Abstract
Introduction
The purpose of this study was to investigate the safety and efficacy of transcorneal electrical stimulation (TES) in patients suffering from retinal artery occlusion (RAO).
Methods
Twelve patients with central and one patient with branch RAO (age 25–84 years, median 74 years) were enrolled in this prospective, randomized, sham-controlled study. RAO was diagnosed 10 days to 17 months prior to study participation. Patients were treated with TES (5 ms positive followed by 5 ms negative biphasic pulses at 20 Hz; applied with DTL electrodes) for 30 min once a week for 6 consecutive weeks. Patients were randomly assigned to TES with 0 mA (sham, n = 3), 66% (n = 5) or 150% (n = 5) of the patient’s individual electrical phosphene threshold (EPT) at 20 Hz. Best corrected visual acuity, ophthalmology examination and EPT (at 3, 6, 9, 20, 40, 60, and 80 Hz) were determined at baseline and at eight follow-up visits over 17 weeks. During four visits (week 1, 5, 9, and 17) kinetic and static visual fields as well as full-field and multifocal electroretinography were measured. The method of restricted maximum likelihood (P < 0.05, Tukey–Kramer) was used to estimate the development of parameters under treatment.
Results
TES was tolerated well; no ocular or systemic adverse events were observed except for foreign-body sensation after TES (n = 3). During the study period the slopes of the scotopic a-wave increased significantly (high-intensity flash white 10 cd.s/m2; P = 0.03) in the 150% treatment group. All other parameters in all other groups remained statistically unchanged.
Conclusions
Although TES was tolerated well, statistically significant improvements were found only for specific a-wave slopes. This is in contradiction to previous smaller, uncontrolled reports. Further studies with larger sample sizes and longer duration might, however, show additional significant effects.
Keywords: Electrophysiology, Growth factors, Neurotrophic factors, Ophthalmology, Retinal artery occlusion, Transcorneal electrical stimulation
Introduction
Retinal artery occlusion (RAO) causes sudden, painless visual loss and leads to irreversible profound retinal damage in the majority of cases [1]. Two types of RAO are commonly found: central RAO (CRAO) and branch RAO (BRAO). CRAO usually results in a dramatic, permanent decrease in visual function [1, 2]. BRAO leads to visual field defects corresponding to the ischemic retinal areas, and if the occlusion does not involve the fovea, visual acuity may not be impaired at all [2].
CRAO is an ophthalmic emergency with treatment often recommended to start as soon as possible, as experimental data show that CRAO lasting for approximately 240 min results in massive, irreversible retinal damage [3]. Since the first detailed description of CRAO in 1859 by von Graefe [4], various therapies have been proposed for the acute phase of nonarteritic CRAO and claimed to be beneficial for visual outcome: anterior chamber paracentesis [5, 6]; ocular massage [7]; intravenous injection of urokinase or plasminogen activator [8]; and also local injection of plasminogen activator [9]. However, Fraser and Siriwardena [10] found that treatments for acute nonarteritic CRAO reported in the literature were of unproven efficacy and concluded that there was currently not enough evidence to determine which, if any, interventions had beneficial effects.
Recently, studies have shown that stimulation with weak electrical currents could have positive effects on degenerative ophthalmologic diseases such as retinitis pigmentosa (RP) [11], nonarteritic anterior ischemic optic neuropathy or traumatic optic neuropathy [12], and also RAO [13–15]. The mechanism for these positive effects has been suggested to be a favorable regulation of neurotropic factors; electrical stimulation has been linked to the upregulation of neurotrophins in the central and peripheral nervous systems [16–18], whereas in animal experiments, electrical stimulation has been shown to be beneficial for the survival of photoreceptors in Royal College of Surgeon’s rats [19] promoting the survival of photoreceptors and improving retinal function in rhodopsin P347L transgenic rabbits [20], rescuing ganglion cells after optic nerve injury in Wistar rats [21, 22], and preserving retinal cells after light-induced retinal damage in Sprague–Dawley rats [23–25].
The neuroprotective effects of electrical stimulation have been attributed to growth factors, such as insulin-like growth factor-1 [21, 26], fibroblast growth factor-2 [27, 28], ciliary neurotrophic factor [29, 30], and brain-derived neurotrophic factor [21, 31]. Also, an overexpression of neuroprotective genes, e.g., B cell lymphoma-2 gene [32], has been reported. Moreover, electrical stimulation leads to reduced expression of some tumor necrosis factor and BAX genes; the latter are related to apoptosis signaling in retinal cells [33].
Another possible mechanism for the positive effects of electrical stimulation could be the associated increase in chorioretinal blood circulation, which has been demonstrated recently [34].
Transcorneal electrical stimulation (TES) in patients with nonarteritic anterior ischemic optic neuropathy and traumatic optic neuropathy [12], longstanding RAO [13, 14] and BRAO [15] has demonstrated positive effects on visual acuity, visual field, and multifocal electroretinography (mfERG) in nonrandomized studies with a small number of patients.
Our recently published favorable results for TES in RP patients [11] inspired us to investigate the efficacy of TES in patients suffering from RAO in a prospective, randomized, sham-controlled, partially blinded, and Good Clinical Practice (GCP)-conforming study.
Materials and Methods
Patients
Patients were recruited from the outpatient clinic and electronic medical records of the Centre for Ophthalmology, University of Tübingen, Germany. Inclusion criteria were age >18 years and best corrected visual acuity (BCVA) between light perception and 0.7 logMAR.
Exclusion criteria were the presence of other ophthalmologic diseases, particularly proliferative retinal diseases, such as diabetic retinopathy, retinal or choroidal neovascularization, exudative age-related macular degeneration, silicone oil tamponade, and severe general diseases. Giant cell arteritis was ruled out by detailed history, normal serum levels of C-reactive protein, and normal age-adjusted blood sedimentation rate.
Study Design
The study was performed at the Centre for Ophthalmology, University of Tübingen, Germany. The protocol was approved by the local ethics committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
The study was conducted according to the standards of GCP, the European Union Directive for Medical Devices, and the German Medical Product Law. The trial was supervised by STZ eyetrial, a clinical trial unit at the Centre for Ophthalmology, University of Tübingen, which is a certified member of the European Vision Institute, Clinical Research Network (European Vision Institute Research Network, Coimbra, Portugal). STZ eyetrial provided study documents, performed regular monitoring visits, and controlled all study documentation including adverse events (AEs). All documents, especially inclusion criteria and study logs, were reviewed; all electronic case report form data were monitored, and source data verification was conducted according to the International Conference on Harmonisation (Geneva, Switzerland) and GCP guidelines.
Patients were seen at nine visits over a period of 17 weeks: one baseline visit (visit 1), followed by six consecutive weekly visits (visits 2–7) including application of TES (dates varied by ±2 days) and two follow-up visits (visits 8 and 9), which occurred 2 weeks (visit 8) and 11 weeks (visit 9) after visit 7 (the last visit varied by ±10 days).
