Abstract
Cystic fibrosis (CF) is the most common genetic disease affecting the Caucasian population. Chronic Pseudomonas aeruginosa pulmonary infection is the major cause of morbidity and mortality in CF patients. Human beta-defensin-2 (hBD-2) is an inducible pulmonary antimicrobial peptide that exerts bacteriostatic activity in a concentration-dependent manner. The decreased expression and compromised function of hBD-2 contributes to the pathogenesis of P. aeruginosa infection in the CF lung. The purpose of this review is to outline the significance of hBD-2 in P. aeruginosa chronic pulmonary infection in CF patients.
Keywords: Antimicrobial peptides, Cathepsins, Cystic fibrosis, Human beta-defensin-2, Innate immunity, Neutrophil infiltration, Pseudomonas aeruginosa, Pulmonary infection, Toll-like receptor tolerance
Introduction
The average human inhales ~10,000 L of air every day. Respiration is a portal of entry for not only atmospheric gases, but also for harmful particulate pervasive in the environment. The pulmonary epithelium is therefore continually exposed to microorganisms, but remains sterile under normal physiologic conditions. This remarkable phenomenon is a testament to the innate immune defenses that provide a silent mode of broad immune protection. The importance of the innate immune system in protecting the lungs from infection is clearly illustrated in the pathologic condition that arises in cystic fibrosis (CF) (mucoviscidosis), which severely damages the pulmonary innate immune defenses [1].
Cystic fibrosis is the most common lethal genetic disorder affecting the Caucasian population, with an incidence of 1 in 2,500 births [2]. CF is caused by an autosomal recessive mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene within chromosome seven [3]. This mutation results in the functional defect in the cyclic adenosine monophosphate stimulated pulmonary chloride pump causing abnormal ion transport in epithelial cells [4, 5]. CF is therefore a disease of ion transport across the epithelium, affecting fluid secretion in exocrine glands and the epithelium of the respiratory, reproductive, and gastrointestinal tracts [6]. Although CF causes a multitude of pathophysiologic effects, the most significant effect is the impaired ciliary clearance that results in the accumulation of mucus in the lung, creating a haven for bacteria. Moreover, the dehydrating conditions in the lung caused by the elevated levels of sodium chloride in the airway secretions severely weaken the host pulmonary innate defenses. The initial acute pulmonary infection of the CF lung is typically a result of colonization by Haemophilus influenzae and Staphylococcus aureus, while the ensuing chronic infection is caused by Pseudomonas aeruginosa [7, 8]. The chronic infection in the lungs of CF patients caused by P. aeruginosa is responsible for the high rate of morbidity and mortality associated with this genetic disease [9].
Pseudomonas aeruginosa is a ubiquitous, antibiotic resistant, Gram-negative opportunistic bacterium [10]. At 6.3 million base pairs, the PAO1 strain of P. aeruginosa has the largest genome sequenced [11]. This large genome provides the genetic machinery that enables P. aeruginosa to readily undergo significant genetic and phenotypic transformations in response to environmental changes, contributing to its versatility and antibiotic resistance potential. Although P. aeruginosa is pervasive in the environment, it only causes infection in immunodeficient hosts, e.g., CF patients, patients with acquired immunodeficiency syndrome, burn victims, etc. Among the many clinical manifestations of P. aeruginosa infection, P. aeruginosa’s opportunistic mode of infection is most known in the chronic pulmonary infection that is the hallmark of CF [12]. Once acquired, P. aeruginosa almost always colonizes the lungs of CF patients for life [13].
Human beta-defensin-2 (hBD-2) is a Major Effector of Innate Immunity
The innate immune system provides the first line of defense against microorganisms pervasive in the environment. Unlike the adaptive immune system, innate immunity is non-specific, lacks memory, and is not influenced by previous exposure. Antimicrobial peptides (AMPs) are cationic endogenous antibiotic proteins expressed throughout the epithelium that are effectors of the innate immune system. AMPs exert antimicrobial activity in a concentration-dependent manner, making their expression a critical factor in host defense [14]. The amphiphathic nature of AMPs contributes to their effectiveness at interacting with hydrophobic and anionic components of the bacterial membrane [15]. Cathelicidins, α-defensins, β-defensins, and θ-defensins are among the major classes of human AMPs [16].
