [48,49]. Histone deacetylase activity in the immunoprecipitated FLAG-tagged APE1 complexes (Fig. 6) was assayed by the release of [3H]acetate from [3H]acetate-labeled peptide corresponding to amino acid residues two to 24 of histone H4. We observed that wildtype APE1 immunoprecipitation had histone deacetylase activity because this activity was significantly sensitive to Trichostatin A (TSA< a specific HDAC inhibitor; Fig. 6). More importantly, the NΔ33 APE1-FLAG immunoprecipitation showed significant less HDAC activity (Fig. 6), indicating again that N-terminal region of APE1 is required for stable association with HDACs in the corepressor complex.
Figure 6.
HDAC activity in APE1/HDAC complex. FLAG-immunoprecipitates from cells expressing ectopic FLAG-wild type (WT) APE1 (lane 2), Trichostatin A (TSA)-treated FLAG-WT APE1 (lane 4) or FLAG-NΔ33 APE1 (lane 3) was tested for HDAC activity as described in methods. *, P<0.05; **, P<0.005.
Kidney renin mRNA level and plasma–renin activity in wild-type vs. apurinic/apyrimidinic-endonuclease 1+/− mice
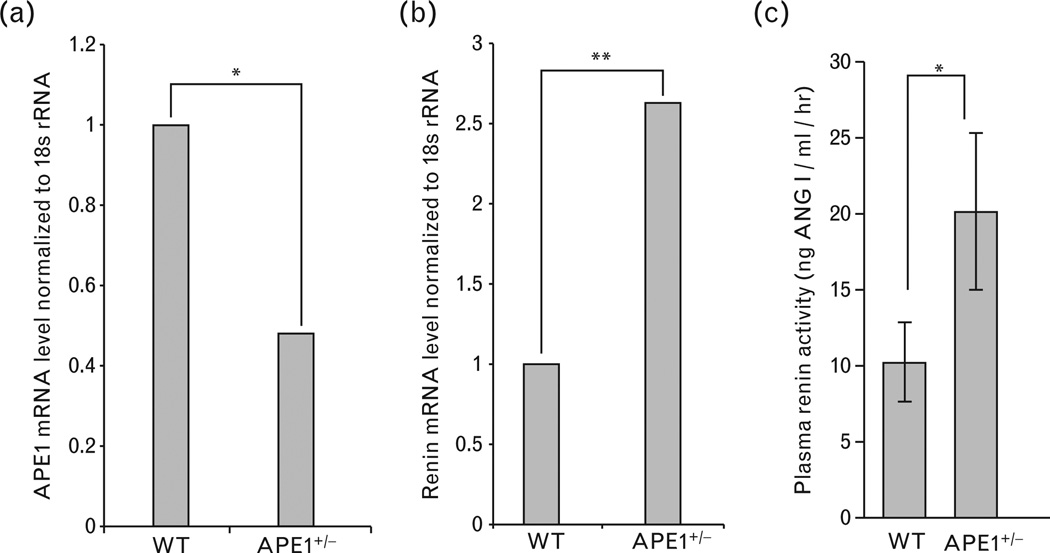
We extended the in-vitro studies on APE1’s repressor role for renin gene to the mouse by comparing wild-type and APE1+/− ice for renin expression and plasma–renin activity. We observed significantly higher renin mRNA levels in the kidneys of APE1+/− ice (n=15) relative to the wild-type mice (n=17; Fig. 7a and b). Plasma–renin activity was indirectly assessed from the relative amount of ANG I cleaved from angiotensinogen by circulating renin. Figure 7c shows that APE1+/− ice had significantly higher plasma–renin activity (20.5 ng ANG I/ml/h) than the wildtype mice (10 ng ANG I/ml/h). These in-vivo data support the evidence for APE1’s repressor function in renin gene regulation.
Figure 7.
Quantization of renin mRNA in kidneys and plasma–renin activity of wild-type (WT) and APE1+/−ice. (a) Kidney APE1 and (b) renin mRNA levels normalized to 18 s rRNA by real-time (RT)-PCR of WT (n=17) and APE1+/− (n=15) C57BL/6 male mice from the same age group. *, P<0.05; **, P<0.005. (c) Mean plasma–renin activity (see methods) in these mice. *, P<0.05.
