Abstract
Proteases have been used in medicine for several decades and are an established and well tolerated class of therapeutic agent. These proteases were sourced from mammals or bacteria that exist or have adapted to moderate temperatures (mesophilic organisms); however, proteases derived from organisms from cold environments—cold-adapted or psychrophilic proteases—generally have high specific activity, low substrate affinity, and high catalytic rates at low and moderate temperatures. Made possible by greater flexibility, psychrophilic enzymes interact with and transform the substrate at lower energy costs. Cold-adapted proteases have been used in a wide range of applications, including industrial functions, textiles, cleaning/hygiene products, molecular biology, environmental bioremediations, consumer food products, cosmetics, and pharmaceutical production. In addition to these applications, they have also shown promise as therapeutic modalities for cosmeceutical applications (by reducing glabellar [frown] lines) and a number of disease conditions, including bacterial infections (by disrupting biofilms to prevent bacterial infection), topical wound management (when used as a debridement agent to remove necrotic tissue and fibrin clots), oral/dental health management (by removing plaque and preventing periodontal disease), and in viral infections (by reducing the infectivity of viruses, such as human rhinovirus 16 and herpes simplex virus). Psychrophilic proteases with greater activity and stability (than the original organism-derived variant) have been developed; this coupled with available manufacturing recombinant production techniques suggests that cold-adapted proteases have a promising future as a distinct therapeutic class with diverse clinical applications.
Keywords: Cold adapted, Proteases, Psychrophiles, Recombinant proteases, Therapeutics
Introduction
Enzymes that cleave peptide bonds in proteins are also known as proteases, proteinases, peptidases, or proteolytic enzymes [1], and function to accelerate the rate of specific biologic reactions by lowering the activation energy of the reaction [2]. Proteases are most often assumed only to be involved in processes relating to digestion, but the fact that over 2% of the human genome encodes protease genes suggests that they play more complex functions than digestion alone [3]. Indeed, proteases have been shown to be involved in the regulation of a number of cellular components from growth factors to receptors, as well as processes including immunity, complement cascades, and blood coagulation [3]. In addition to involvement in homeostatic processes, increased or dysregulated activity of proteases has been implicated in cancer via its link with tumor growth and invasion [4].
Briefly, proteases are initially produced as inactive precursors, or zymogens, and are distributed in specific organs or locations, where they have little catalytic ability until they are activated by proteolytic cleavage [5]. Further posttranslational mechanisms to control the activity of proteases include phosphorylation, cofactor binding, and segregation of enzyme and/or substrate in vesicles or granules. In addition, the effective concentration of active enzyme can also be strictly regulated by protease inhibitors, which can reduce functional efficacy by forming a complex with the protease and effectively “balance” proteolytic activity [6]. In this short review, the therapeutic uses and future outlook for proteases (notably cold-adapted proteases) will be discussed.
Therapeutic Use of Proteases
Proteases have been used in medicine for several decades and are an established and well tolerated class of therapeutic agent [3]. Early documented use of proteases in the published literature appeared over 100 years ago [7–9]. In general, proteases have been used therapeutically in four areas: the management of gastrointestinal disorders with orally administered agents, as anti-inflammatory agents, as thrombolytic agents for thromboembolic disorders, and as locally administered agents for wound debridement [10]. Since the first approval of a protease drug in 1978 (urokinase, a serine protease indicated for thrombolysis and catheter clearing), a further 11 drugs have been approved for therapeutic use by the US Food and Drug Administration (FDA) [3]. The majority of these are indicated for the treatment of blood disorders and include thrombolytics: alteplase, reteplase, and tenecteplase; and procoagulants: factor IX, factor VIIa, thrombin, and topical thrombin in bandages. The other approved protease therapeutics are indicated for digestion (pancrelipase), muscle spasms, and as cosmeceuticals (cosmetic products with biologically active ingredients intended to have medicinal or drug-like benefits; botulinum toxin A and botulinum toxin B) [3].
The use of topical proteases as a tool for selective tissue destruction (i.e., ablation of diseased tissue to expedite improvement or cure) is an attractive one. Epidermally confined dermatologic disorders, i.e., those for which the primary disease is confined to the epidermis (e.g., verruca or actinic keratosis), are known to be cured using superficial destructive techniques to remove diseased skin, allowing the regeneration of healthy tissue from adjacent/accessory structures [2]. Precise tissue destruction is also a desirable property and is possible with topically administered proteases. For example, the ideal method to destroy an epidermal neoplasm would involve selective elimination of malignant tissue without causing damage to healthy tissue or deeper structures. Therefore, exploiting the unique ability of proteases to cause selective epidermal separation is an attractive approach to achieve such desired precision. However, such a level of precision is currently not achievable using conventional methods of therapeutic tissue destruction, such as cryosurgery (with liquid nitrogen), electrosurgery, laser surgery, chemosurgery, and cold-steel surgery, which can produce tissue damage (to varying degrees) that extend unnecessarily beyond the epidermis, which can result in delayed healing, scar formation, and alterations to pigmentation [2]. As a consequence, there has been great interest in using the selective properties of enzymes and, thus, proteases have been examined for effectiveness in a number of such topical applications, including animal models of acne vulgaris, wound healing, epidermal ablation, and debridement of necrotic ulcers.