Beta-defensins are at the interface between the adaptive and innate immune systems; beta-defensins exhibit chemotactic function towards immature dendritic cells, memory T cells expressing the chemokine receptor CCR6, neutrophils primed with tumor necrosis factor (TNF)-α, and mast cells [17, 18]. Individual beta-defensins have specific antimicrobial activity. Among the various types of defensin AMPs, only the expression of human beta-defensin-2 (hBD-2) and human beta-defensin-3 (hBD-3) is increased following stimulation by pro-inflammatory cytokines; all other defensin AMPs are continuously expressed [19]. However, although the expression of hBD-2 and hBD-3 can be stimulated by pro-inflammatory cytokines, e.g., TNF-α, interleukin (IL)-1β, IL-17, and IL-22, these antimicrobial peptides are still expressed in unstimulated cells in basal amounts [20, 21]. An additional difference between these two AMPs that are induced by humoral stimulation is that hBD-2 primarily targets Gram-negative bacteria, such as P. aeruginosa, while hBD-3 exerts broad bacteriostatic activity against both Gram-positive and Gram-negative bacteria [22]. hBD-2, like all defensins, is found throughout the epithelium of mammals. However, hBD-2 is most concentrated in the epithelia of the lung, tonsils, and trachea, and therefore plays a critical role in the prevention of pulmonary infection [23, 24]. The inducible properties of hBD-2 suggest it plays a significant role in innate immune defense.
Human beta-defensin-2 is a cationic, 41 amino acid, 4 kDa, AMP intricately involved in the innate immune response of vertebrates that works synergistically with other antimicrobial molecules, such as lactoferrin and lysozyme [24, 25]. Like other beta-defensins, hBD-2 is a monomeric protein containing six conserved cysteine residues forming three core disulfide bonds [26]. The initial contact between hBD-2 and invading microorganisms is an electrostatic amphipathic attraction between the cationic AMP and the negatively charged phospholipid groups of the bacterium’s phospholipid bilayer [27, 28]. Following initial electrostatic attraction, hBD-2 exerts its antimicrobial effects through insertion within the phospholipid bilayer disrupting the membrane integrity of the invading bacteria resulting in the collapse of membrane potential and death of the invading pathogen [29]. Nuclear magnetic resonance (NMR) analysis of the crystal structures of hBD-2 suggests that the formation of a hBD-2 octamer is a prerequisite to the binding of the bacteria cell surface and subsequent increases in membrane permeability [30].
Decreased hBD-2 Expression Occurs in Chronic P. aeruginosa Infection
A common theme in pathogen—host interactions is the selection against virulence factors required for the establishment of infection, as the stage the infection shifts from acute to chronic. Genetic variants are selected that promote long-term survivability and clonal expansion, while variants that no longer provide a survival advantage are selected against. In the CF lung, P. aeruginosa undergoes significant genetic and phenotypic transformations in response to changes in the pulmonary milieu. P. aeruginosa mutates to a mucoid, flagella-deficient phenotype over the course of chronic pulmonary infection [31, 32]. The changes in the expression of P. aeruginosa virulence factors affect the expression of hBD-2 in the pulmonary epithelium that weakens the innate immune defense of the lung [33].
Flagellum is a structure common to most Gram-negative bacteria derived from flagellin monomers that confers motility, promotes adhesion, and consequently is a significant bacterial virulence factor [34]. Flagellum is a bacterial ligand that is detected by toll-like receptor (TLR) 5 [35]. The activation of TLR5 by flagellum initiates an inflammatory response that includes the up-regulation of hBD-2 via a nuclear factor (NF)-κB dependent pathway in airway epithelial cells [21]. The loss of flagella expression during the transition to the mucoid phenotype allows P. aeruginosa to evade the antimicrobial activity of hBD-2 through decreased TLR5 stimulation, contributing to P. aeruginosa’s pathogenesis in the CF lung [21, 35].
Some bacterial virulence factors remain expressed throughout different stages of infection. Although P. aeruginosa isolates from the chronic stage of pulmonary infection are flagella-deficient, other virulence factors, which are TLR agonists and stimulate hBD-2 expression, remain expressed. For example, lipopolysaccharide (LPS) is an endotoxin attached to the outer membrane of Gram-negative bacteria that is an agonist of TLR 4 [36]. Although LPS expression does not decrease as pulmonary infection shifts from the acute to chronic stage, the cellular responsiveness to LPS decreases.