After the screening visit, patients were randomly assigned to TES with 0 mA (sham), 66% of the patient’s individual electrical phosphene threshold (EPT) at 20 Hz (66%), or 150% of the patient’s individual EPT (150%) at 20 Hz.
Patients and technicians who performed static and kinetic perimetry, as well as full-field ERG and mfERG, were blinded to the treatment group for the entire study period. The physicians who performed all other examinations and TES were not blinded to study treatment because they were responsible for setting the stimulation parameters.
Clinical Examinations
At all nine visits, ophthalmologic examinations were performed: BCVA assessment using a Snellen projector (SCP-660®, Nidek Inc., Freemont, CA, USA) at a viewing distance of 6 m; retro-illuminated Early Treatment Diabetic Retinopathy Study charts (ETDRS, ETDRS® Visual Acuity Tester, Steinbeis-Transferzentrum, Tübingen, Germany) at a viewing distance of 2 m distance; for both Snellen and ETDRS examinations, a viewing distance of 1 m was used if the visual acuity at 2 and 6 m was immeasurable. Also performed were slit-lamp biomicroscopy, fundus examination, and Goldmann applanation tonometry (AT 900®, Haag-Streit, Wedel, Germany). At visits 1, 2, 5, 8, and 9, static and kinetic perimetry were recorded. Perimetry was performed with an Octopus 900® perimeter (Haag-Streit, Wedel, Germany); background luminance was 10 cd/m² using white-on-white stimuli and a fast threshold strategy (German Adaptive Thresholding Estimation) [35] up to 85° eccentricity. For kinetic perimetry, white stimuli (Goldmann III4e with constant angular velocity of 3°/s) up to 90° eccentricity were presented every 15°. Isopter and scotoma areas (in degrees²) were quantified using the machine’s built-in software algorithm. As a quality criterion for kinetic perimetry, the blind spot was detected with at least five stimuli (Goldmann I4e) at 2°/s.
All patients underwent initial visual field testing at the screening visit and once again at visit 1. Generally, only the test at visit 1 was recorded.
At visits 1, 5, 8, and 9, ERG and mfERG were conducted according to the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV) [36, 37]. ERG was recorded using an Espion E2® (Diagnosys LLC, Cambridge, UK) and a ColorDome® (Diagnosys LLC, Cambridge, UK). The protocol consisted of six steps with stimulus strengths from 0.01 to 10 cd.s/m2 and 4 ms duration (white 6500K). Three single-flash stimuli were used under scotopic conditions (stimulus strength 0.01 cd.s/m2; rod response), 3 cd.s/m2 (standard flash, combined rod-cone response), and 10 cd.s/m2 (high-intensity flash) as a scotopic protocol. A single flash stimulus with 3 cd.s/m2 (single-flash cone response) and a 30 Hz flicker were chosen as a photopic protocol. The impedance level was <10 kΩ. A wide-range band-pass filter (from 0.3 to 300 Hz) was applied using the machine’s built-in algorithm. ERG potentials <5 μV were excluded from the analysis.
To perform mfERG, a VerisTM®-System (Electro-Diagnostic Imaging Inc., Redwood City, CA, USA; software version Veris Science TM 5.1) and a 21′′ screen (“UHR21L,” Nortech Imaging Technologies, Plymouth, MN, USA; resolution 1,024 × 768) positioned 32 cm in front of the patient were used. The stimulus consisted of 61 scaled hexagonal elements presented with alternating black (5 cd/m2) and white (100 cd/m2) fields covering a central visual field of 60 × 55°. The built‐in algorithm allowed recordings between 10 and 100 Hz, amplified by a factor of 200,000.
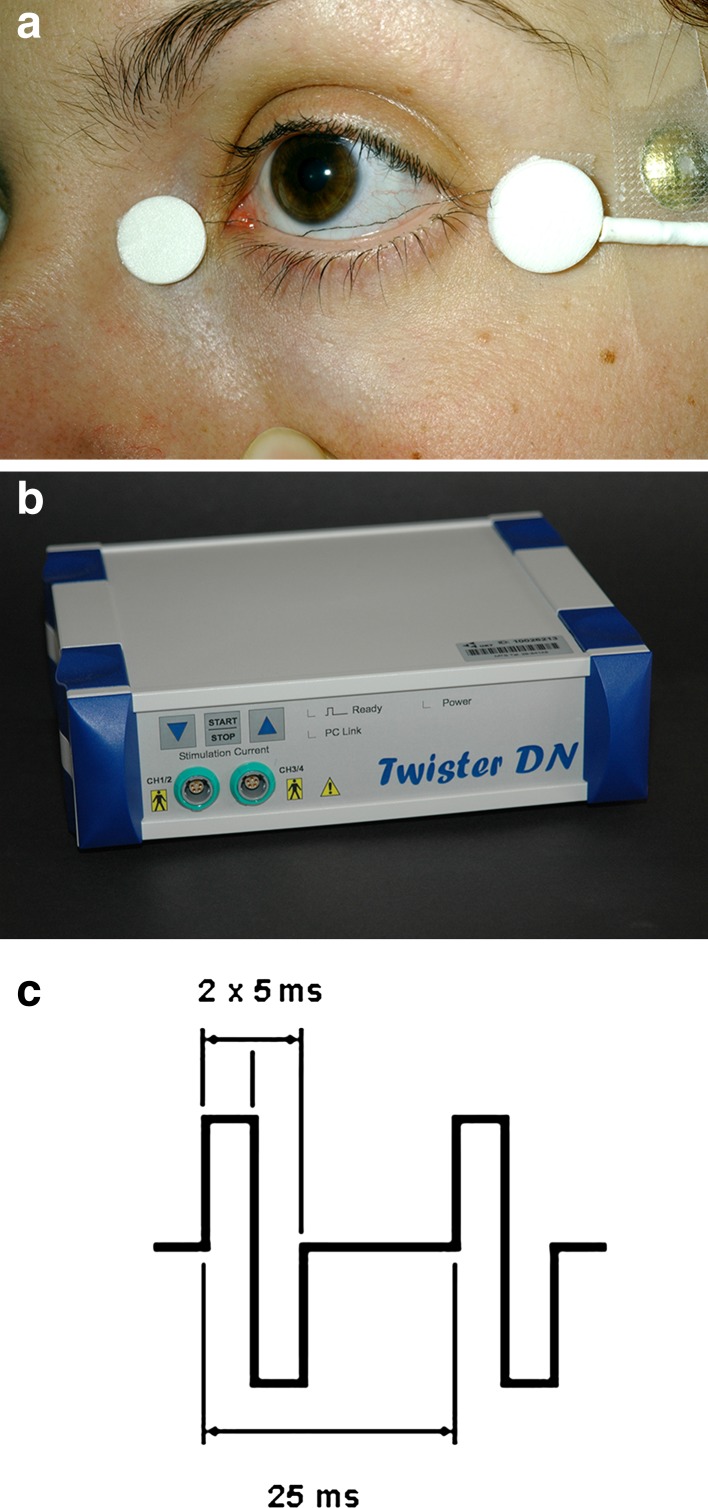
All electrophysiologic examinations and electrical stimulation were performed using a single-use sterile DTL electrode [38] (Diagnosys, Liverpool, UK) and a gold-plated cup electrode (LKC Technologies Inc., Gaithersburg, MD, USA) as a counter electrode (Fig. 1).
