Abstract
Introduction
The aims of this study were: (1) to investigate the association of vascular endothelial growth factor isoform A (VEGF-A) concentration in the anterior chamber liquid (ACL) with vascular proliferation in patients with diabetic retinopathy (DR) who had undergone surgical treatment for cataract and neovascular glaucoma; (2) to analyze the association of VEGF-A level in ACL with the cataract surgery outcomes.
Materials and Methods
Undiluted aqueous fluid samples were obtained from 207 eyes of patients who underwent intraocular surgery, 136 patients with diabetes mellitus (DM) and 22 patients without DM. The ACL samples were obtained during operation. The VEGF-A levels were analyzed by enzyme-linked immunosorbent assay.
Results
The lowest VEGF-A levels were in diabetic patients without signs of DR [22.75 pg/mL (10.78; 63.36)]. More severe DR tended to occur in diabetic patients with higher VEGF-A levels in ACL. In diabetic patients with proliferative DR (PDR), VEGF-A levels were significantly higher [336.6 pg/mL (232.3; 410.74)] than in patients without DR P < 0.0001. In patients with terminal stage of DR [neovascular glaucoma (NG)], VEGF-A levels were dramatically higher and attained 1,634.01 pg/mL (610.69; 2657.33). In non-diabetic patients, VEGF-A levels were 95.07 pg/ml (60.92; 129.22). The best visual acuity results in post-operative period were observed in the group of diabetic patients without DR. In the group of patients with PDR, post-operative visual acuity [0.26 (0.1; 0.42)] was similar to visual acuity before operation [0.29 (0.13; 0.44)]. There was no significant increase in visual acuity due to cataract surgery. In 52.4% patients, no complications had occurred by the end of the follow-up period. In 40% patients, retinal laser coagulation was performed, and in 7.6% patients NG had developed.
Conclusion
VEGF-A level in ACL increases with DR progression and may be of prognostic value in evaluating the potential risk of further neovascularization progression in diabetic patients.
Keywords: Cataract, Cataract surgery, Diabetes mellitus, Diabetic retinopathy, Ophthalmology, Vascular endothelial growth factor
Introduction
Ophthalmological complications of diabetes mellitus (DM) remain a major public health problem. Despite a significant progress in investigating the causes of diabetic retinopathy (DR) and the development of therapeutic methods, DR remains to be a leading cause of blindness among young persons suffering from DM; among working-age adults, it causes a dramatic loss in visual acuity in 5% of cases [1]. DR is caused by chronic hyperglycemia which results in the activation of the sorbitol shunt, oxidative damages, accumulation of end-products of glycation, microcirculation impairment, hypercoagulation, increased vascular permeability, endothelial dysfunction, activation of apoptosis, etc. [2, 3]. Multicenter clinical trials have shown the efficacy of preventive measures aimed primarily at good glycemic control, which permit to achieve 75% reduction in the risk of visual loss [4]. However, once DR has developed it usually cannot be reversed. Accumulation of pathobiochemical and pathophysiological changes resulting in retinal damages, its hypoperfusion and ischemia, which ultimately trigger the production of cytokines and growth factors [5–7]. A key growth factor mediating vascular permeability and neovascularization is a vascular endothelial growth factor (VEGF), isoform A of which is the most active and exerts many effects [8]. Retinal cells (retinal pigment epithelium, pericytes, and astrocytes) are those which, in response to hypoxic damage, actively produce VEGF [9–11] which, in turn, causes its edema and neovascularization. Eye structures of patients with DM and DR have been shown to contain higher amounts of VEGF [12], the levels of which are decreased following retinal laser coagulation (RLC) [13].
Nowadays, anti-VEGF agent widely used for the treatment of DR as well as the age-related macular degeneration [14, 15], it demonstrated reducing the macular edema and blockage new vessels grow. On the other hand, VEGF mediates normal physiological processes (reparation and wound healing, ensuring cell survival, normal course of pregnancy, glomerulogenesis, vasodilatation, etc.,), the suppression of which may result in serious adverse reactions in patients with DM in particular.