Trypsin demonstrated antiaging properties and a comedolytic effect (i.e., opening up of clogged pores and lysis of comedones [hard plugs of keratin and sebum within hair follicles]) in a murine model of acne [11]. The principle physiological change that leads to acne vulgaris is the process of a sebaceous follicle transforming to a comedone via hyper-cornification and hyper-keratinization of the infundibulum (i.e., the funnel in which the hair follicle grows). A murine model was used to quantify the effects of daily topical trypsin over 5 days’ treatment and resulted in improved skin plasticity, increased cell layers in the dermis and epidermis, as well as increased skin elasticity when compared with control treatment. In addition, when compared with controls, trypsin induced follicular-epidermal differentiation and also prevented extensive programmed cell death and apoptosis [11].
Collagenase ointment has also shown benefits in wound healing by achieving selective debridement in porcine models [12]. Partial or full-thickness wounds in Yorkshire pigs were contaminated with Staphylococcus aureus and Pseudomonas aeruginosa, then treated with Clostridium collagenase ointment (Santyl®; Healthpoint Ltd., Fort Worth, Texas, USA), or controls of white petrolatum or moist dressing and untreated wounds. Following treatment over 8 days, collagenase ointment achieved complete re-epithelialization in 85% of animals with partial-thickness wounds compared with only 10% using petrolatum and 0% using moist dressing and untreated wounds. Furthermore, significantly less inflammation and less neutrophil infiltration was observed by histology in the animals treated with collagenase, and re-epithelialization was enhanced, compared with petrolatum [12].
The potential of topically applied proteases for epidermal ablation has also been demonstrated through the in vitro and in vivo use of subtilisin, trypsin, and dispase in murine and human skin samples. These proteases target keratin, desmosomes, and collagen IV, respectively. Following application, they all demonstrated subcorneal separation, intraepidermal acantholysis, and subepidermal dissociation [2]. Furthermore, topical application of a 2.5% (w/v) solution of bovine trypsin to two seborrheic keratosis for 15 min on the trunk of a human participant destroyed the lesions after 1 month, and after 3 months there was no evidence of scarring, pigment changes, or residual seborrhoea keratosis [2].
The use of streptokinase-streptodomase or crystalline trypsin (Trypure®; Novo Nordisk, Bagsvaerd, Denmark) impregnated in wound dressings was examined in patients with necrotic varicose or arteriosclerotic leg ulcers. Treatment with either protease resulted in a significant reduction in pus and debris associated with the ulcers, as well as a significant increase in tissue granulation (P < 0.01 in both groups). Compared with trypsin, the streptokinase-streptodomase formulation was associated with less pain (P < 0.01) [13].
In the face of increasing antibiotic resistance among bacteria, development of therapeutics has broadened to compounds that target virulence factors rather than viability. Antivirulence strategies would be less likely to result in the emergence of mutations leading to resistance, due to the reduced impact on the level of selective pressure on the bacterial population [14]. A virulence factor recognized as a tremendous burden on our healthcare system is the formation of bacteria into biofilm. Biofilms, complex structures notoriously difficult to disassemble, protect the colonizing bacteria from the host’s immune system and from antibiotic therapy. Much research effort to develop new prophylactic and therapeutic agents has focused on the inhibition of biofilm formation or dispersal of established biofilms, as this would release the bacteria into their planktonic phenotype, which is much more sensitive to antibiotics and to the immune defenses [14].
Efforts to discover effective antibiofilm therapeutic alternatives to antibiotics have been plentiful, and much of that effort has focused on enzyme-based treatments. For example, proteinase K and trypsin were shown to be effective in disrupting biofilm formed by certain staphylococcal strains [15]. The overexpression of bacterial extracellular proteases inhibited biofilm formation [16], and esperase HPF (subtilisin) is effective against multispecies biofilms [17].
Psychrophilic or Cold-Adapted Proteases
The proteases so far approved by the US FDA are sourced from a range of mammals or bacteria that exist or have adapted to moderate temperatures—i.e., mesophilic organisms. In the pursuit of more effective and more flexible proteases, the therapeutic potential of molecules derived from organisms from cold environments has been examined. Those organisms from the three domains of life (bacteria, archaea, eucarya) that thrive in cold environments (i.e., psychrophiles) have developed enzymes that generally have high specific activity, low substrate affinity, and high catalytic rates at low and moderate temperatures [18–20]. In general, when compared with mesophilic variants, the property of greater flexibility in psychrophilic enzymes allows the protease to interact with and transform the substrate at lower energy costs. The comparative ease of interaction is possible because the catalytic site of the psychrophilic protease can accommodate the substrate more easily [20]. However, this increased flexibility is often accompanied by a trade-off in stability [21]. Therefore, in contrast to mammalian analogs, psychrophilic proteases are more sensitive to inactivation by heat, low pH, and autolysis [18, 19, 21–25].