Fig. 1.
Transcorneal electrical stimulation was performed using a a sterile single-use DTL electrode and b a commercially-available neurostimulator programmed with c biphasic current pulses of 10 ms duration (5 ms positive, directly followed by 5 ms negative) at a frequency of 20 Hz
Cornea and conjunctiva were anesthetized with 0.4% oxybuprocaine hydrochloride (Conjuncain EDO®, Bausch & Lomb, Rochester, NY, USA). Pupils were dilated with 0.5% tropicamide (Mydriaticum Stulln®, Stulln, Germany).
Determination of EPT and TES
TES and assessment of EPT were performed using a neurostimulator (Twister®; Dr. Langer GmbH, Waldkirch, Germany) and a single-use sterile DTL-electrode (Fig. 1). A gold-plated cup electrode was used as a counter electrode, and fixed on the ipsilateral temple (Fig. 1). EPT was determined at all nine visits over a range of frequencies.
The threshold was defined as the minimal electric current that elicited phosphene perception anywhere in the visual field. An alternative forced choice method was used for verification. The neurostimulator was modified by the manufacturer to limit current output to 10 mA with increments starting from 1 μA.
Impedance of the electrodes was tested each time prior to stimulation and at various intervals during stimulation using the machine’s built-in algorithm; impedance did not exceed 5 kΩ.
Measurements were performed in darkness with a very dim indirect light produced by the shielded computer screen. To avoid dark adaptation, the light was switched on periodically (ca. every 60–90 s) [39]. Full darkness was necessary to allow perception of the very subtle phosphenes. The threshold at 20 Hz was determined three times, and the average was taken at each study visit for the calculation of individual stimulation strength according to the treatment group. TES was applied with 10 ms rectangular biphasic current pulses (5 ms positive, directly followed by 5 ms negative) at 20 Hz for 30 min once a week for 6 consecutive weeks.
Data Analysis
Statistical analyses were performed using the JMP® statistical software (version 8.02, SAS Institute, Cary, NC, USA). ERG data analysis was performed using previously described software [40]; the slope of the initial part of the a-wave was calculated and compared to evaluate photoreceptor activity [41]. Intra-individual differences were calculated for each patient between baseline and follow-up visits. A comparison between treatment groups was then performed using the method of restricted maximum likelihood (REML) to estimate the development of parameters under treatment for each group. To compare groups, the Tukey–Kramer post hoc test analysis was applied with a significance level of P < 0.05.
Results
Thirteen patients with nonarteritic RAO were enrolled (12 patients with CRAO and one patient with BRAO, age range 25–84 years, median 74 years). In total, 20 patients were screened for study participation and seven patients were excluded from the study because they were not able to deliver repeatable results in the visual field examination due to poor fixation. RAO was diagnosed 10 days to 17 months (median 11 months) prior to inclusion. Table 1 shows detailed characteristics for all patients. The numbers of patients assigned to the treatment groups were: 0 mA TES (sham), n = 3; 66% of the patients’ individual EPT at 20 Hz (60%), n = 5, and 150% of the patients’ individual EPT at 20 Hz (150%), n = 5.
Table 1.
Patient characteristics
| Patient | Age (years) | Gender | Ophthalmologic diagnosis | Treated eye | Treatment group (% of EPT) | Time from RAO to TES (days) | VA (Snellen), visit 1 | VA (Snellen), visit 9 | General disorders/characteristics |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | F | CRAO | R | 150% | 18 | HM | 1/35 | Arterial hypertension, hypercholesterolemia |
| 2 | 74 | F | CRAO | R | 150% | 64 | PLO | 1/20 | Diabetes mellitus type II, arterial hypertension, tobacco smoking |
| 3 | 69 | M | CRAO | R | 150% | 112 | 1/20 | 1/20 | Diabetes mellitus type II, arterial hypertension, coronary heart disease, two coronary stents |
| 4 | 84 | F | CRAO | L | 150% | 10 | HM | 1/35 | Diabetes mellitus type II, arterial hypertension, obesity, heart failure, pacemaker |
| 5 | 65 | F | CRAO | L | 150% | 142 | 1/10 | 1/15 | Aortic and mitral valve biologic prostheses |
| 6 | 60 | M | CRAO | L | 66% | 218 | 1/25 | 1/20 | Arterial hypertension |
| 7 | 75 | F | CRAO | L | 66% | 20 | HM | HM | Diabetes mellitus type II, arterial hypertension, coronary heart disease, three coronary stents, pacemaker |
| 8 | 75 | M | CRAO | R | 66% | 30 | 6/60 | 6/15 | Arterial hypertension |
| 9 | 25 | M | CRAO | L | 66% | 510 | 1/20 | 6/120 | Patent foramen ovale |
| 10 | 69 | F | CRAO | R | 66% | 153 | 1/15 | 1/10 | Diabetes mellitus type II, arterial hypertension, carotid stenosis |
| 11 | 71 | F | CRAO | R | Sham | 27 | HM | 1/15 | Diabetes mellitus type II, arterial hypertension, early myocardial infarction, absolute arrhythmia |
| 12 | 81 | F | BRAO | L | Sham | 14 | 6/7.5 | 6/7.5 | Diabetes mellitus type II, arterial hypertension, mitral regurgitation, aortic valve stenosis, carotid stenosis |
| 13 | 65 | F | CRAO | R | Sham | 11 | HM | 1/15 | Arterial hypertension |
VA (Snellen at 6 m or 1 m) was recorded as perception of light only (PLO), detection of hand movement (HM), or ability to count fingers (CF) at baseline (visit 1) and 9 weeks after TES (visit 9)
BRAO branch retinal artery occlusion, CRAO central retinal artery occlusion, EPT electrical phosphene threshold, F female, L left, M male, R right, RAO retinal artery occlusion, TES transcorneal electrical stimulation, VA visual acuity
All 13 patients completed the entire study. TES using DTL-electrodes was well tolerated, as reported previously [11, 39]. No ocular or systemic AEs were observed, except for foreign-body sensation after TES (n = 3).
All raw data and REML values are listed in Table 2; excerpts follow in this section. At the beginning, three patients reported phosphenes only at some of the tested frequencies (20 and 40 Hz) despite stimulation currents up to 4 mA; a level at which unpleasant sensations, such as a twitch in the eyelid, were reported. In the course of the study in two of these patients, determination of EPT was possible at all tested frequencies, whereas in one patient phosphenes could only be evoked at 20 Hz at the end of the study.