The aims of this study were: (1) to investigate the association of VEGF-A concentration in the anterior chamber liquid (ACL) with vascular proliferation in patients with DR who had undergone surgical treatment for cataract and neovascular glaucoma; (2) to analyze the association of VEGF-A level in ACL with the cataract surgery outcomes.
Materials and Methods
This study was a cross-sectional study evaluating the levels of VEGF-A and glucose in ACL of DM patients with cataract and neovascular glaucoma (NG), with a 12-month follow-up period after cataract extraction.
From 2007 to 2009, at the Department of Diabetic Retinopathy and Eye Surgery, 120 DM patients were operated on for cataract and 16 DM patients with NG were operated for non-controlled pain glaucoma by valve implantation. The control group included 22 non-diabetic patients operated on for age-related cataract.
The patients over 16 years old who had signed the informed consent form were included in the study. Exclusion criteria were previous vitreous, retinal or glaucoma surgery, eye infections, age-macular degeneration, chorio-retinal neovascularization, chronic or immune uveitis, systemic immune-suppressing therapy.
For all patients, the levels of VEGF-A and glucose were measured in ACL, which was collected at baseline (before surgery). The samples were centrifuged, separated from the precipitate and stored at −80 °C. The levels of VEGF-A were measured by the enzyme-linked immunosorbent assay (ELISA) and the glucose levels by a glucose oxidation method. Patients were examined before surgery, the day after surgery and 1, 3, 6, and 12 months after surgery. To assess the long-term visual outcomes, the patients were monitored for 12 months after surgery. At examination performed immediately after cataract extraction (on next day), DM patients were assigned to the groups according to the verified stage of DR. The stage of DR was determined according to World Health Organization (WHO) classification [16]: non-proliferative (NPDR), pre-proliferative (PPDR), and proliferative (PDR) (Table 1). Ophthalmic examination included best-corrected visual acuity (estimation of visual acuity was performed using the Golovin–Sivtsev table from a 5-m distance), intraocular pressure, biomicroscopy, ophthalmoscopy, ultrasound B-scan, perimetry, and color fundus photography. For assessment, the diabetes condition glycated hemoglobin (HbA1C), blood glucose, blood pressure, microalbuminuria, and glomerular filtration rate (GFR) were measured.
Table 1.
Clinical and demographic parameters of patients with different DR stages
| Variable | No DM | DM without DR | DM with NPDR | DM with PPDR | DM with PDR | DM with NG | Significance test (P value) |
|---|---|---|---|---|---|---|---|
| Number of operated eyes (n) | 27 | 13 | 61 | 62 | 27 | 17 | |
| Patients’ age in group (years)a | 71 (65; 77) | 59 (52; 62) | 68 (59; 74) | 69 (57; 73) | 68 (61; 70) | 60 (53; 65) | 0.0081 |
| Duration of DM (yearsa) | – | 6 (5; 22) | 11 (8; 17) | 17.5 (12; 22) | 16 (14; 21) | 12.5 (6.5; 20.5) | 0.021 |
| HbAa1C (%) | – | 6.3 (5.9; 7.0) | 8.1 (7.4; 9.6) | 7.6 (6.8; 8.6) | 8.0 (7.4; 8.8) | 7.8 (7.4; 8.9) | 0.0016* |
| Visual acuity before surgeryb | 0.24 (0.13; 0.36) | 0.33 (0.14; 0.52) | 0.24 (0.17; 0.32) | 0.18 (0.12; 0.24) | 0.29 (0.13; 0.44) | <0.01 | 0.31** |
| History of RLCc | 0 | 0 | 45.2 | 61.1 | 43.8 | – | |
| Concurrent glaucomac | 4.2 | 11.1 | 17.1 | 14.3 | 16.7 | – | – |
| Presence of comorbidities and DM complicationsc | |||||||
| Microalbuminuria/proteinuria | – | 11.1 | 22 | 45.2 | 77.8 | 56.3 | – |