DISCUSSION
In this study, we have documented APE1’s function as a corepressor of renin by using a renin-expressing mouse renal cell line. We further established APE1’s role in [Ca2+]imediated renin repression via recruitment of the HDAC1 corepressor complex on the renin enhancer. Finally, we showed the physiological role of APE1 in renin expression in a mouse model system.
The molecular mechanism of renin suppression by elevated calcium has long been a puzzle. In human parathyroid cells and renin-producing juxtaglomerular cells, increased [Ca2+]i suppresses PTH and renin expression and secretion, respectively [50]. The regulation of renin secretion by calcium via adenylyl cyclase has been well established [51,52]. However, the mechanism by which Ca2+ s involved in negative regulation of renin expression at the transcription level is unknown. Earlier studies from group of Okazaki et al. [27] and our laboratory showed that calcium-dependent repression is mediated by binding of transacting factors along with APE1 to nCaRE in the human PTH promoter [29]. Subsequently, Fuchs et al. [35] identified APE1-bound identical nCaRE in human renin promoter. However, no nCaRE-type element could be identified in 4.2 kb region upstream to the mouse renin transcription start site that includes both an enhancer involved in calcium-mediated renin repression as well as the proximal promoter region [16,17]. In this study we have established APE1’s binding to this enhancer region, presumably to a non-nCaRE. Furthermore, we have shown that APE1 depletion significantly relieved thapsigargin-mediated suppression of renin expression, an increase in [Ca2+]i significantly enhanced APE1’s association with HDAC1 corepressor complex on this region and HDAC1 complex recruitment to this region is significantly reduced in APE1-depleted cells. These collectively confirm APE1’s direct role in renin repression in mouse.
In spite of APE1’s binding to the renin-enhancer region, APE1 by itself does not have affinity for a specific cis-element; rather, it affects promoter activity by binding to transacting factors that recognize cognate cis-elements in promoter regions [29,37,38]. Thus, APE1 is likely to be recruited to specific cis-elements in the renin-enhancer region by unidentified transacting repressor protein/complexes that may bind to multiple regulatory sequences present in this region. This region contains a CREB/CREMbinding cyclic AMP-responsive element (CRE) and USF1/USF2-binding E-Box, which are capable of synergistically activating renin transcription [53]. Furthermore, TNF-αmediated inhibition of renin expression was shown to be indirectly mediated via this CRE [54,55]. However, the role of CRE appears to be complex because vitamin D was also reported to be involved in renin repression [56] by inhibiting transactivating complex formation with CBP at the CRE site [57]. Additionally, nuclear orphan receptor (Ear2) may repress renin by binding to the steroid hormone receptor-binding site in this enhancer region [58]. Hence, it is possible that increased [Ca2+]i inhibits binding of one or more transactivating factors to the enhancer region, whereas simultaneously recruiting transrepressor protein(s) including the APE1/HDAC1 corepressor complex to the enhancer region. Deacetylation of acetylated histones bound to the promoter/enhancer could cause transcription inhibition [48,49,59]. HDAC1 and HDAC2 generally coexist with mSin3A in a multiprotein corepressor complex, which is recruited to the promoter of target genes [48,49,59]. Consistent with this, we have shown that APE1-immunoprecipitation has significant histone deacetylase activity due to associated HDACs whose binding was enhanced by thapsigargin treatment. However, the key factor that recruits APE1/HDAC1 corepressor complex to the cognate cis-element(s) remains to be identified.
We made another significant observation regarding the role of APE1’s N-terminal region in its corepressor function. This positively charged, intrinsically disordered N-terminal region (amino acid 1–61), absent in the Escherichia coli prototype Xth [24,60] is necessary for APE1’s interaction with various binding partners, for example, XRCC1 [61], YB-1 [37], NPM1 [62] and STAT3 [63] involved in both repair and transcriptional regulation. We showed earlier that the N-terminal 33 residues are required for YB-1-mediated transcriptional activation of multidrug resistance gene, MDR1 [37,38] and STAT3-mediated IL-6-inducible expression of hepatic acute-phase reactant proteins [63]. In this study, our observations on lack of association of NΔ33 APE1 with HDAC1, HDAC2 and msin3A and associated histone deacetylase activity, and its reduced occupancy on the renin-enhancer region underscore the essentiality of this region for binding to the renin–repressor complex.