Table 2.
Raw data for the tested parameters for each of the three treatment groups (transcorneal electrical stimulation with sham, 66% and 150% of the individual phosphene threshold at 20 Hz, respectively) as well as the changes detected under treatment (calculated using a REML method; P < 0.05, Tukey–Kramer)
| Test | Subtest [unit raw data; unit REML] | Group | Raw dataa | REML | |||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1b | Visit 5 | Visit 8 | Visit 9 | P value | Mean | 95% CI interval | |||
| Scotopic ERG | SF a-wave ampl. [μV; μV] | Sham | 109.29 ± 11.01 | 124.69 ± 30.30 | 128.37 ± 36.15 | 170.40 ± 20.26 | 0.08 | 32.25 | 3.53, 60.96 |
| 66% | 162.86 ± 96.46 | 166.22 ± 88.94 | 163.26 ± 107.9 | 143.92 ± 30.08 | −5.61 | −28.62, 17.40 | |||
| 150% | 145.50 ± 40.52 | 147.68 ± 43.92 | 141.36 ± 21.72 | 139.28 ± 30.79 | −5.31 | −28.96, 18.18 | |||
| SF a-wave impl. [ms; ms] | Sham | 19.67 ± 2.08 | 20.33 ± 1.53 | 19.67 ± 2.08 | 19.00 ± 3.00 | 0.55 | −0.01 | −0.69, 0.69, | |
| 66% | 18.00 ± 1.87 | 17.40 ± 1.67 | 18.40 ± 2.79 | 18.00 ± 2.12 | −0.08 | −0.61, 0.45 | |||
| 150% | 19.40 ± 0.89 | 19.00 ± 1.87 | 19.80 ± 1.10 | 18.20 ± 0.84 | −0.41 | −0.94, 0.12 | |||
| SF b-wave ampl. [μV; μV] | Sham | 179.35 ± 93.88 | 198.72 ± 122.1 | 187.97 ± 76.25 | 223.57 ± 109.9 | 0.39 | 24.10 | −16.33, 64.53 | |
| 66% | 248.40 ± 172.7 | 235.44 ± 168.2 | 231.04 ± 139.9 | 249.06 ± 83.70 | −10.30 | −42.22, 21.63 | |||
| 150% | 135.47 ± 62.43 | 132.27 ± 56.51 | 142.48 ± 57.46 | 127.97 ± 50.30 | −2.28 | −34.58, 30.01 | |||
| SF b-wave impl. [ms; ms] | Sham | 61.67 ± 6.51 | 63.33 ± 8.96 | 55.67 ± 4.62 | 49.33 ± 5.03 | 0.11 | −5.76 | −11.16, −0.35 | |
| 66% | 55.20 ± 4.82 | 53.20 ± 4.92 | 55.80 ± 11.99 | 52.60 ± 9.71 | −1.51 | −5.09, 2.07 | |||
| 150% | 53.60 ± 8.29 | 47.40 ± 8.79 | 59.90 ± 3.56 | 55.20 ± 5.89 | 1.06 | −2.54, 4.65 | |||
| Photopic ERG | SF a-wave ampl. [μV; μV] | Sham | 18.80 ± 4.40 | 23.18 ± 15.22 | 21.51 ± 13.81 | 24.06 ± 9.62 | 0.40 | 4.11 | −5.05, 13.28 |
| 66% | 18.26 ± 11.78 | 32.28 ± 27.09 | 17.57 ± 10.29 | 18.84 ± 6.67 | 4.56 | −2.65, 11.78 | |||
| 150% | 19.93 ± 7.49 | 19.03 ± 5.50 | 17.96 ± 7.70 | 17.86 ± 3.46 | −1.80 | −9.09, 5.50 | |||
| SF a-wave impl. [ms; ms] | Sham | 18.00 ± 1.00 | 18.00 ± 1.00 | 17.00 ± 2.65 | 17.33 ± 1.53 | 0.21 | −0.56 | −1.97, 0.85 | |
| 66% | 15.80 ± 2.28 | 16.40 ± 2.07 | 16.20 ± 1.30 | 15.80 ± 2.05 | 0.35 | −0.76, 1.46 | |||
| 150% | 16.00 ± 1.00 | 17.20 ± 0.45 | 18.00 ± 1.41 | 16.00 ± 1.22 | 1.05 | −0.07, 2.17 | |||
| SF b-wave ampl. [μV; μV] | Sham | 47.12 ± 26.33 | 65.42 ± 57.39 | 59.79 ± 21.19 | 67.64 ± 34.63 | 0.11 | 17.15 | 2.19, 32.11 | |
| 66% | 67.28 ± 25.86 | 60.11 ± 35.60 | 56.11 ± 26.92 | 85.10 ± 45.11 | −0.02 | −11.51, 11.46 | |||
| 150% | 35.59 ± 18.54 | 32.93 ± 17.25 | 35.94 ± 14.01 | 31.09 ± 14.73 | −1.97 | −13.54, 9.59 | |||
| SF b-wave impl. [ms; ms] | Sham | 37.67 ± 4.51 | 38.33 ± 4.73 | 36.00 ± 2.65 | 36.33 ± 2.52 | 0.25 | −0.78 | −2.08, 0.51 | |
| 66% | 34.00 ± 3.54 | 34.00 ± 3.08 | 34.00 ± 3.74 | 33.60 ± 3.78 | −0.14 | −1.14, 0.87 | |||
| 150% | 36.80 ± 3.96 | 36.40 ± 5.50 | 35.40 ± 1.95 | 34.60 ± 3.13 | −1.32 | −2.33, −0.31 | |||
| Visual acuity | Snellen [logMAR; logMAR] | Sham | 1.40 ± 1.08 | 1.00 ± 0.78 | 1.07 ± 0.85 | 1.00 ± 0.82 | 0.89 | 0.30 | 0.01, 0.60 |
| 66% | 1.34 ± 0.42 | 1.14 ± 0.30 | 1.00 ± 0.41 | 1.12 ± 0.60 | 0.23 | 0.01, 0.46 | |||
| 150% | 1.68 ± 0.50 | 1.44 ± 0.30 | 1.18 ± 0.27 | 1.32 ± 0.20 | 0.29 | 0.06, 0.52 | |||
| VF | Area [deg2; deg2] | Sham | 7,975 ± 3,230b | 6,595 ± 5,501 | 7,361 ± 5,271 | 6,471 ± 5,473 | 0.24 | −9.10 | −1,739.00, 1,720.00 |
| 66% | 9,941 ± 3,630 | 9,138 ± 3,641 | 9,743 ± 3,384 | 11,522 ± 2,630 | 1,091 | 59.00, 2,123.00 | |||
| 150% | 1,387 (n = 1)b | 2,077 ± 1,961 | 2,203 ± 2,896 | 3,862 ± 3,759 | NA | NA | |||
| Mean sensitivity [dB; dB] | Sham | 5.90 ± 6.08 | 3.87 ± 7.49 | 6.90 ± 6.03 | 4.40 ± 7.71 | 0.28 | 1.50 | −0.80, 3.77 | |