| Hypertension | 16.7 | 55.6 | 85.4 | 97.7 | 100 | 100 | – |
| Cardiac failure | 8.7 | 22.2 | 26.8 | 30 | 38.9 | 25 | – |
| GFR (mL/min; Cockcroft–Gault formula) | 81.8 (62.1; 92.0) | 73.3 (63.4; 80.3) | 79.2 (61.9; 105.1) | 56 (43.2; 81.0) | 63.8 (54.7; 81.5) | 55.4 (32.6; 70.3) | 0.0316* |
| Analysis of ACLa | |||||||
| Glucose (mmol/L) | 2.45 (2.07; 2.91) | 3.08 (2.9; 3.26) | 3.14 (2.14; 3.79) | 3.03 (2.57; 4.71) | 4.51 (3.27; 6.32) | 4.85 (4.6; 5.9) | 0.027* |
| VEGF-A (pg/mL) | 95.07 (60.92; 129.22) | 22.75 (10.78; 63.36) | 52.5 (44.52; 88.95) | 75.84 (71.7; 123.58) | 336.6 (232.3; 410.74) | 1,634.01 (610.69; 2,657.33) | <0.001*** |
ACL anterior chamber liquid, DM diabetes mellitus, DR diabetic retinopathy, NPDR non-proliferative diabetic retinopathy, PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, NG neovascular glaucoma, VEGF-A vascular endothelial growth factor A, NG neovascular glaucoma, HbA 1C glycated hemoglobin, RLC retinal laser coagulation, GFR glomerular filtration rate
* Comparisons were performed using Kruskal–Wallis test between groups of DM patients only
** Comparisons were performed using Kruskal–Wallis test between groups of patients with DM and without DM operated for cataract
*** Comparisons were performed using Kruskal–Wallis test between all groups of patients
aData are presented as median (25th percentile; 75th percentile)
bData are presented as median (95% CI)
cData are presented as weight percentage in group (%)
After the end of the follow-up period, DR stage and visual acuity were assessed and all complications were registered.
Statistical Analysis
The descriptive statistics are presented as median (25th percentile; 75th percentile) as well as weight percentage (%) for the prevalence parameters. Visual acuity data are presented as median (95% CI). To compare more than two independent groups, Kruskal–Wallis test was used; then pairwise comparisons using Mann–Whitney test with the Bonferroni adjustment was performed. Qualitative data were compared and contingency tables evaluated using Chi-square test. The dependent groups were compared using Wilcoxon test. Correlation analysis was performed using Spearman’s rank test (R). To identify the risk factors in the follow-up groups at the completion of the prospective phase, risk (I R), risk difference (RD), relative risk (RR), and odds ratio (OR) with regard to the occurrence of an endpoint were calculated using the contingency tables. Kaplan–Meier survival curve was displayed based on the prospective follow-up data, and the significance of the difference was analyzed using a Cox proportional hazards regression model.
The critical significance level (P) for statistical hypothesis testing was set at <0.05.
The study protocol was approved by local ethics committee and was conducted in accordance with the guidelines of the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Results
The cohort comprised 120 patients operated from cataract: 19 had DM type 1 [age, 51 (31; 59); DM duration, 15 (8; 35); average HbA1C, 8.6% (7.5; 10.6)] and 101 had DM type 2 [age, 70 (64; 73); DM duration, 14 (10; 20), average HbA1C, 7.9% (6.9; 8.8)]. There were 16 patients with DM and uncontrolled NG, operated due to high intraocular pressure [age, 60 (53; 67); DM duration, 12.5 (6.5; 20.5), average HbA1C, 7.7% (7.4; 8.9)]. 22 patients without diabetes, which operated from age-related cataract, were 72 years old (65; 78). The baseline best-corrected visual acuity was 0.24 (0.14; 0.33) in patients with DM type 1, 0.23 (0.19; 0.27) in patients with DM type 2, and 0.24 (0.13; 0.36) in patients without DM.