Chronic hypertension in APE1+/− ice is accompanied by decreased basal arterial nitric oxide (NO) production by endothelial NO synthase (eNOS) [34]. Jeon et al. [34] have shown that APE1-mediated upregulation of H-ras results in phosphoionositide-3 kinase/Akt kinase-dependent stimulation of NO production by calcium sensitization of eNOS activity in order to maintain vascular homeostasis. Given the role of Ca2+ n maintaining vascular tone [34] and APE1’s involvement in Ca2+-mediated transcription regulation [27,29], it is logical to consider APE1’s direct participation in multiple nuclear events for controlling blood pressure [64]. In support of this notion, we observed that APE1+/− ice have significantly higher kidney renin mRNA levels and plasma–renin activity as compared to wild-type mice. Thus, apart from regulating eNOS activity, APE1 is involved in renin repression by which it also plays an important role in blood pressure homeostasis. Identification of APE1-interacting transcription factor(s) in Ca2+-mediated downregulation of renin gene and elucidation of the molecular mechanism of this regulation should lay the foundation for future experiments involving pharmacological modulation of APE1’s repressor activity. This could also help design novel and effective approaches for treatment of hypertension.
ACKNOWLEDGEMENTS
Sources of Funding: American Heart Association Grant # 0565008Y, NIH Grant # RO1 CA148941 and Sealy Center for Molecular Medicine Pilot Project to K.K.B. and NIH Grants # RO1 ESO8457 and RO1 CA53791 to S.M.
Abbreviations
- [Ca2+]i
intracellular calcium ion concentration
- ANG
angiotensin
- APE1
apurinic/pyrimidinic-endonuclease 1
- APE1+/−
APE1 heterozygous
- BER
base excision repair
- ChIP
chromatin immunoprecipitation
- CRE
cyclic AMP response element
- eNOS
endothelial NO synthase
- HDAC
histone deacetylase
- IP
immunoprecipitate
- NΔ33
N terminal 33 aa deletion
- nCaRE
negative calcium response element
- PTH
parathyroid hormone
- RAS
renin–angiotensin system
- RT-PCR
reverse transcriptase PCR
- TSA
Trichostatin A
- WT
wild-type
Footnotes
The study was previously presented at American Heart Association Meeting 2006; Abstract # 751; Circulation. 2006; 114:II_129.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 2.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system: an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 3.Sigmund CD. Structural biology: on stress and pressure. Nature. 2010;468:46–47. doi: 10.1038/468046a. [DOI] [PubMed] [Google Scholar]
- 4.Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin–angiotensin system. Circulation. 1993;87:1816–1828. doi: 10.1161/01.cir.87.6.1816. [DOI] [PubMed] [Google Scholar]
- 5.Haber E. The renin–angiotensin system and hypertension. Kidney Int. 1979;15:427–444. doi: 10.1038/ki.1979.55. [DOI] [PubMed] [Google Scholar]
- 6.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 7.Hackenthal E, Taugner R. Hormonal signals and intracellular messengers for renin secretion. Mol Cell Endocrinol. 1986;47:1–12. doi: 10.1016/0303-7207(86)90010-9. [DOI] [PubMed] [Google Scholar]
- 8.Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension. 2009;54:1240–1247. doi: 10.1161/HYPERTENSIONAHA.109.138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 10.Baumbach L, Leyssac PP. Studies on the mechanism of renin release from isolated superfused rat glomeruli: effects of calcium, calcium ionophore and lanthanum. J Physiol. 1977;273:745–764. doi: 10.1113/jphysiol.1977.sp012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumbach L, Skott O. Renin release from isolated rat glomeruli: seasonal variations and effects of D600 on the response to calcium deprivation. J Physiol. 1981;310:285–292. doi: 10.1113/jphysiol.1981.sp013549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fray JS. Stimulation of renin release in perfused kidney by low calcium and high magnesium. Am J Physiol. 1977;232:F377–F382. doi: 10.1152/ajprenal.1977.232.4.F377. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz A, Pfeilschifter J, Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984;124:359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz A, Pfeilschifter J, Hutter A, Buhrle C, Nobiling R, Taugner R, et al. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- 15.Della Bruna R, Pinet F, Corvol P, Kurtz A. Opposite regulation of renin gene expression by cyclic AMP and calcium in isolated mouse juxtaglomerular cells. Kidney Int. 1995;47:1266–1273. doi: 10.1038/ki.1995.181. [DOI] [PubMed] [Google Scholar]
