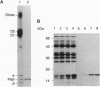
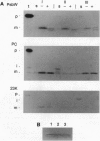
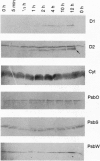
Abstract
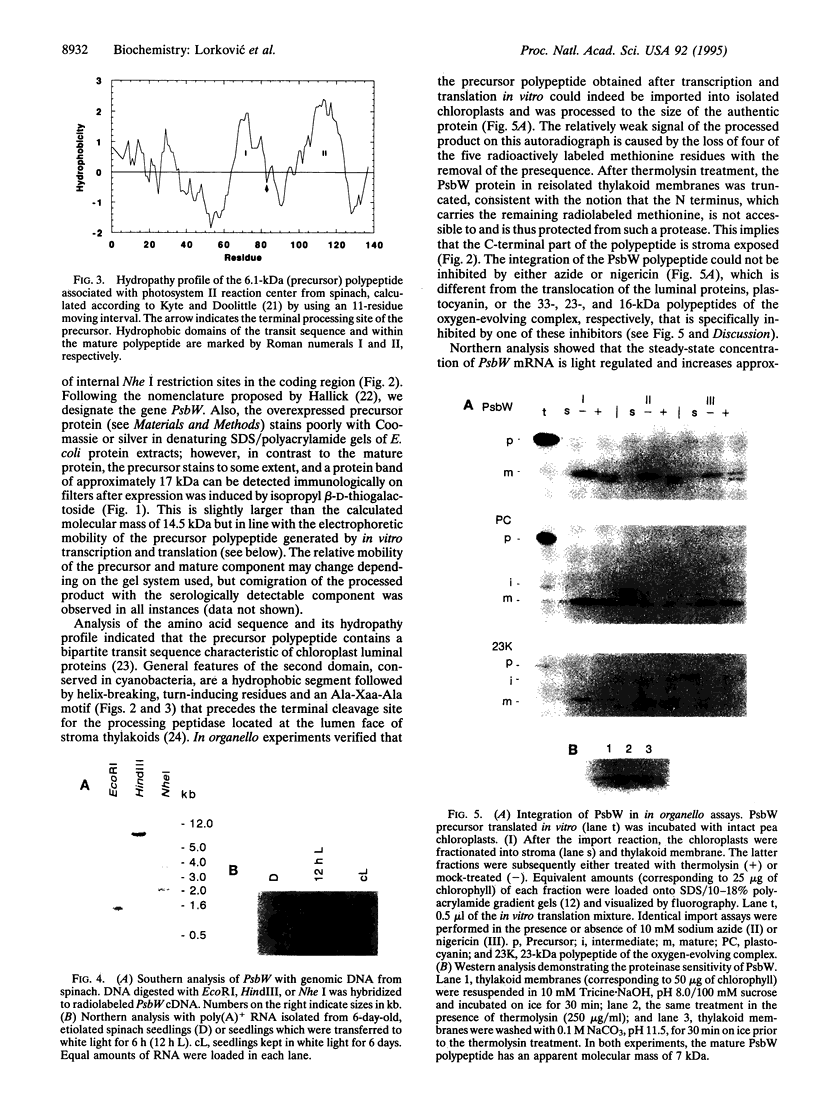
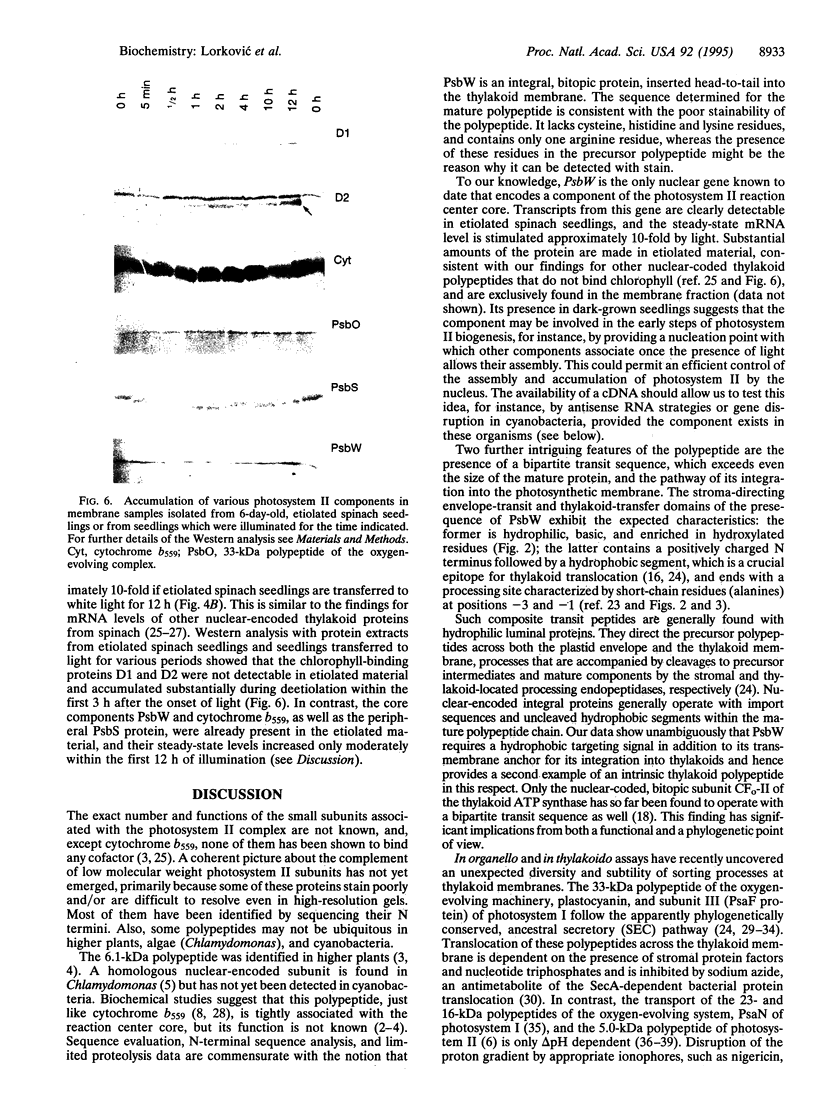
We describe the isolation and characterization of cDNAs encoding the precursor polypeptide of the 6.1-kDa polypeptide associated with the reaction center core of the photosystem II complex from spinach. PsbW, the gene encoding this polypeptide, is present in a single copy per haploid genome. The mature polypeptide with 54 amino acid residues is characterized by a hydrophobic transmembrane segment, and, although an intrinsic membrane protein, it carries a bipartite transit peptide of 83 amino acid residues which directs the N terminus of the mature protein into the chloroplast lumen. Thylakoid integration of this polypeptide does not require a delta pH across the membrane, nor is it azide-sensitive, suggesting that the polypeptide chain inserts spontaneously in an as yet unknown way. The PsbW mRNA levels are light regulated. Similar to cytochrome b559 and PsbS, but different from the chlorophyll-complexing polypeptides D1, D2, CP43, and CP47 of photosystem II, PsbW is present in etiolated spinach seedlings.