| 66% | 5.25 ± 3.52 | 7.10 ± 5.35 | 8.48 ± 6.68 | 9.43 ± 7.58 | 2.70 | 0.98, 4.43 | |||
| 150% | 1.50 (n = 1)b | 7.37 ± 7.74 | 6.55 ± 10.02 | 7.80 ± 10.48 | −0.08 | −4.39, 4.24 | |||
| Mean defect [dB; dB] | Sham | 13.05 ± 7.85 | 15.90 ± 9.39 | 14.10 ± 9.72 | 15.33 ± 9.68 | 0.25 | −1.53 | −2.92, −0.12 | |
| 66% | 15.60 ± 4.07 | 14.25 ± 6.49 | 13.18 ± 8.52 | 12.63 ± 8.81 | −1.69 | −3.25, −1.17 | |||
| 15% | 20.90 (n = 1)b | 14.33 ± 8.88 | 15.50 ± 9.55 | 12.87 ± 10.89 | −1.18 | −0.11, 1.05 | |||
| Phosphene threshold | Sensitivity at 20 Hz [mA; mA] | Sham | 0.65 ± 0.48 | 0.52 ± 0.35 | 0.57 ± 0.44 | 0.57 ± 0.48 | 0.51 | 0.12 | −0.18, 0.41 |
| 66% | 0.66 ± 0.65 | 0.46 ± 0.33 | 0.43 ± 0.28 | 0.51 ± 0.41 | 0.16 | −0.04, 0.36 | |||
| 150% | 1.41 ± 1.50 | 1.37 ± 1.51 | 0.91 ± 0.43 | 0.77 ± 0.40 | 0.29 | 0.09, 0.49 | |||
| Multifocal ERG | Amp. Ring 1 [μV; μV] | Sham | 21.03 ± 18.51 | 13.93 ± 14.53 | 12.40 ± 19.33 | 8.63 ± 9.57 | 0.13 | −9.38 | −18.40, −0.35 |
| 66% | 11.52 ± 8.02 | 12.04 ± 7.17 | 11.34 ± 8.27 | 10.46 ± 10.55 | −0.24 | −7.23, 6.75 | |||
| 150% | 5.38 ± 7.07 | 5.08 ± 6.22 | 8.64 ± 7.89 | 8.10 ± 7.07 | 1.89 | −5.10, 8.88 | |||
| Impl. Ring 1 [ms; ms] | Sham | 27.73 ± 14.67 | 28.87 ± 15.79 | 30.00 ± 15.21 | 28.03 ± 15.30 | 0.82 | 1.23 | −12.69, 15.16 | |
| 66% | 30.16 ± 10.25 | 31.14 ± 11.20 | 33.48 ± 11.77 | 29.48 ± 8.02 | 1.21 | −9.58, 11.99 | |||
| 150% | 18.98 ± 19.59 | 21.82 ± 12.06 | 26.48 ± 15.33 | 24.00 ± 14.46 | 5.12 | −5.67, 15.91 | |||
| Amp. Ring 2 [μV; μV] | Sham | 8.40 ± 7.31 | 7.30 ± 6.00 | 6.03 ± 7.89 | 7.90 ± 7.11 | 0.29 | −1.32 | −4.12, 1.47 | |
| 66% | 7.72 ± 2.68 | 7.96 ± 1.48 | 5.82 ± 4.04 | 6.01 ± 3.78 | −1.09 | −3.26, 1.07 | |||
| 150% | 3.16 ± 3.46 | 3.06 ± 3.23 | 4.74 ± 3.82 | 4.38 ± 3.02 | 0.90 | −1.27, 3.07 | |||
| Impl. Ring 2 [ms; ms] | Sham | 28.60 ± 12.47 | 26.10 ± 12.47 | 28.30 ± 13.01 | 29.97 ± 17.02 | 0.64 | −0.48 | −14.29, 13.33 | |
| 66% | 32.82 ± 3.92 | 34.48 ± 3.91 | 33.98 ± 12.30 | 29.82 ± 9.27 | −0.06 | −10.76, 10.64 | |||
| 150% | 20.14 ± 18.91 | 22.16 ± 10.03 | 28.16 ± 13.46 | 25.00 ± 15.15 | 5.63 | −5.06, 16.33 | |||
| Amp. Ring 3 [μV; μV] | Sham | 6.03 ± 2.97 | 5.50 ± 5.16 | 5.00 ± 2.88 | 6.17 ± 5.06 | 0.56 | −0.48 | −1.87, 0.91 | |
| 66% | 4.96 ± 1.98 | 5.52 ± 0.94 | 3.64 ± 3.00 | 3.52 ± 2.41 | −0.73 | −1.81, 0.34 | |||
| 150% | 3.14 ± 2.03 | 2.64 ± 1.30 | 3.24 ± 1.81 | 3.58 ± 2.38 | 0.01 | −1.06, 1.09 | |||
| Impl. Ring 3 [ms; ms] | Sham | 36.90 ± 2.55 | 25.23 ± 13.91 | 36.50 ± 3.78 | 37.73 ± 5.65 | 0.93 | −3.74 | −12.28, 4.79 | |
| 66% | 34.64 ± 2.65 | 34.80 ± 5.99 | 33.16 ± 11.57 | 29.30 ± 12.30 | −2.22 | −8.83, 4.39 | |||
| 150% | 34.32 ± 13.89 | 32.32 ± 10.15 | 34.64 ± 14.63 | 30.32 ± 11.77 | −1.89 | −8.50, 4.72 | |||
| Amp. Ring 4 [μV; μV] | Sham | 4.27 ± 2.19 | 4.97 ± 2.27 | 3.90 ± 1.87 | 3.90 ± 3.46 | 0.82 | −0.01 | −1.14, 1.12 | |
| 66% | 4.10 ± 1.86 | 4.12 ± 1.21 | 2.86 ± 1.86 | 4.20 ± 1.81 | −0.37 | −1.25, 0.50 | |||
| 150% | 2.62 ± 1.84 | 1.92 ± 1.29 | 2.54 ± 1.80 | 2.26 ± 1.73 | −0.38 | −1.26, 0.50 | |||
| Impl. Ring 4 [ms; ms] | Sham | 36.37 ± 3.18 | 35.97 ± 4.88 | 36.40 ± 6.72 | 37.73 ± 5.88 | 0.44 | 0.33 | −3.76, 4.43 | |
| 66% | 36.30 ± 4.27 | 36.98 ± 4.81 | 32.30 ± 10.24 | 35.96 ± 4.82 | −1.22 | −4.39, 1.95 | |||
| 150% | 36.82 ± 16.26 | 34.14 ± 14.40 | 35.32 ± 14.28 | 32.80 ± 13.31 | −2.73 | −5.90, 0.44 | |||
| Amp. Ring 5 [μV; μV] | Sham | 3.67 ± 2.51 | 4.03 ± 2.18 | 3.90 ± 2.21 | 3.33 ± 1.93 | 0.66 | 0.09 | −1.38, 1.59 | |
| 66% | 4.06 ± 1.73 | 3.60 ± 1.31 | 3.26 ± 0.96 | 3.70 ± 1.15 | −0.54 | −1.68, 0.60 | |||
| 150% | 2.70 ± 2.23 | 1.68 ± 1.23 | 2.26 ± 1.51 | 2.20 ± 1.59 | −0.65 | −1.79, 0.49 | |||
| Impl. Ring 5 [ms; ms] | Sham | 36.93 ± 4.90 | 37.70 ± 4.69 | 37.77 ± 4.86 | 40.53 ± 8.24 | 0.50 | 1.73 | −3.91, 7.38 | |
| 66% | 38.14 ± 4.54 | 38.48 ± 6.60 | 35.30 ± 4.15 | 36.00 ± 4.56 | −1.55 | −5.92, 2.82 | |||
| 15% | 36.14 ± 16.73 | 33.98 ± 14.33 | 34.32 ± 13.02 | 34.14 ± 12.20 | −1.99 | −6.36, 2.38 | |||