- 16.Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension. 2005;46:1340–1346. doi: 10.1161/01.HYP.0000192025.86189.46. [DOI] [PubMed] [Google Scholar]
- 17.Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension. 2005;45:3–8. doi: 10.1161/01.HYP.0000149717.55920.45. [DOI] [PubMed] [Google Scholar]
- 18.Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflugers Arch. 2002;444:499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 19.Ryan MJ, Black TA, Millard SL, Gross KW, Hajduczok G. Endothelin-1 increases calcium and attenuates renin gene expression in As4.1 cells. Am J Physiol Heart Circ Physiol. 2002;283:H2458–H2465. doi: 10.1152/ajpheart.00295.2002. [DOI] [PubMed] [Google Scholar]
- 20.Shricker K, Holmer S, Kramer BK, Riegger GA, Kurtz A. The role of angiotensin II in the feedback control of renin gene expression. Pflugers Arch. 1997;434:166–172. doi: 10.1007/s004240050379. [DOI] [PubMed] [Google Scholar]
- 21.Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair (Amst) 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S, Hazra TK, Roy R, Ikeda S, Biswas T, Lock J, et al. Complexities of DNA base excision repair in mammalian cells. Mol Cells. 1997;7:305–312. [PubMed] [Google Scholar]
- 23.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free Radic Biol Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 24.Bhakat KK, Mantha AK, Mitra S. Transcriptional Regulatory Functions of Mammalian AP-endonuclease (APE1/Ref-1), an Essential Multifunctional Protein. Antioxid Redox Signal. 2009;11:621–638. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bapat A, Fishel ML, Kelley MR. Going ape as an approach to cancer therapeutics. Antioxid Redox Signal. 2009;11:651–668. doi: 10.1089/ars.2008.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Chung U, Nishishita T, Ebisu S, Usuda S, Mishiro S, et al. A redox factor protein, ref1, is involved in negative gene regulation by extracellular calcium. J Biol Chem. 1994;269:27855–27862. [PubMed] [Google Scholar]
- 28.Izumi T, Henner WD, Mitra S. Negative regulation of the major human AP-endonuclease, a multifunctional protein. Biochemistry. 1996;35:14679–14683. doi: 10.1021/bi961995u. [DOI] [PubMed] [Google Scholar]
- 29.Bhakat KK, Izumi T, Yang SH, Hazra TK, Mitra S. Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J. 2003;22:6299–6309. doi: 10.1093/emboj/cdg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, et al. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 2010;38:832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izumi T, Brown DB, Naidu CV, Bhakat KK, Macinnes MA, Saito H, et al. Two essential but distinct functions of the mammalian a basic endonuclease. Proc Natl Acad Sci U S A. 2005;102:5739–5743. doi: 10.1073/pnas.0500986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meira LB, Devaraj S, Kisby GE, Burns DK, Daniel RL, Hammer RE, et al. Heterozygosity for the mouse Apex gene results in phenotypes associated with oxidative stress. Cancer Res. 2001;61:5552–5557. [PubMed] [Google Scholar]
- 33.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci U S A. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs S, Philippe J, Corvol P, Pinet F. Implication of Ref-1 in the repression of renin gene transcription by intracellular calcium. J Hypertens. 2003;21:327–335. doi: 10.1097/00004872-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Sigmund CD, Okuyama K, Ingelfinger J, Jones CA, Mullins JJ, Kane C, et al. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem. 1990;265:19916–19922. [PubMed] [Google Scholar]