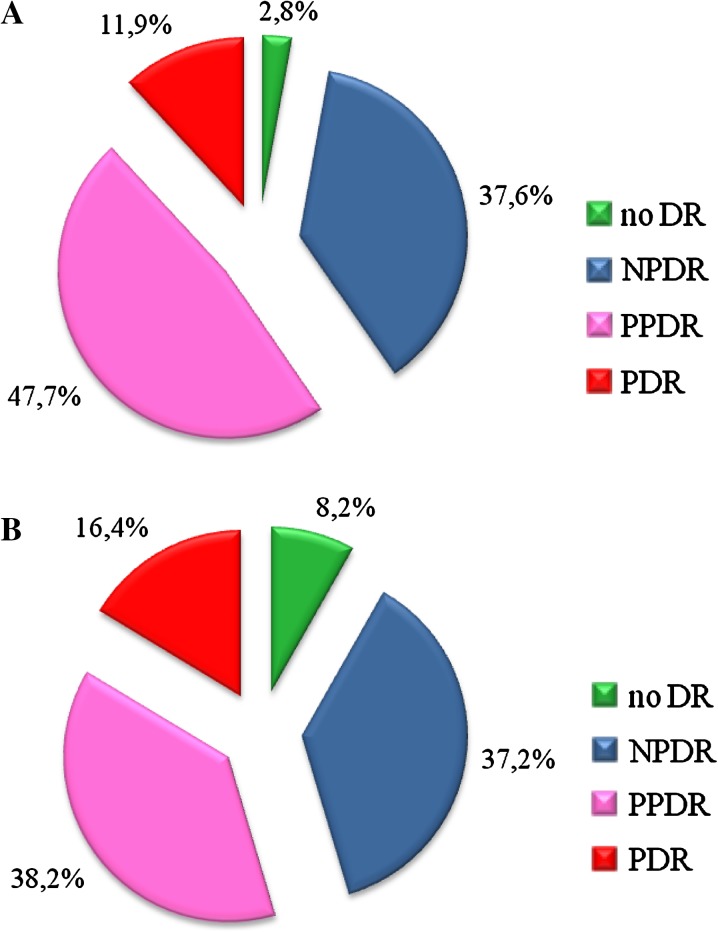
The prevalence of DR among the operated DM patients with cataract is shown in Fig. 1. Post-operative examination (5–7 days after operation) has shown that no signs of DR were present in 8.2% of patients, and NPDR, PPDR, and PDR were present in 37.2%, 38.2%, and 16.4% of patients, respectively. The likelihood of PDR detection post-operation was higher by 4.5%. It should also be noted that before operation PDR was correctly diagnosed in 50% cases only because of lens opacity interfered the adequate fundus examination.
Fig. 1.
Distribution of operated diabetic patients with cataract according to DR type [ examinations have been performed before (a) and after surgery (b)]. No DR no signs of diabetic retinopathy were detected, NPDR non-proliferative diabetic retinopathy, PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy
Glucose Levels in ACL
On the day of surgery, ACL glucose levels and fasting plasma glucose were similar in different groups of DM patients. However, the median of ACL glucose levels was slightly increased in the groups with severe forms of DR (from 3.08 in patients without DR to 4.85 in DM patients with neovascular glaucoma). A direct relationship exists between ACL glucose levels and fasting plasma glucose (R = 0.49, P < 0.001), Table 1. HbA1C levels in the different groups of diabetic patients were also similar, except for the group of patients without DR (P < 0.001) (in this group, DM was compensated to the highest possible degree as the patients had the lowest glucose levels both in blood and in ACL).