Ampl. amplitude, CI confidence interval, ERG electroretinography, Impl. implicit time, REML restricted maximum likelihood, SF standard flash, VF visual field
aResults from visit 1, 5, 8, and 9 are given as the mean ± standard deviation
bFor VF, some values are given for visit 2 because VF was not recordable in five patients at baseline. REML estimates are given as the mean and 95% upper and lower confidence intervals. Electrical stimulation was performed between visits 2 and 7. SF = 3 cd.s/m2
At all visits and in all patients, the lowest EPT was found at 20 Hz. EPT (mean ± standard deviation [SD]) at baseline was 0.97 ± 1.06 mA at 20 Hz, 1.43 ± 1.36 mA at 40 Hz, 1.83 ± 1.51 mA at 60 Hz, 1.63 ± 1.42 mA at 80 Hz, 1.82 ± 1.50 mA at 3 Hz, 1.59 ± 1.36 mA at 6 Hz, and at 1.82 ± 1.50 mA 9 Hz. The stimulation current ranged from 0.1 to 1.75 mA.
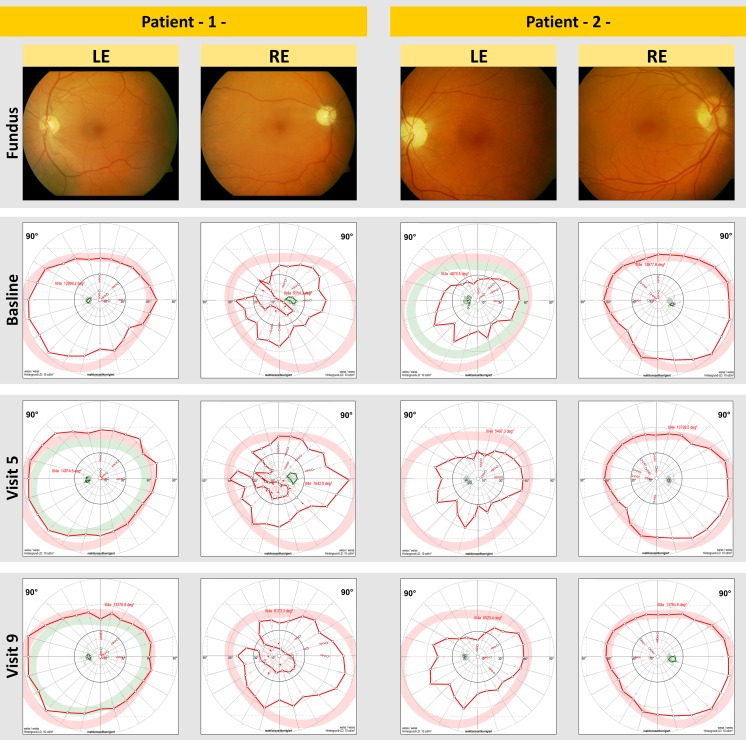
No statistically significant differences were detected in visual field parameters (Table 2). The mean defect, the mean sensitivity in static visual field, and the area of kinetic visual field did not differ significantly between the groups; calculation of the changes in the area of the kinetic visual field in the 150% treatment group failed because the visual field was not recordable in four patients at baseline (Table 2). However, in some of the treated patients, an enlargement of visual field area was observed. Figure 2 shows fundus photographs and the development of visual field area in two selected patients in the 150% treatment group. RAO was diagnosed in the first of these patients at 7 months and in the second of these patients at 6 months before study enrollment.
Fig. 2.
Fundus photographs and development of visual field (VF) area in two selected patients from the group treated with 150% of the individual electric phosphene threshold at 20 Hz. In the treated patients, an enlargement of VF area was observed, but statistical significance was not reached. Retinal artery occlusion was diagnosed in patient 1 (left) at approximately 7 months and in patient 2 (right) at approximately 6 months prior to study enrollment. LE right eye, RE right eye
During study visits no significant differences were observed between the groups for amplitudes and implicit times in the ERG measurements under dark- or light-adapted conditions (Table 2). There were, however, statistically significant changes (P = 0.03) in the a-wave slope of the high-intensity flash (10 cd.s/m²) under scotopic conditions with increased values in the follow-up as compared to baseline in the 150% treatment group with mean ± standard error (SE) of 0.46 ± 0.7 μV/ms, and decreased values in the 66% and sham treatment groups of −2.1 ± 0.7 and −1.74 ± 0.9 μV/ms, respectively.
Discussion
Recently, studies with TES in humans have shown that TES is safe and can exhibit positive effects in patients with degenerative retinal diseases [11–15]. In particular, a sham-controlled pilot study in RP showed some significant effects after only 6 weeks of TES treatment [11].
In 2007, Inomata et al. [13] published a case description of three patients with longstanding RAO. Patients were treated with TES once a month for a period of 3 months (stimulation current up to 1.1 mA, 20 Hz biphasic pulses, 30 min, contact lens electrode). After treatment the authors noted an improvement in visual acuity of >0.2 logMAR in two cases and an enlargement of the visual field in all three cases.
In 2010, Robles-Camarillo et al. [14] investigated the effects of TES on low vision in patients with CRAO. In the 2-month study, nine patients were split into three groups: control, TES treatment for 45 min twice per month, and TES treatment for 45 min once per week. TES was applied with contact lens electrodes, although stimulation parameters were not given in this publication. The group’s results showed an improvement in visual acuity and increased responses of the a- and b-wave in the ERG data in all stimulated patients.