Full text
PDF
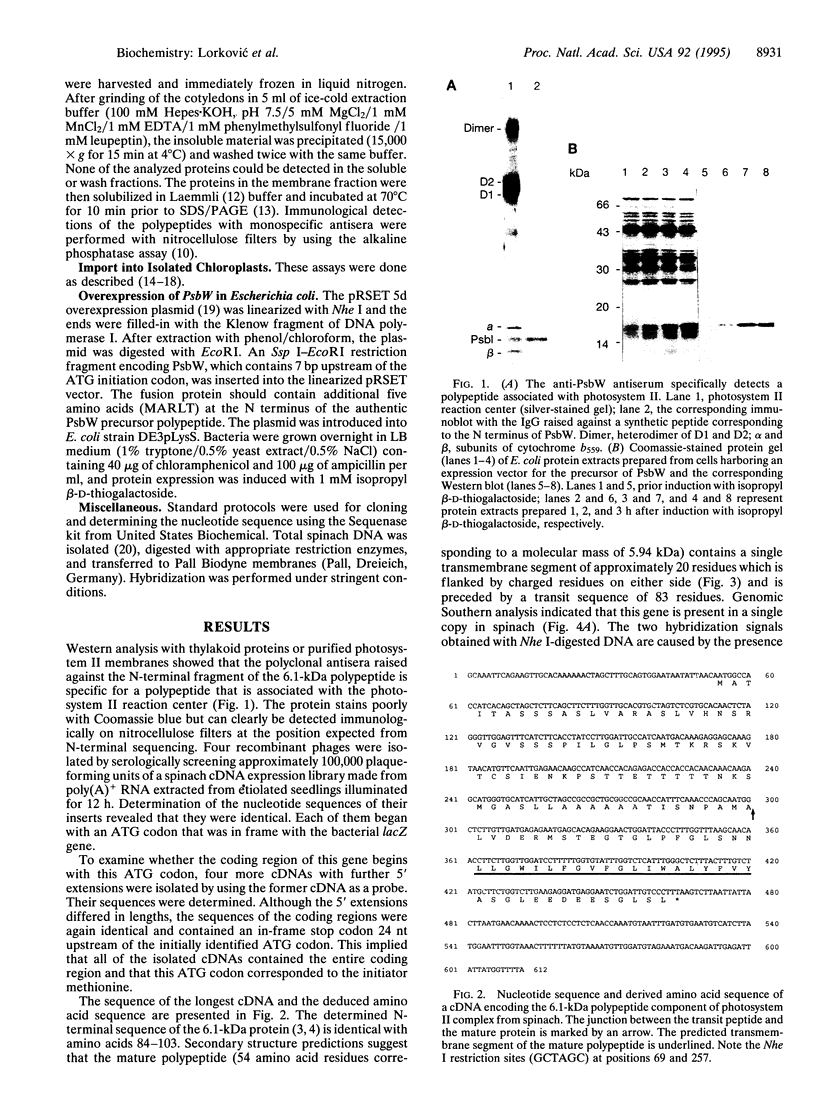

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs L. M., Pecoraro V. L., McIntosh L. Copper-induced expression, cloning, and regulatory studies of the plastocyanin gene from the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1990 Oct;15(4):633–642. doi: 10.1007/BF00017837. [DOI] [PubMed] [Google Scholar]
- Brock I. W., Hazell L., Michl D., Nielsen V. S., Møller B. L., Herrmann R. G., Klösgen R. B., Robinson C. Precursors of one integral and five lumenal thylakoid proteins are imported by isolated pea and barley thylakoids: optimisation of in vitro assays. Plant Mol Biol. 1993 Nov;23(4):717–725. doi: 10.1007/BF00021527. [DOI] [PubMed] [Google Scholar]
- Chitnis P. R., Purvis D., Nelson N. Molecular cloning and targeted mutagenesis of the gene psaF encoding subunit III of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1991 Oct 25;266(30):20146–20151. [PubMed] [Google Scholar]
- Clausmeyer S., Klösgen R. B., Herrmann R. G. Protein import into chloroplasts. The hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993 Jul 5;268(19):13869–13876. [PubMed] [Google Scholar]
- Cline K., Ettinger W. F., Theg S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992 Feb 5;267(4):2688–2696. [PubMed] [Google Scholar]
- Cline K., Henry R., Li C., Yuan J. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 1993 Nov;12(11):4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flieger K., Tyagi A., Sopory S., Cseplö A., Hermann R. G., Oelmüller R. A 42 bp promoter fragment of the gene for subunit III of photosystem I (psaF) is crucial for its activity. Plant J. 1993 Jul;4(1):9–17. doi: 10.1046/j.1365-313x.1993.04010009.x. [DOI] [PubMed] [Google Scholar]
- Henry R., Kapazoglou A., McCaffery M., Cline K. Differences between lumen targeting domains of chloroplast transit peptides determine pathway specificity for thylakoid transport. J Biol Chem. 1994 Apr 8;269(14):10189–10192. [PubMed] [Google Scholar]
- Hulford A., Hazell L., Mould R. M., Robinson C. Two distinct mechanisms for the translocation of proteins across the thylakoid membrane, one requiring the presence of a stromal protein factor and nucleotide triphosphates. J Biol Chem. 1994 Feb 4;269(5):3251–3256. [PubMed] [Google Scholar]
- Ikeuchi M., Takio K., Inoue Y. N-terminal sequencing of photosystem II low-molecular-mass proteins. 5 and 4.1 kDa components of the O2-evolving core complex from higher plants. FEBS Lett. 1989 Jan 2;242(2):263–269. doi: 10.1016/0014-5793(89)80482-x. [DOI] [PubMed] [Google Scholar]
- Karnauchov I., Cai D., Schmidt I., Herrmann R. G., Klösgen R. B. The thylakoid translocation of subunit 3 of photosystem I, the psaF gene product, depends on a bipartite transit peptide and proceeds along an azide-sensitive pathway. J Biol Chem. 1994 Dec 30;269(52):32871–32878. [PubMed] [Google Scholar]