VEGF-A Levels in ACL
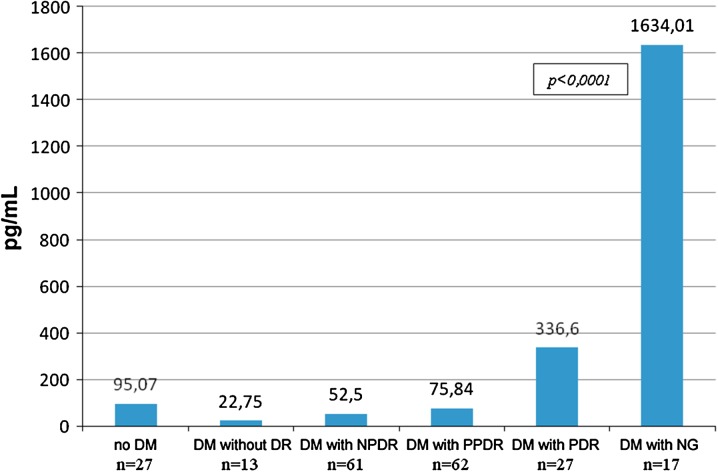
VEGF-A levels in ACL of diabetic patients were increased in the groups with more severe stages of DR. The lowest VEGF-A levels in ACL were seen in diabetic patients without signs of DR [22.75 pg/mL (10.78; 63.36)]; they were even lower than those in the control group [95.07 pg/mL (60.92; 129.22)], and the difference was significant (P = 0.008). In the groups of patients with a more severe stage of DR, VEGF-A levels in ACL were higher, with 52.5 pg/mL (44.52; 88.95) in patients with NPDR and 75.84 pg/mL (71.7; 123.58) in those with PPDR (the difference with a group of patients without DR was significant, P = 0.007). In patients with PDR, VEGF-A levels in ACL attained the highest values [336.6 pg/mL (232.3; 410.74); P < 0.0001]. Therefore, in diabetic patients with PDR, VEGF-A levels in ACL were three times higher than those in control groups and 15 times higher than those in diabetic patients without DR (Fig. 2). Of interest, VEGF-A levels in ACL continue to increase with further DR progression. Indeed, in diabetic patients with NG (terminal stage of DR) these levels attained 1,634.01 pg/mL (610.69; 2657.33) and were 4.8 times higher than in diabetic patients with PDR and 17 times higher than in the control group (differences with all groups were significant, P < 0.05).
Fig. 2.
Median of VEGF-A levels in ACL in operated patients (pg/mL) as function of DR severity. Axis Y, VEGF-A in ACL. ACL anterior chamber liquid, DM diabetes mellitus, DR diabetic retinopathy, NPDR non-proliferative diabetic retinopathy, PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, NG neovascular glaucoma, VEGF-A vascular endothelial growth factor A
Correlation analysis has shown association between VEGF-A levels and DR severity (R = 0.59, P < 0.001). However, no correlations have been found between VEGF-A levels in ACL and the level of HbA1C, GFR, blood pressure.
Visual Function in Patients with Cataract
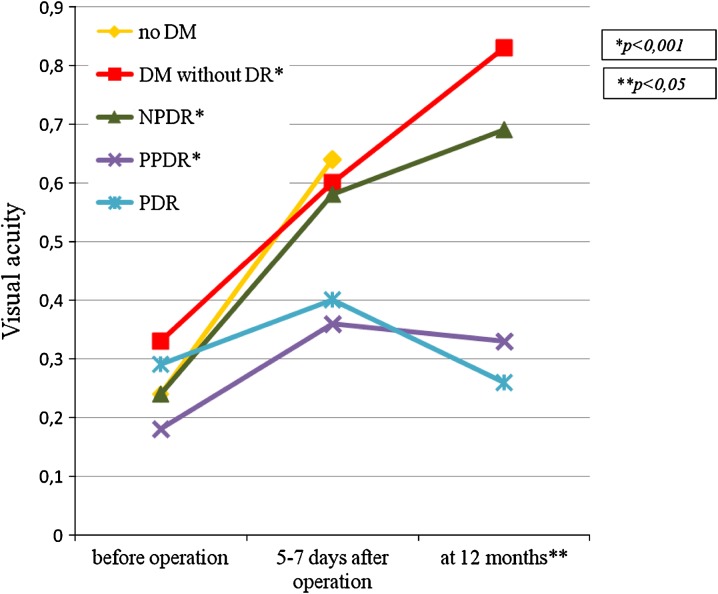
Visual function assessments in operated diabetic patients with cataract on the next day, at 5th–7th day and at 1st, 3rd, 6th, and 12th month after surgery in the post-operative period have shown that the best results were achieved in the groups of patients with less severe stages of DR (P < 0.001). Pre-operative visual acuity in groups of patients with different DR stage was similar (P = 0.31). This fact could be explained by the significant influence of lens opacity on visual acuity. The majority of patients had the hard, progressive, cortical or posterior subcapsular cataract. In groups of diabetic patients without DR, with NPDR and with PPDR, visual acuity was significantly increased post-operation and maintained at high level over 12 months (P < 0.05). However, in patients with severe stages of DR, visual acuity in the delayed post-operative period gradually decreased and returned to baseline values at 12 months after operation (Fig. 3).