Comparisons between psychrophilic and mesophilic trypsins suggested that there are a number of structural features that are unique to the cold-adapted trypsins that give greater efficiency, but also reduced stability. Their greater efficiency and catalytic ability arise because of deletions from the surrounding loop regions of the structure. This increased flexibility is generally most pronounced around the site of catalytic activity and enables the protease to move and facilitate reactions at low temperatures, and in a low energy environment [26]. The increased catalytic activity is thought to result from optimization of the electrostatic forces (hydrogen bonds, van der Waals interactions, and ion pairs) at the active site [27]; for cold-adapted serine proteases, this is thought to result from the lower electrostatic potential of the S1 binding pocket caused by the lack of hydrogen bonds adjacent to the catalytic triad [25].
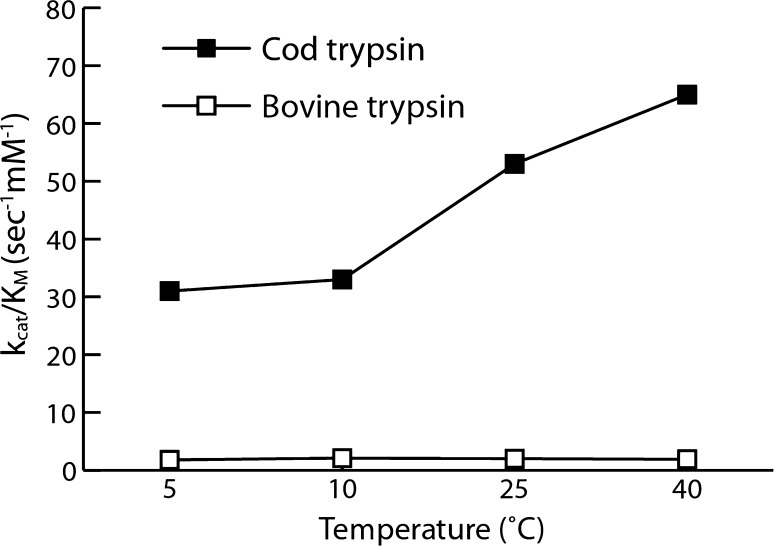
Catalytic activity or enzyme efficiency is often expressed as kcat/KM (i.e., the specificity constant), where kcat represents the catalytic production of a product under ideal conditions (i.e., turnover number) and KM represents the Michaelis constant or the affinity of the protease for the substrate. Generally, the high catalytic rates observed in cold-adapted proteases are the result of modifications in enthalpy favoring higher turnover numbers. However, when looking at proteases that have adapted through strong KM improvement, such as trypsin (that does not only increase kcat but also increases its catalytic efficiency by lowering its KM), the distinction between these mesophilic and psychrophilic proteases become more pronounced. An example of this is seen by a 17 times greater catalytic efficiency with trypsin from Atlantic cod, compared with trypsin from bovine sources (Fig. 1) [22]. Detailed examination of the temperature performance of cod and bovine trypsin demonstrated that the cod-derived protease displayed a twofold increase in kcat and a more than eightfold improvement (reduction) in KM. Practically, the main implication of a lower KM is that a lesser amount of enzyme is required to gain a high catalytic efficiency. Furthermore, in a study comparing Atlantic cod trypsin with bovine trypsin [28], the cod trypsin cleaved proteins more effectively across a range of temperatures. For example, at temperatures up to 25°C, cod trypsin more effectively cleaved intercellular adhesion molecule 1, myoglobin, lactoferrin, and lysozyme when compared with bovine trypsin. At lower temperatures (4°C), this difference in effect was even more pronounced. Overall, it appears that for cold-adapted proteases, the enzyme activity curve as a function of temperature is shifted toward low temperature (compared with their mesophile counterparts). Therefore, either due to improved kcat or KM, the catalytic activity (kcat/KM) values are higher for psychrophilic proteases than their mesophilic counterpart over a temperature range from 0°C to at least 30°C. In fact, many cold-adapted enzymes have temperature optima in the range of, or even closer to, the temperature range in which mesophilic enzymes operate naturally, than mesophilic enzymes themselves [18, 22]. However, the greater efficacy is accompanied by a reduced thermal stability, evident in the fast denaturation at moderate temperatures [18, 27]. Variations in the flexibility and rigidity of the psychrophilic protein may explain the greater adaptability and efficacy at lower temperatures, and also the reduced stability. Structural changes, such as fewer hydrogen bonds, fewer salt bridges, and poorer van der Waals packing interactions in the core, are evident in psychrophilic proteases [25]. However, this is not a widespread rule; while some psychrophilic proteases have lower stability than mesophilic analogs, some have decreased stability only at the sites of substrate binding and catalysis [10, 29].