A study involving the exposure of airway epithelial cells to a regime of two discrete bacterial infections demonstrated reduced TLR responsiveness in the second bacterial challenge due to down-regulation of the IRAK1 signaling protein, which is involved in NF-κB activation [37]. IRAK1 phosphorylation leads to the activation of NF-κB and AP-1, which are two transcription factors that induce the up-regulation of IL-8 and hBD-2 in airway epithelial cells [38]. Although this in vitro model only measured the production of IL-8, not hBD-2, these results provide a mechanistic explanation for the reduced levels of hBD-2 expression in the chronic stage of pulmonary infection in CF patients [39]. Furthermore, the reduced expression of hBD-2 in the lung in advanced chronic pulmonary infection (owing to decreased TLR responsiveness) provides further insight as to why P. aeruginosa only colonizes the lung post-S. aureus and H. influenzae infection. Moreover, this underscores the potential influence of hBD-2 in the progression of chronic pulmonary infection in CF patients. The down-regulation of TLR4 expression in the airway epithelia in response to acute infection may result in reduced hBD-2 expression, promoting P. aeruginosa colonization [40].
Neutrophil and Macrophage Infiltration Contribute to Degradation of hBD-2 in the CF Lung
Inflammation is a protective tissue response to infection or injury. In the context of the CF lung, the inflammatory responses induced by P. aeruginosa severely damage the pulmonary epithelium. Exposure of the airway epithelium to P. aeruginosa induces the expression of the potent neutrophil chemokine IL-8, initiating neutrophil infiltration [41].
Neutrophils are granulocytic polymorphonuclear leukocytes that play a key role in innate defense [42]. However, in the CF lung the abnormal accumulation and persistence of neutrophils produces an inflammatory response that severely damages the lung [43, 44]. The quorum-sensing controlled production of rhamnolipid by P. aeruginosa induces rapid necrotic killing of invading neutrophils, which explains why the neutrophils do not significantly contribute to the elimination of P. aeruginosa in the CF lung [45–47]. In the CF lung, infiltrating neutrophils and most P. aeruginosa strains secrete elastase—a serine protease that exerts diverse biological effects that contribute significantly to the progression of pulmonary CF disease [48, 49]. Elastase is a potent protease that exerts antimicrobial activity against most Gram-negative bacteria, but not against P. aeruginosa [50]. The viability and morphology of P. aeruginosa remains unaltered even when exposed to neutrophil elastase (NE) concentrations as high as 25 μM, which is commonly present in the CF lung [51]. After a short life span, neutrophils succumb to apoptosis and subsequent phagocytotic clearance by macrophages [13].
Cathepsins are cysteine proteases secreted by macrophages that are involved in the remodeling of the extracellular matrix [52]. Pulmonary macrophage influx occurs in response to the elevated levels of apoptotic neutrophils in the lungs of CF patients resulting in cathepsin secretion into the bronchoalveolar fluid (BAF) of the CF lung [51, 53]. Beta-defensins have a conserved core structure of three disulfide bridges, which are susceptible to proteolytic cleavage by cathepsins present in the BAF [54]. Specifically, cathepsins B, L, and S have been found to cleave the disulfide bonds of hBD-2 and hBD-3 resulting in their degradation and loss of antimicrobial activity [30]. In addition to the high concentrations of cathepsins in the BAF of the CF lung, the low pH of the CF BAF promotes optimal enzymatic activity for cathepsin proteolytic activity; most cathepsins have optimal proteolytic function in acidic pH and lose their proteolytic properties at physiologic pH [52]. The BAF of CF patients is acidic because of impaired bicarbonate transport across the pulmonary epithelium caused by the CFTR mutation [55]. Furthermore, the elevated [Cl−] present in the BAF resulting from the functional CFTR defect reduces the efficacy of hBD-2 due to the reduced electrostatic interaction between the cationic hBD-2 peptide and the anionic resting membrane potential of invading microorganisms [24]. The overexpression of cathepsins during chronic pulmonary infection may cause increased degradation of hBD-2, promoting bacterial colonization and infection [30].