Fig. 3.
Visual acuity changes in patients operated for cataract. DM diabetes mellitus, DR diabetic retinopathy, NPDR non-proliferative diabetic retinopathy, PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, NG neovascular glaucoma. *P < 0.001—the significance of the differences between baseline visual acuity and visual acuity at 12 months after surgery (Wilcoxon test). **P < 0.05—the significance of the differences of the visual acuity at 12 months between groups (Mann–Whitney test with the Bonferroni adjustment). The authors checked the best-corrected visual acuity by the Golovin–Sivtsev table from a 5-m distance. It had been estimated in conventional units. For example, visual acuity 1.0 (Golovin–Sivtsev table) is equivalent to 20/20 (Snellen chart); visual acuity 0.1 (Golovin–Sivtsev table) is equivalent to 20/200 (Snellen chart)
Analysis of Cataract Surgery Outcomes
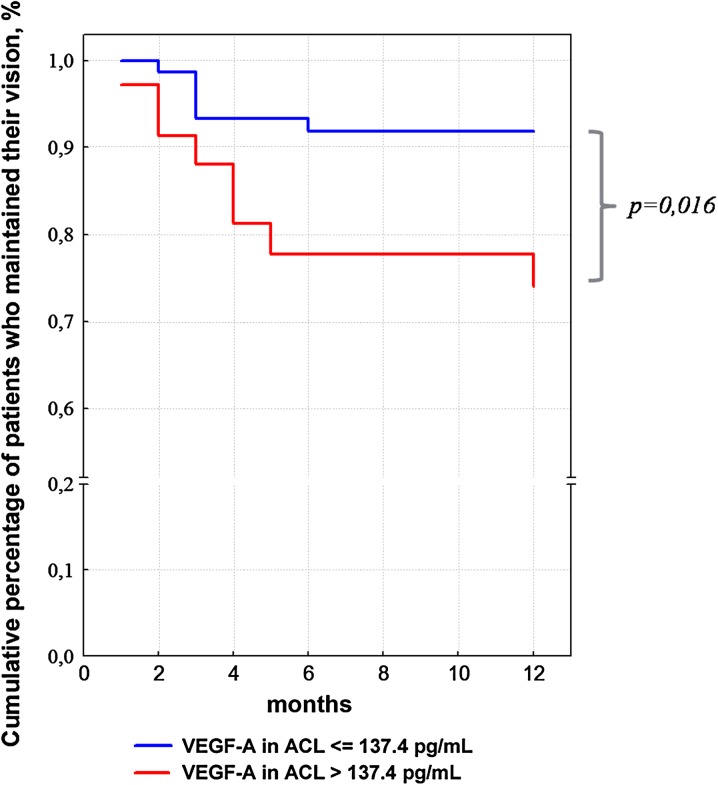
There is an ongoing discussion on the high-risk of complications after cataract surgery in diabetic patients. Therefore, the authors followed the patients operated for cataract for 12 months after surgery by measuring visual acuity, assessing DR stage, and monitoring the complications. Kaplan–Meier survival curve was displayed based on data obtained in the observation period: follow-up outcomes were a decrease in the visual acuity by more than 0.2, vision loss and development of NG (or rubeosis iridis). Patients treated with RLC (who maintained their visual acuity), patients lost to follow-up, and patients who required eye surgery for other indications (different from follow-up outcomes) were not censored (i.e., not taken into account as patients with vision loss). As seen in Fig. 4, the incidence of vision loss was higher in patients with high baseline VEGF-A levels in ACL. The highest incidence of these episodes was observed from month 3 to 5. In diabetic patients with the highest VEGF-A levels in ACL (25% of all patients from the upper quartile, corresponding to patients with VEGF-A levels in ACL >137.4 pg/mL), vision loss at 12 months was observed in 25% of cases, while in patients with VEGF-A levels in ACL <137.4 pg/mL, only in 8–9% of cases. As was shown using a Cox proportional hazards regression model, these differences were statistically significant (P = 0.016).