Fig. 1.
Example of the large impact of temperature on the specificity constant kcat/KM on a cold-adapted protease, here determined for Atlantic cod trypsin and its mesophilic homolog, bovine trypsin, using the synthetic substrate, benzoyl-l-arginine p-nitroanilide. The same pattern also applies to other substrates. k cat turnover number, K M Michaelis constant. Adapted with permission from Asgeirsson et al. [22]
Clearly, if a psychrophilic protease were to be the most effective in a mesophilic environment, there is the obvious requirement to enhance its fundamental stability and functionality. Before applying the thermal stability traits of a mesophilic protease to a psychrophilic analog, an understanding of the relationship between stability, static and dynamic flexibility or plasticity, and catalytic efficiency of cold-adapted proteases is required. Site-directed mutagenesis and directed evolution are among the methods expected to produce proteases that exhibit the stability of a mesophilic product while retaining the efficiency of a psychrophilic molecule [21, 30–33].
Using random mutagenesis, saturation mutagenesis, and in vitro recombination/DNA shuffling, Miyazaki and colleagues [31] generated mutant libraries of the psychrophilic protease, subtilisin S41. Of the resulting proteases, one variant (3-2G7) had an optimal operating temperature increased by 10°C, without compromising activity at low temperatures, and exhibited threefold greater catalytic efficiency. Subsequent generations of this protease have also been developed and have demonstrated even greater levels of activity and stability [32]. One of the authors postulated that a protease with increased activity at low temperature and stability at higher temperatures can exist physically, but it had not been found naturally due to the course of evolution [31].
While it has been shown that it is possible to modify psychrophilic proteases to be more stable at higher temperatures, the opposite is also true: existing mesophilic proteases can be engineered to achieve improved function at low temperatures. For example, based on subtilisin BPN’, an alkaline serine protease, sequential in vitro mutagenesis was employed to produce a cold-adapted mutant. Using three mutations in the structure of subtilisin, two that enhanced activity and one that reduced activity, a cold-adapted variant was produced that had a 100% increase in activity compared with the wild type. The increase in activity was primarily attributed to increased affinity of the mutant variant for the substrate [33].
That the cold-adapted proteases exhibit reduced stability at moderate temperatures need not be considered a disadvantage; in fact, it could prove to be an important property for exploitation if considered for therapeutic use, in particular, topical administration. Therefore, while psychrophilic proteases have high activity and efficiency when applied locally at ambient temperatures, because they are optimized for activity at lower temperatures, they will lose activity and be degraded once equilibrium with the warmer in vivo environment is reached; thereby limiting the duration of activity and ensuring that the protease only exhibits a controlled localized effect. Another factor favoring cold-adapted proteases with regard to safety in therapeutic use is that the high catalytic efficiency requires exposure to a smaller amount of enzyme. This is particularly true for proteases with a low KM, such as cod trypsin. Furthermore, the inherent greater flexibility of cold-adapted proteases has been reported to be particularly useful in conditions, such as low water conditions (e.g., targeting lipid membrane proteins, lipid layer of mucus), wherein the activity of mesophilic and thermophilic enzymes is severely impaired by the high level of structural rigidity [34].
In the event that an extended half-life or greater exposure may be required, proteases can be administered in their inactive zymogen form (to be subsequently activated in vivo). Furthermore, greater tolerability may be achieved by engineering the protease to have reduced antigenicity and immunogenicity [35].
While psychrophilic proteases have been obtained from biological sources, such as Atlantic cod (Gadus morhua) or Antarctic krill (Euphausia superba), the large-scale production of suitable quantities of homogenous cold-adapted proteases could be obtained using recombinant technologies. A wide variety of fish enzymes and proteases has already been identified, cloned, and expressed in microorganisms [36]. In the production of other proteases for therapeutic purposes, non-human sources or production hosts are preferred so that the potential for contamination can be avoided. Recombinant technologies are thus widely employed to produce approved mammalian (recombinant) therapeutic proteins, such as blood clotting factors (from recombinant Chinese hamster ovary or baby hamster kidney cells), thrombolytics (from Escherichia coli), or botulinum toxin (Clostridium botulinum) [3]. Therefore, it would appear logical to explore the possibility of producing cold-adapted proteases through recombinant technology. There have been several, more or less successful, attempts to do this in the laboratory. However, large-scale production of recombinant cold-adapted enzymes is associated with several complicating factors, such as the short half-life and autolytic activity of cold-adapted enzymes, which makes production difficult under more standardized industrial conditions and temperatures.