Conclusion
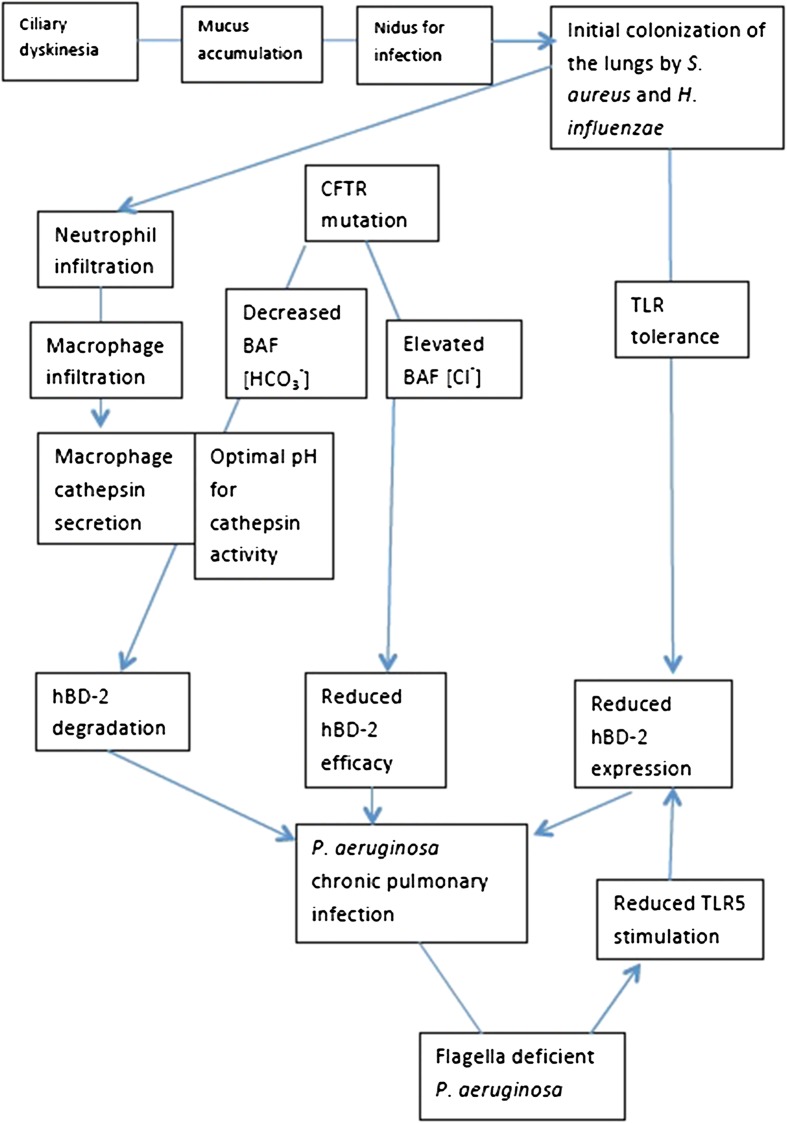
Many factors contribute to the pathogenesis of P. aeruginosa in the lungs of CF patients (Fig. 1). It is becoming increasingly evident that the regulation of hBD-2 expression and degradation has profound implications in pulmonary infections. hBD-2 is an indicator of inflammation and an essential component of the innate immune system. The regulation of hBD-2 activity, expression, and prevention of degradation (cathepsin inhibitors) are potential therapeutic options for CF patients that may decrease the high rates of morbidity and mortality associated with this common genetic disease.
Fig. 1.
Compromised hBD-2 function in the CF lung promotes chronic pulmonary infection by the opportunistic pathogen P. aeruginosa
Acknowledgments
This project was funded by an Undergraduate Student Research Award from the Natural Sciences and Engineering Research Council of Canada. Dalcin is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Dalcin and Dr Ulanova declare no conflict of interest.
Compliance with ethics
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodge JA, Morison S, Lewis PA, et al. Incidence, population, and survival of cystic fibrosis in the UK, 1968–95. Arch Dis Child. 1997;77:493–496. doi: 10.1136/adc.77.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rommens JM, Lannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 4.Bobadilla JL, Macek M, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Song K, Painter RG, et al. Cystic fibrosis transmembrane conductance regulatory recruitment to phagosomes in neutrophils. J Innate Immun. 2013;5:219–230. doi: 10.1159/000346568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 7.Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect. 2002;17:47–56. doi: 10.1053/srin.2002.31690. [DOI] [PubMed] [Google Scholar]
- 8.Hoiby N, Frederiksen B. Microbiology of cystic fibrosis. In: Hodson ME, Geddes DM, editors. Cystic Fibrosis. London: Arnold; 2000, p. 83–107.