More recently, Oono et al. [15] in 2011 described an improvement in mfERG responses in five patients with BRAO. A single TES treatment was delivered using contact lens electrodes; the current strength ranged between 0.5 and 0.9 mA with biphasic pulses at 20 Hz while the duration of each session was 30 min.
To the authors’ knowledge, the present study is the first randomized, sham-controlled, and partially blinded study to investigate TES as a potential treatment in patients with RAO. The specific stimulation parameter was chosen due to the fact that the lowest EPT was observed at 20 Hz. This trough for EPT at 20 Hz was also found in healthy subjects, and patients with other retinal diseases, such as RP, nonarteritic anterior ischemic optic neuropathy, Stargardt’s disease, and primary open-angle glaucoma [42].
The stimulation current, calculated from the individual EPT at 20 Hz, ranged in the present study from 0.10 to 1.75 mA. In their studies with RAO patients, Inomata et al. [13] and Oono et al. [15] used stimulation currents ranging from 0.50 to 1.10 mA. These values are, remarkably, comparable to the values used in the present study, despite significant differences in techniques. The stimulation time of 30 min in the present study was based on that used in previous studies [11–13, 15]. The frequency of application in the present study (once weekly in 6 consecutive weeks) was selected on the basis that more intensive stimulation (vs. the three-times monthly TES treatment used by Inomata et al. [13]) could potentially have more positive effects.
In the 150% treatment group, a statistically significant increase in the scotopic a-wave slope was detected. As the a-wave slope is regarded as the electrophysiologic representation of the phototransduction process by the photoreceptors [43–46], these results indicate an improvement in photoreceptor activity during TES treatment. All other tested parameters failed to show statistically significant improvements. A failure to reach statistical significance may have been due to the very small sample size, with only three to five patients in each group. It may, however, also have been due to the short study period of only six stimulation sessions in an attempt to treat very profound retinal damage; such damage usually has a very poor prognosis. In contrast to this, in the present study with 24 RP patients [11], significant improvements were found in clinically relevant parameters, such as visual field area and scotopic b-wave amplitude, under the same study parameters, but had the chance to examine a considerably larger study population. Statistical significance would not have been reached with smaller sample sizes as recalculations of the present data have shown. In the present study subgroup analysis of data was not done because the groups were small, although an improvement in two longstanding cases is in accordance with previous reports by Inomata et al. [13]. It should be emphasized here that the cases included in the Inomata et al. [13] study and all other previous studies mentioned [14, 15] were based on uncontrolled series with a limited strength of evidence. The possibility that the present findings may have been biased by a potential imbalance in the study groups, such as differing extent and severity of retinal damage, and inconsistent timing of TES treatment, cannot be completely eliminated.
As a statistically significant clinical effect from the present data could not be shown, TES cannot be currently recommended as a treatment for CRAO or BRAO. Hayreh and Zimmerman [1] reported that without any treatment, an improvement in visual acuity and visual field is observed in only 6% of eyes with nonarteritic CRAO. However, the visual outcome in CRAO patients using existing treatments is unsatisfactory; various studies of new treatments for CRAO have shown no significant differences in the visual outcome between treated and untreated patients [6, 47–49]. Considering the lack of scientifically justifiable treatment options for BRAO and CRAO, and taking into account the limitations of the present study (short duration, small sample size) and the statistically significant improvement detected in a-wave slopes, further investigation is warranted to explore the potential of TES for these conditions. Any further studies on the therapeutic value of TES should retain the stringent use of a sham control, as used in the present study, and maintain study groups as homogeneously as possible. It may be possible that the initiation of treatment with TES very shortly after the onset of RAO might have a clearer effect on the course of the disease.
In conclusion, TES provides several potential advantages over intravitreal injections or surgical interventions, e.g., minimal invasiveness. Furthermore, TES most probably generates low treatment costs, and the simplicity of the concept also means considerable ease of use for both the examiner and the patient. Therefore, it is conceivable that patients may be allowed a stimulation device at home.
Acknowledgments
The study was supported financially by Okuvision GmbH, Reutlingen, Germany. Dr. Naycheva is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–391. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116:1188–94, e1181–4. [DOI] [PMC free article] [PubMed]
- 3.Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp Eye Res. 2004;78:723–736. doi: 10.1016/S0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 4.Von Graefe A. Über Embolie der Arteria centralis retinae als Ursache plötzlicher Erblindung. Graefes Arch Clin Exp Ophthalmol. 1859;5:136–185. doi: 10.1007/BF02720764. [DOI] [Google Scholar]
- 5.Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol. 1980;64:913–917. doi: 10.1136/bjo.64.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–2034. doi: 10.1016/s0161-6420(95)30758-0. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt D. Ocular massage in a case of central retinal artery occlusion the successful treatment of a hitherto undescribed type of embolism. Eur J Med Res. 2000;5:157–164. [PubMed] [Google Scholar]
- 8.Schmidt D, Schumacher M, Wakhloo AK. Microcatheter urokinase infusion in central retinal artery occlusion. Am J Ophthalmol. 1992;113:429–434. doi: 10.1016/s0002-9394(14)76167-7. [DOI] [PubMed] [Google Scholar]
- 9.Richard G, Lerche RC, Knospe V, Zeumer H. Treatment of retinal arterial occlusion with local fibrinolysis using recombinant tissue plasminogen activator. Ophthalmology. 1999;106:768–773. doi: 10.1016/S0161-6420(99)90165-3. [DOI] [PubMed] [Google Scholar]
- 10.Fraser S, Siriwardena D. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 2002;CD001989. [DOI] [PubMed]
- 11.Schatz A, Röck T, Naycheva L, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52:4485–4496. doi: 10.1167/iovs.10-6932. [DOI] [PubMed] [Google Scholar]
- 12.Fujikado T, Morimoto T, Matsushita K, Shimojo H, Okawa Y, Tano Y. Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Jpn J Ophthalmol. 2006;50:266–273. doi: 10.1007/s10384-005-0304-y. [DOI] [PubMed] [Google Scholar]