The Use of Cold-Adapted Proteases as Therapeutics
To date, cold-adapted proteases have been used in a wide range of applications, including industrial functions, textiles, cleaning/hygiene products (detergents), molecular biology, environmental bioremediations (reducing contamination), consumer food products (dairy manufacturing and preparation), cosmetics, and pharmaceuticals (as biocatalysis in organic synthesis of drugs and/or intermediates in their generation) [1, 10, 29].
Cosmeceuticals and Dermatology
The use of proteases for cosmeceuticals is of great interest and potential. One example of such a protease is the FDA-approved botulinum toxin A indicated for muscle spasms. Botulinum toxin A disrupts neurotransmission by inhibiting acetylcholine release and inactivates soluble N-ethylmaleimide-sensitive factor-attachment protein 25 (SNAP-25). Although indicated for the treatment of muscle spasms, botulinum toxins are probably best known for their utility in reducing glabellar (frown) lines [3].
The removal of necrotic tissue and fibrin clots is considered a critical phase in wound care and, as such, proteases are considered to have the potential to be important in the removal of barriers to tissue regeneration and tissue healing [37]. Investigations have shown that proteolytic enzymes from Antarctic krill (acidic endopeptidases [trypsin and chymotrypsin-like enzymes] and exopeptidases [carboxypeptidase A and B]) were shown to be superior to saline control in facilitating recovery in a standard porcine model of wound management [38]. Electrokeratome and trichloracetic acid were used to create necrotic ulcers in a model of wound recovery in domestic pigs, which were then treated twice daily with dressings impregnated with various concentrations of krill or saline control for 7 days. The krill proteases (at a concentration of ≥3.0 casein units/mL) were found to be an effective debridement tool that, when compared with the control treatment, significantly reduced the degree of necrotic tissue (P < 0.05), improved tissue granulation, and enhanced wound healing [38]. In addition, the krill proteases achieved wound cleaning 3–4 days earlier when compared with control treatment.
Periodontal Disease
Tooth decay or dental cavities are caused by the build-up of bacterial plaque and can lead to oral disease. Formed through a number of steps whereby “pioneer” bacteria adhere to dental pellicle (the protein film on the surface of tooth enamel) and subsequent bacteria adhere to the pioneer colonizers, a matrix is formed of salivary components and bacteria. If not adequately managed by mechanical removal (e.g., by brushing or flossing), the toxic bacterial products from accumulated plaque can lead to gingivitis and periodontal disease, the most common oral disorder in industrialized populations [39, 40]. Preventive measures to reduce periodontal disease and the requirement for dental treatments would have obvious benefits.
Krill-derived proteases have shown potential in the management of periodontal disease [41]. In vitro examination indicated that krill enzymes were able to inhibit the binding of oral bacteria to saliva-covered surfaces and detach bacterial plaque; accumulated plaque was effectively removed from dentures without having an effect on the normal (beneficial) microbial flora of the oral environment [39]. Furthermore, when used in a chewing gum formulation, krill proteases were shown to reduce gingivitis. In addition to regular dental care, krill proteases (0.06 or 6.0 U) were delivered via a chewing gum (for 10 min, four times a day for 10 days) to healthy volunteers. Compared with placebo gum, the krill protease-containing gum disrupted bacterial adherence to dental pellicle and a significant reduction (54%, P < 0.05) in gingival bleeding was seen with a therapeutic dose of 0.06 U krill enzymes compared with placebo chewing gum [41]. The gum containing proteolytic enzymes was found to be well tolerated as none of the subjects reported any adverse reactions or events during the entire trial period.
Viral Infections
Acute nasopharyngitis, or the common cold, caused by any one of a large number of antigenically distinct viruses and as one of the most common infectious syndromes in humans, is associated with significant health burden, both in terms of financial and quality of life outcomes [42, 43]. Pathogens of the enterovirus family (human rhinoviruses and Coxsackie A virus serotypes) are the principal causative agent in viral infections and can result in symptoms such as sore throat, sneezing and rhinorrhea, and secondary bacterial infections, as well as more severe symptoms by exacerbating asthma, chronic obstructive pulmonary disease, and cystic fibrosis [42, 43].
Rhinovirus, the most common cause of colds and acute respiratory tract illness [34], gains entry into host cells of the nose and throat by interacting with the human intercellular adhesion molecule 1 (or CD54) [15]. This suggests that proteases that target these molecules, such as those from cod trypsin [28], may have therapeutic potential in the management of viral infections. Indeed, in vitro studies have shown that exposing viruses to trypsins results in a reduction in infectivity/activation [44, 45]. Furthermore, data from postmarket studies suggest that the use of ColdZyme® (Enzymatica AB, Lund, Sweden) mouth spray, an oral solution containing glycerol and a cold-adapted cod trypsin, can reduce the incidence of the common cold [46]. Marketed for use as a moisturizer and to improve oral hygiene, users of ColdZyme noted a reduced occurrence of cold symptoms. The ColdZyme mouth spray creates a thin film in the mouth and throat cavity that acts as an active surface barrier with proteolytic activity. Furthermore, the cold-adapted trypsin used in ColdZyme mouth spray has shown high efficiency in reducing the infectivity of human rhinovirus 16 [46] and herpes simplex virus 1 in vitro [47].