- Klösgen R. B., Brock I. W., Herrmann R. G., Robinson C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992 Mar;18(5):1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Reddy K. J., Sherman L. A. Nucleotide sequence of the gene from the cyanobacterium Anacystis nidulans R2 encoding the Mn-stabilizing protein involved in photosystem II water oxidation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8230–8234. doi: 10.1073/pnas.84.23.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michl D., Robinson C., Shackleton J. B., Herrmann R. G., Klösgen R. B. Targeting of proteins to the thylakoids by bipartite presequences: CFoII is imported by a novel, third pathway. EMBO J. 1994 Mar 15;13(6):1310–1317. doi: 10.1002/j.1460-2075.1994.tb06383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould R. M., Robinson C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991 Jul 5;266(19):12189–12193. [PubMed] [Google Scholar]
- Mould R. M., Shackleton J. B., Robinson C. Transport of proteins into chloroplasts. Requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991 Sep 15;266(26):17286–17289. [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen V. S., Mant A., Knoetzel J., Møller B. L., Robinson C. Import of barley photosystem I subunit N into the thylakoid lumen is mediated by a bipartite presequence lacking an intermediate processing site. Role of the delta pH in translocation across the thylakoid membrane. J Biol Chem. 1994 Feb 4;269(5):3762–3766. [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Cai D., Hulford A., Brock I. W., Michl D., Hazell L., Schmidt I., Herrmann R. G., Klösgen R. B. The presequence of a chimeric construct dictates which of two mechanisms are utilized for translocation across the thylakoid membrane: evidence for the existence of two distinct translocation systems. EMBO J. 1994 Jan 15;13(2):279–285. doi: 10.1002/j.1460-2075.1994.tb06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Klösgen R. B. Targeting of proteins into and across the thylakoid membrane--a multitude of mechanisms. Plant Mol Biol. 1994 Oct;26(1):15–24. doi: 10.1007/BF00039516. [DOI] [PubMed] [Google Scholar]
- Seibert M., Picorel R., Rubin A. B., Connolly J. S. Spectral, Photophysical, and Stability Properties of Isolated Photosystem II Reaction Center. Plant Physiol. 1988 Jun;87(2):303–306. doi: 10.1104/pp.87.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- de Vitry C., Diner B. A., Popo J. L. Photosystem II particles from Chlamydomonas reinhardtii. Purification, molecular weight, small subunit composition, and protein phosphorylation. J Biol Chem. 1991 Sep 5;266(25):16614–16621. [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]