- 13.Inomata K, Shinoda K, Ohde H, et al. Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol. 2007;245:1773–1780. doi: 10.1007/s00417-007-0610-9. [DOI] [PubMed] [Google Scholar]
- 14.Robles-Camarillo D, Nino-de-Rivera L, Calleja-Arriaga W, Quiroz-Mercado H, Lopez-Miranda MJ. Effects of wavelets transcorneal-stimulation on low vision patients with central retinal artery occlusion CRAO. Telecommun Radio Eng. 2010;69:727–732. doi: 10.1615/TelecomRadEng.v69.i8.90. [DOI] [Google Scholar]
- 15.Oono S, Kurimoto T, Kashimoto R, Tagami Y, Okamoto N, Mimura O. Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin Ophthalmol. 2011;5:397–402. doi: 10.2147/OPTH.S17751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews RJ. Neuroprotection trek—the next generation: neuromodulation II. Applications—epilepsy, nerve regeneration, neurotrophins. Ann N Y Acad Sci. 2003;993:14–24. doi: 10.1111/j.1749-6632.2003.tb07507.x. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto T, Fujikado T, Choi JS, et al. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Invest Ophthalmol Vis Sci. 2007;48:4725–4732. doi: 10.1167/iovs.06-1404. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto T, Kanda H, Kondo M, Terasaki H, Nishida K, Fujikado T. Transcorneal electrical stimulation promotes survival of photoreceptors and improves retinal function in rhodopsin P347L transgenic rabbits. Invest Ophthalmol Vis Sci. 2012;53:4254–4261. doi: 10.1167/iovs.11-9067. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto T, Miyoshi T, Matsuda S, Tano Y, Fujikado T, Fukuda Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci. 2005;46:2147–2155. doi: 10.1167/iovs.04-1339. [DOI] [PubMed] [Google Scholar]
- 22.Miyake K, Yoshida M, Inoue Y, Hata Y. Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest Ophthalmol Vis Sci. 2007;48:2356–2361. doi: 10.1167/iovs.06-1329. [DOI] [PubMed] [Google Scholar]
- 23.Ni YQ, Gan DK, Xu HD, Xu GZ, Da CD. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol. 2009;219:439–452. doi: 10.1016/j.expneurol.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Messias A, Schatz A, Zrenner E, Bartz-Schmidt K, Gekeler F. Effect of transcorneal electrical stimulation on rat retinal function after bright light exposure. ARVO Meeting Abstracts. 2009;50:3615. [Google Scholar]
- 25.Schatz A, Arango-Gonzales B, Fischer D, et al. Transcorneal electrical stimulation shows neuroprotective effects in retinas of light-exposed rats. Invest Ophthalmol Vis Sci. 2012;53:5552–5561. doi: 10.1167/iovs.12-10037. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Fujikado T, Morimoto T, Matsushita K, Harada T, Tano Y. Effect of electrical stimulation on IGF-1 transcription by L-type calcium channels in cultured retinal Muller cells. Jpn J Ophthalmol. 2008;52:217–223. doi: 10.1007/s10384-008-0533-y. [DOI] [PubMed] [Google Scholar]
- 27.Ciavatta VT, Kim M, Wong P, et al. Retinal expression of Fgf2 in RCS rats with subretinal microphotodiode array. Invest Ophthalmol Vis Sci. 2009;50:4523–4530. doi: 10.1167/iovs.08-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, Lee TS, Takamatsu F, Fujikado T. Induction of fibroblast growth factor-2 by electrical stimulation in cultured retinal Mueller cells. NeuroReport. 2008;19:1617–1621. doi: 10.1097/WNR.0b013e3283140f25. [DOI] [PubMed] [Google Scholar]
- 29.Kent TL, Glybina IV, Abrams GW, Iezzi R. Chronic intravitreous infusion of ciliary neurotrophic factor modulates electrical retinal stimulation thresholds in the RCS rat. Invest Ophthalmol Vis Sci. 2008;49:372–379. doi: 10.1167/iovs.07-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen R, Song Y, Cheng T, et al. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, Fujikado T, Lee TS, Tano Y. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Muller cells. Invest Ophthalmol Vis Sci. 2008;49:4641–4646. doi: 10.1167/iovs.08-2049. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/S0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 33.Willmann G, Schaferhoff K, Fischer MD, et al. Gene expression profiling of the retina after transcorneal electrical stimulation in wild-type Brown Norway rats. Invest Ophthalmol Vis Sci. 2011;52:7529–7537. doi: 10.1167/iovs.11-7838. [DOI] [PubMed] [Google Scholar]
- 34.Kurimoto T, Oono S, Oku H, et al. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol. 2010;4:1441–1446. doi: 10.2147/OPTH.S14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiefer U, Pascual JP, Edmunds B, et al. Comparison of the new perimetric GATE strategy with conventional full-threshold and SITA standard strategies. Invest Ophthalmol Vis Sci. 2009;50:488–494. doi: 10.1167/iovs.08-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 37.Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y. Guidelines for basic multifocal electroretinography (mfERG) Doc Ophthalmol. 2003;106:105–115. doi: 10.1023/A:1022591317907. [DOI] [PubMed] [Google Scholar]
- 38.Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18:988–991. [PubMed] [Google Scholar]
- 39.Gekeler F, Messias A, Ottinger M, Bartz-Schmidt KU, Zrenner E. Phosphenes electrically evoked with DTL electrodes: a study in patients with retinitis pigmentosa, glaucoma, and homonymous visual field loss and normal subjects. Invest Ophthalmol Vis Sci. 2006;47:4966–4974. doi: 10.1167/iovs.06-0459. [DOI] [PubMed] [Google Scholar]
- 40.Messias A, Gekeler F, Wegener A, Dietz K, Kohler K, Zrenner E. Retinal safety of a new fluoroquinolone, pradofloxacin, in cats: assessment with electroretinography. Doc Ophthalmol. 2008;116:177–191. doi: 10.1007/s10633-007-9081-x. [DOI] [PubMed] [Google Scholar]
- 41.Hood DC, Birch DG. Human cone receptor activity: the leading edge of the a-wave and models of receptor activity. Vis Neurosci. 1993;10:857–871. doi: 10.1017/S0952523800006076. [DOI] [PubMed] [Google Scholar]
- 42.Naycheva L, Schatz A, Rock T, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53:7440–7448. doi: 10.1167/iovs.12-9612. [DOI] [PubMed] [Google Scholar]
- 43.Hood DC, Birch DG. Light adaptation of human rod receptors: the leading edge of the human a-wave and models of rod receptor activity. Vision Res. 1993;33:1605–1618. doi: 10.1016/0042-6989(93)90027-T. [DOI] [PubMed] [Google Scholar]
- 44.Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci. 1994;35:2948–2961. [PubMed] [Google Scholar]
- 45.Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- 47.Mueller AJ, Neubauer AS, Schaller U, Kampik A. Evaluation of minimally invasive therapies and rationale for a prospective randomized trial to evaluate selective intra-arterial lysis for clinically complete central retinal artery occlusion. Arch Ophthalmol. 2003;121:1377–1381. doi: 10.1001/archopht.121.10.1377. [DOI] [PubMed] [Google Scholar]
- 48.Beatty S, Au Eong KG. Local intra-arterial fibrinolysis for acute occlusion of the central retinal artery: a meta-analysis of the published data. Br J Ophthalmol. 2000;84:914–916. doi: 10.1136/bjo.84.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Framme C, Spiegel D, Roider J, et al. Central retinal artery occlusion. Importance of selective intra-arterial fibrinolysis [In German] Ophthalmologe. 2001;98:725–730. doi: 10.1007/s003470170079. [DOI] [PubMed] [Google Scholar]