A summary of the proteases can be found in Table 1 [2, 3, 11–13, 38, 39, 41, 46, 47].
Table 1.
| FDA approved | Use | Protease description |
|---|---|---|
| Cardiovascular/hematology | Thrombolytics | Serine proteases urokinase; alteplase, reteplase, and tenecteplase |
| Procoagulants | Factor IX, factor VIIa, thrombin, and topical thrombin in bandages | |
| Dermatology | Wound healing | Collagenase |
| Digestion | Digestion aid | Pancrelipase |
| Neuromuscular | Muscle spasms | Botulinum toxin A and botulinum toxin B |
| Other | Protease action/effect | Protease |
|---|---|---|
| Dermatology | Anti-aging properties and comedolytic effect | Trypsin |
| Epidermal ablation | Subtilisin, trypsin, and dispase | |
| Wound healing | Streptokinase-streptodomase | |
| Wound debridement and management | Acidic endopeptidases (trypsin and chymotrypsin-like enzymes) and exopeptidases (carboxypeptidase A and B)a | |
| Periodontal disease | Reducing plaque and gingivitis | Acidic endopeptidases (trypsin and chymotrypsin-like enzymes) and exopeptidases (carboxypeptidase A and B)a |
| Infection | Prevent contracting a cold (rhinovirus) | Cod trypsin |
| Biofilm disruption/antibacterial | Proteinase K and trypsin |
FDA US Food and Drug Administration
aFrom Arctic krill
Conclusion and Future Scenarios
Proteases, a recognized, effective, and well tolerated drug class that have been used therapeutically over a number of years, illustrate great potential in the management of a number of disease conditions. This recognition is a stimulus for the investigation of promising proteolytic enzyme variants, including cold-adapted proteases (and other enzymes), for further therapeutic applications. Cold-adapted proteases, therefore, have a promising future as a distinct therapeutic class with diverse clinical applications. This is illustrated by their ability to catalyze biological processes more effectively than mesophilic analogs at lower temperatures, demonstrate good safety profiles, have efficacy in topical applications with a relatively localized effect, and be readily manufactured through recombinant production processes.
As our understanding of their structure and function has broadened, proteases with greater efficacy and stability have been produced while retaining high specificity constants, which provides a tantalizing insight into how they might be employed as therapeutics in the future. Applications in which proteases may hold promise in the future include the prevention of infection and disease, enhancing the management of peripheral artery disease and thrombosis, dermatology, and wound care.
It is imperative that we continue investigating ways in which potent candidates such as cold-adapted proteases can offer competent alternatives to traditional pharmaceutical therapy, in particular when systemically active agents, such as antibiotics, are used to treat local bacterial or viral infections. Therefore, the authors strongly propose the consideration of cold-adapted proteases as an emerging class of therapeutics for the treatment of infectious diseases.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Matt Weitz, inScience Communications, Springer Healthcare. Support for this assistance was funded by Enzymatica AB. Dr Clarsund is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Both authors are employees of Enzymatica AB, as stated in their affiliations.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Kuddus M, Ramteke PW. Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol. 2012;38:330–338. doi: 10.3109/1040841X.2012.678477. [DOI] [PubMed] [Google Scholar]
- 2.Fein H, Maytin EV, Mutasim DF, Bailin PL. Topical protease therapy as a novel method of epidermal ablation: preliminary report. Dermatol Surg. 2005;31:139–147. doi: 10.1111/j.1524-4725.2005.31034. [DOI] [PubMed] [Google Scholar]
- 3.Craik CS, Page MJ, Madison EL. Proteases as therapeutics. Biochem J. 2011;435:1–16. doi: 10.1042/BJ20100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 5.Scott CJ, Taggart CC. Biologic protease inhibitors as novel therapeutic agents. Biochimie. 2010;92:1681–1688. doi: 10.1016/j.biochi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings ND, Tolle DP, Barrett AJ. Evolutionary families of peptidase inhibitors. Biochem J. 2004;378:705–716. doi: 10.1042/BJ20031825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris RT. The action of trypsin, pancreatic extract and pepsin upon sloughs, coagula, and mucopus. NY Med J. 1891;53:424–426. [Google Scholar]