- 37.Chattopadhyay R, Das S, Maiti AK, Boldogh I, Xie J, Hazra TK, et al. Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol Cell Biol. 2008;28:7066–7080. doi: 10.1128/MCB.00244-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta S, Mantha AK, Mitra S, Bhakat KK. Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1. Oncogene. 2011;30:482–493. doi: 10.1038/onc.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci U S A. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S, Chattopadhyay R, Bhakat KK, Boldogh I, Kohno K, Prasad R, et al. Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress. J Biol Chem. 2007;282:28474–28484. doi: 10.1074/jbc.M704672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhakat KK, Hazra TK, Mitra S. Acetylation of the human DNA glycosylase NEIL2 and inhibition of its activity. Nucleic Acids Res. 2004;32:3033–3039. doi: 10.1093/nar/gkh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhakat KK, Yang SH, Mitra S. Acetylation of human AP-endonuclease 1, a critical enzyme in DNA repair and transcription regulation. Methods Enzymol. 2003;371:292–300. doi: 10.1016/S0076-6879(03)71022-2. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig DL, MacInnes MA, Takiguchi Y, Purtymun PE, Henrie M, Flannery M, et al. A murine AP-endonuclease gene-targeted deficiency with postimplantation embryonic progression and ionizing radiation sensitivity. Mutat Res. 1998;409:17–29. doi: 10.1016/s0921-8777(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 44.Bost KL, Mason MJ. Thapsigargin and cyclopiazonic acid initiate rapid and dramatic increases of IL-6 mRNA expression and IL-6 secretion in murine peritoneal macrophages. J Immunol. 1995;155:285–296. [PubMed] [Google Scholar]
- 45.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 46.Itani HA, Liu X, Pratt JH, Sigmund CD. Functional characterization of polymorphisms in the kidney enhancer of the human renin gene. Endocrinology. 2007;148:1424–1430. doi: 10.1210/en.2006-1381. [DOI] [PubMed] [Google Scholar]
- 47.Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics. 2008;35:243–253. doi: 10.1152/physiolgenomics.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci. 2002;115(Pt 4):689–698. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 49.Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 50.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 51.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, et al. Stimulation of Renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 2011;57:460–468. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, et al. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem. 2001;276:45530–45538. doi: 10.1074/jbc.M103010200. [DOI] [PubMed] [Google Scholar]
- 54.Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, et al. Role of CREB1 and NF{kappa}B-p65 in the downregulation of renin gene expression by tumor necrosis factor {alpha} J Biol Chem. 2005;280:24356–24362. doi: 10.1074/jbc.M502968200. [DOI] [PubMed] [Google Scholar]
- 55.Itani H, Liu X, Sarsour EH, Goswami PC, Born E, Keen HL, Sigmund CD. Regulation of renin gene expression by oxidative stress. Hypertension. 2009;53:1070–1076. doi: 10.1161/HYPERTENSIONAHA.109.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigmund CD. Regulation of renin expression and blood pressure by vitamin D(3) J Clin Investig. 2002;110:155–156. doi: 10.1172/JCI16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Huang X, Sigmund CD. Identification of a nuclear orphan receptor (Ear2) as a negative regulator of renin gene transcription. Circ Res. 2003;92:1033–1040. doi: 10.1161/01.RES.0000071355.82009.43. [DOI] [PubMed] [Google Scholar]
- 59.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strauss PR, Holt CM. Domain mapping of human apurinic/apyrimidinic endonuclease. Structural and functional evidence for a disordered amino terminus and a tight globular carboxyl domain. J Biol Chem. 1998;273:14435–14441. doi: 10.1074/jbc.273.23.14435. [DOI] [PubMed] [Google Scholar]
- 61.Vidal AE, Boiteux S, Hickson ID, Radicella JP. XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions. EMBO J. 2001;20:6530–6539. doi: 10.1093/emboj/20.22.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vascotto C, Fantini D, Romanello M, Cesaratto L, Deganuto M, Leonardi A, et al. APE1/Ref-1 interacts with NPM1 within nucleoli and plays a role in the rRNA quality control process. Mol Cell Biol. 2009;29:1834–1854. doi: 10.1128/MCB.01337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray S, Lee C, Hou T, Bhakat KK, Brasier AR. Regulation of signal transducer and activator of transcription 3 enhanceosome formation by apurinic/apyrimidinic endonuclease 1 in hepatic acute phase response. Mol Endocrinol. 2010;24:391–401. doi: 10.1210/me.2009-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson C. Blood pressure control goes nuclear. Circ Res. 2004;95:849–851. doi: 10.1161/01.RES.0000147313.80351.92. [DOI] [PubMed] [Google Scholar]