- 9.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 10.Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27:93–99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 11.Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 12.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1253. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 13.Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax. 2005;60:659–664. doi: 10.1136/thx.2004.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol. 2004;186:545–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro da Silva F, Machado MC. Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides. 2012;36:308–314. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 18.Boniotto M, Jordan WJ, Eskdale J, et al. Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2006;50:1433–1441. doi: 10.1128/AAC.50.4.1433-1441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsustumi-Ishii Y, Nagaoka I. NF-kappa B-mediated transcriptional regulation of human beta-defensin-2 gene following lipopolysaccharide stimulation. J Leukoc Biol. 2002;71:154–162. [PubMed] [Google Scholar]
- 20.Bals H, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005;73:7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harder J, Meyer-Hoffert U, Teran LM, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1 beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 24.Bals R, Wang X, Wu Z, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schröder JM, Harder J. Human beta-defensin-2. Int JBiochem Cell Biol. 1999;31:645–651. doi: 10.1016/S1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 26.Schibli DJ, Hunter HN, Aseyev V, et al. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J Biol Chem. 2002;277:8279–8289. doi: 10.1074/jbc.M108830200. [DOI] [PubMed] [Google Scholar]
- 27.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 28.Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Corrales-Garcia LL, Possani LD, Corzo G. Expression systems of human β defensins: vectors, purification and biological activities. Amino Acids. 2011;40:5–13. doi: 10.1007/s00726-010-0493-7. [DOI] [PubMed] [Google Scholar]
- 30.Taggart CC, Greene CM, Smith SG, et al. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–937. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 31.Smith EE, Buckley DG, Wu Z, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jelsbak L, Johansen HK, Frost AL, et al. Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:2214–2224. doi: 10.1128/IAI.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobb LM, Mychaleckyj JC, Wozniak DJ, Lopez-Boado YS. Pseudomonas aeruginosa flagellin and alginate elicit very different gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease. J Immunol. 2004;173:5659–5670. doi: 10.4049/jimmunol.173.9.5659. [DOI] [PubMed] [Google Scholar]
- 34.Soutourina OA, Bertin PN. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol Rev. 2006;274:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 36.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;288:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 37.Wu Q, Lu Z, Verghese MW, Randell SH. Airway epithelial cell tolerance to Pseudomonas aeruginosa. Respir Res. 2005;6:26. doi: 10.1186/1465-9921-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehkamp J, Harder J, Wehkamp K, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros. 2004;3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.MacRedmond R, Greene C, Taggart CC, McElvaney N, O’Neill S. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir Res. 2005;6:1–11. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene CM, Carroll TP, Smith SG, et al. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 42.Baggiolini M, Dewald B. The neutrophil. Int Arch Allergy Immunol. 1985;76:13–20. doi: 10.1159/000233730. [DOI] [PubMed] [Google Scholar]
- 43.Doring G. The role of neutrophil elastase in chronic inflammation. Am JRespir Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 44.Dunlevy FK, Martin SL, de Courcey F, Elborn JS, Ennis M. Anti-inflammatory effects of DX-890, a human neutrophil elastase inhibitor. J Cyst Fibros. 2012;11:300–304. doi: 10.1016/j.jcf.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Jensen PO, Bjarnsholt T, Phipps R, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 46.Alhede M, Bjarnsholt T, Jensen PO, et al. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology. 2009;155:3500–3508. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
- 47.Van Gennip M, Christensen LD, Alhede M, et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS. 2009;117:537–546. doi: 10.1111/j.1600-0463.2009.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wretlin B, Pavlovskis OR. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. RevInfect Dis. 1983;5(Suppl 5):S998–S1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]
- 49.Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–614. doi: 10.1358/dnp.2006.19.10.1068008. [DOI] [PubMed] [Google Scholar]
- 50.Sonawane A, Jyot J, During R, Ramphal R. Neutrophil elastase, an innate immunity effector molecule, represses flagellin transcription in Pseudomonas aeruginosa. 2006. Infect Immun. 2006;74:6682–6689. doi: 10.1128/IAI.00922-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger M. Inflammation in the lung in cystic fibrosis. A vicious cycle that does more harm than good? Clin Rev Allergy. 1991;9:119–142. doi: 10.1007/978-1-4612-0475-6_8. [DOI] [PubMed] [Google Scholar]
- 52.Wolters PJ, Chapman HA. Importance of lysosomal cysteine proteases in lung disease. Respir Res. 2000;1:170–177. doi: 10.1186/rr29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich M, Worlitzsch D, Viglio S, et al. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoover DM, Rajashankar KR, Blumenthal R, et al. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275:32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 55.Tate S, MacGregor G, Davis M, Innes JA, Greening AP. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002;57:926–929. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]