- 8.Morani AD. Trypsin therapy in the management of chronic surface ulcers. Plast Reconstr Surg. 1953;11:372–379. doi: 10.1097/00006534-195305000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport C. The use of trypsin in the therapy of tuberculous lymphadenitis and tuberculous fistulae. Dis Chest. 1958;34:154–161. doi: 10.1378/chest.34.2.154. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsdottir A, Palsdottir HM. Atlantic cod trypsins: from basic research to practical applications. Mar Biotechnol. 2005;7:77–88. doi: 10.1007/s10126-004-0061-9. [DOI] [PubMed] [Google Scholar]
- 11.Seiberg M, Siock P, Wisniewski S, Cauwenbergh G, Shapiro SS. The effects of trypsin on apoptosis, utriculi size, and skin elasticity in the Rhino mouse. J Investig Dermatol. 1997;109:370–376. doi: 10.1111/1523-1747.ep12336244. [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Carson D. Collagenase Santyl ointment: a selective agent for wound debridement. J Wound Ostomy Cont Nurs. 2009;36(Suppl.):S12–S16. doi: 10.1097/WON.0b013e3181bfdd1a. [DOI] [PubMed] [Google Scholar]
- 13.Hellgren L. Cleansing properties of stabilized trypsin and streptokinase-streptodornase in necrotic leg ulcers. Eur J Clin Pharmacol. 1983;24:623–628. doi: 10.1007/BF00542211. [DOI] [PubMed] [Google Scholar]
- 14.Brooks JL, Jefferson KK. Staphylococcal biofilms: quest for the magic bullet. Adv Appl Microbiol. 2012;81:63–87. doi: 10.1016/B978-0-12-394382-8.00002-2. [DOI] [PubMed] [Google Scholar]
- 15.Chaignon P, Sadovskaya I, Ragunah C, Ramasubbu N, Kaplan JB, Jabbouri S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl Microbiol Biotechnol. 2007;75:125–132. doi: 10.1007/s00253-006-0790-y. [DOI] [PubMed] [Google Scholar]
- 16.Marti M, Trotonda MP, Tormo-Mas MA, et al. Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect. 2010;12:55–64. doi: 10.1016/j.micinf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hangler M, Burmolle M, Schneider I, Allermann K, Jensen B. The serine protease Esperase HPF inhibits the formation of multispecies biofilm. Biofouling. 2009;25:667–674. doi: 10.1080/08927010903096008. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui KS, Cavicchioli R. Cold-adapted enzymes. Ann Rev Biochem. 2006;75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723. [DOI] [PubMed] [Google Scholar]
- 19.Lonhienne T, Gerday C, Feller G. Psychrophilic enzymes: revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim Biophys Acta. 2000;1543:1–10. doi: 10.1016/S0167-4838(00)00210-7. [DOI] [PubMed] [Google Scholar]
- 20.Aghajari N, Van Petegem F, Villeret V, et al. Crystal structures of a psychrophilic metalloprotease reveal new insights into catalysis by cold-adapted proteases. Proteins. 2003;50:636–647. doi: 10.1002/prot.10264. [DOI] [PubMed] [Google Scholar]
- 21.Gerday C, Aittaleb M, Bentahir M, et al. Cold-adapted enzymes: from fundamentals to biotechnology. Trends Biotechnol. 2000;18:103–107. doi: 10.1016/S0167-7799(99)01413-4. [DOI] [PubMed] [Google Scholar]
- 22.Asgeirsson B, Fox JW, Bjarnason JB. Purification and characterization of trypsin from the poikilotherm Gadus morhua. Eur J Biochem. 1989;180:85–94. doi: 10.1111/j.1432-1033.1989.tb14618.x. [DOI] [PubMed] [Google Scholar]
- 23.Osnes KK, Mohr V. On the purification and characterization of three anionic, serine-type peptide hydrolases from antarctic krill, Euphausia superba. Comp Biochem Physiol B. 1985;82:607–619. [Google Scholar]
- 24.Stefansson B, Helgadottir L, Olafsdottir S, Gudmundsdottir A, Bjarnason JB. Characterization of cold-adapted Atlantic cod (Gadus morhua) trypsin I—kinetic parameters, autolysis and thermal stability. Comp Biochem Physiol B: Biochem Mol Biol. 2010;155:186–194. doi: 10.1016/j.cbpb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Leiros HK, Willassen NP, Smalas AO. Structural comparison of psychrophilic and mesophilic trypsins. Elucidating the molecular basis of cold-adaptation. Eur J Biochem. 2000;267:1039–1049. doi: 10.1046/j.1432-1327.2000.01098.x. [DOI] [PubMed] [Google Scholar]
- 26.Collins T, Roulling F, Piette F, et al. Fundamentals of cold-adapted enzymes. Psychrophiles: from biodiversity to biotechnology. Berlin: Springer; 2008. pp. 211–227. [Google Scholar]
- 27.Smalas AO, Leiros HK, Os V, Willassen NP. Cold adapted enzymes. Biotechnol Ann Rev. 2000;6:1–57. doi: 10.1016/S1387-2656(00)06018-X. [DOI] [PubMed] [Google Scholar]
- 28.Johannsdottir UB. Activity of Atlantic cod trypsin towards cytokines and other proteins. PhD thesis, University of Iceland; 2009.
- 29.Huston AL. Biotechnological aspects of cold-adapted enzymes. Psychrophiles: from biodiversity to biotechnology. Berlin: Springer; 2008. pp. 347–363. [Google Scholar]
- 30.Marx JC, Collins T, D’Amico S, Feller G, Gerday C. Cold-adapted enzymes from marine Antarctic microorganisms. Mar Biotechnol. 2007;9:293–304. doi: 10.1007/s10126-006-6103-8. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH. Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol. 2000;297:1015–1026. doi: 10.1006/jmbi.2000.3612. [DOI] [PubMed] [Google Scholar]
- 32.Wintrode PL, Miyazaki K, Arnold FH. Patterns of adaptation in a laboratory evolved thermophilic enzyme. Biochim Biophys Acta. 2001;1549:1–8. doi: 10.1016/S0167-4838(01)00226-6. [DOI] [PubMed] [Google Scholar]
- 33.Taguchi S, Ozaki A, Momose H. Engineering of a cold-adapted protease by sequential random mutagenesis and a screening system. Appl Environ Microbiol. 1998;64:492–495. doi: 10.1128/aem.64.2.492-495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karan R, Capes MD, Dassarma S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat Biosyst. 2012;8:4. doi: 10.1186/2046-9063-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lollar P. Mapping factor VIII inhibitor epitopes using hybrid human/porcine factor VIII molecules. Haematologica. 2000;85(Suppl.):26–28. [PubMed] [Google Scholar]
- 36.Macouzet M, Simpson BK, Lee BH. Cloning of fish enzymes and other fish protein genes. Crit Rev Biotechnol. 1999;19:179–196. doi: 10.1080/0738-859991229233. [DOI] [PubMed] [Google Scholar]
- 37.Lee SG, Koh HY, Lee HK, Yim JH. Possible roles of Antarctic krill proteases for skin regeneration. Ocean Polar Res. 2008;30:467–472. doi: 10.4217/OPR.2008.30.4.467. [DOI] [Google Scholar]
- 38.Mekkes JR, Le Poole IC, Das PK, Bos JD, Westerhof W. Efficient debridement of necrotic wounds using proteolytic enzymes derived from Antarctic krill: a double-blind, placebo-controlled study in a standardized animal wound model. Wound Repair Regen. 1998;6:50–57. doi: 10.1046/j.1524-475X.1998.60108.x. [DOI] [PubMed] [Google Scholar]
- 39.Berg CH, Kalfas S, Malmsten M, Arnebrant T. Proteolytic degradation of oral biofilms in vitro and in vivo: potential of proteases originating from Euphausia superba for plaque control. Eur J Oral Sci. 2001;109:316–324. doi: 10.1034/j.1600-0722.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- 40.Hellgren K. Assessment of Krillase chewing gum for the reduction of gingivitis and dental plaque. J Clin Dent. 2009;20:99–102. [PubMed] [Google Scholar]
- 41.Hellgren K. Krill enzymes (Krillase) an important factor to improve oral hygiene. In: Virdi MS, editor. Oral health care—pediatric, research, epidemiology and clinical practice. Croatia: InTech; 2012.
- 42.Wang ES, Dobrikova E, Goetz C, Dufresne AT, Gromeier M. Adaptation of an ICAM-1-tropic enterovirus to the mouse respiratory tract. J Virol. 2011;85:5606–5617. doi: 10.1128/JVI.01502-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wat D. The common cold: a review of the literature. Eur J Intern Med. 2004;15:79–88. doi: 10.1016/j.ejim.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Angelo M, Visintin JA, Richtzenhain LJ, Goncalves RF. Evaluation of trypsin treatment on the inactivation of bovine herpesvirus type 1 on in vitro produced pre-implantation embryos. Reprod Dom Anim. 2009;44:536–539. doi: 10.1111/j.1439-0531.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 45.Piirainen L, Hovi T, Roivainen M. Variability in the integrity of human enteroviruses exposed to various simulated in vivo environments. Microb Pathog. 1998;25:131–137. doi: 10.1006/mpat.1998.0221. [DOI] [PubMed] [Google Scholar]
- 46.ColdZyme [product information]. Lund, Sweden: Enzymatica AB; 2011.
- 47.Hilmarsson H, Stefansson B, Bjarnason JB, Gudmundsdottir A. Virucidal activities of Penzyme against Herpes Simplex veiru type 1 (poster 928). COST (European Cooperation in Science and Technology) 928; March 2–4, 2010; Naples, Italy.