Fig. 4.
Percentages of diabetic patients with different VEGF-A ACL levels in whom visual acuity at 12 months were maintained at the same level as post-operation. ACL anterior chamber liquid, VEGF-A vascular endothelial growth factor A
In the overall follow-up group, 7.6% of patients developed NG by 12 months after surgery, 40% patients were treated with RLC due to severe forms of DR, and only 52.4% of patients had no complications during the post-operative period.
To evaluate the risks of NG development in diabetic patients after cataract surgery, the authors performed a variance analysis assessing each factor’s contribution (Table 2). The whole population was divided according to the presence of the factor studied, and the risk parameters were calculated for each factor. Patients with high VEGF-A levels in ACL (>137.4 pg/mL) had a RR with regard to NG development of 9.62 and OR of 12.3, i.e., the risk of NG development in the post-operative period was 9.62–12.3 higher in these patients than in the others (the differences were statistically significant, P = 0.0004). Patients with low GFR (<60 mL/min) had 5.9–7 times higher risk than those who had GFR higher than 60 mL/min (P = 0.009). Other risk factors (such as, the presence of concurrent open-angle glaucoma, history of RLC, HbA1C higher than 7.5%, marked hypertension, the presence of microalbuminuria or proteinuria) also increased the risk of NG development by 1.65–3.5 times; however, the differences did not reach statistical significance P > 0.05.
Table 2.
Estimated values of risk (IR), risk difference (RD), relative risk (RR), and odds ratio (OR) for the occurrence of NG in diabetic patients operated for cataract
| Variable | I R NG (%) | RDNG (%) | RRNG | ORNG | χ 2 NG (P value) |
|---|---|---|---|---|---|
| VEGF-A >137.4 pg/mL | 25 | 22.4 | 9.62 | 12.3 | 0.0004 |
| HbA1C >7.5% | 8.3 | 5.9 | 3.5 | 4.8 | 0.12 |
| Presence of open-angle glaucoma | 17.6 | 11.9 | 3.09 | 3.6 | 0.09 |
| History of RLC | 10.7 | 4.2 | 1.65 | 1.6 | 0.58 |
| Stage II or III hypertension | 9.1 | 4.1 | 1.82 | 1.8 | 0.58 |
| Microalbuminuria/proteinuria | 12.8 | 8.3 | 2.8 | 3.1 | 0.12 |
| GFR <60 mL/min | 17 | 14.1 | 5.9 | 7.0 | 0.009 |
NG neovascular glaucoma, VEGF-A vascular endothelial growth factor A, NG neovascular glaucoma, HbA 1C glycated hemoglobin, RLC retinal laser coagulation, GFR glomerular filtration rate
Discussion
Concomitant presence of DR and cataract worsens the visual prognosis in diabetic patients and makes it difficult to perform therapeutic interventions. This study has shown that lenticular opacities prevent accurate and prompt verification of DR stage, with PDR being accurately verified in 50% of cases only.
Hyperglycemia is clearly a trigger for DR development and, combined with other pathophysiological mechanisms, results in progression of retinopathy. In this study, the authors have not found any differences in ACL glucose levels, glycemia, and HbA1C in patients with different DR stages, except for diabetic patients without DR in whom ACL glucose values and HbA1C were at the lowest levels (differences in HbA1C were statistically significant). This may be explained by the fact that good metabolic control prevents the occurrence of DR signs, however, if DR has already developed, the markers of carbohydrate metabolism do not correlate well with the severity of DR anymore. Because the cause of the retina neovascularization is the severe ischemia the use of some medicines (statins, antiplatelets) should be investigated carefully.
At the same time, VEGF-A levels in ACL reflected the severity of DR. These levels were significantly different in various groups of diabetic patients. VEGF-A levels in ACL increased with DR progression; the values achieved were 16-fold higher than in patients without DM and 65-fold higher than in diabetic patients without DR. Similar results were shown in previous studies [17–21], which found the elevation the vitreous and aqueous concentration of VEGF in patients with DR. It proved the essential role of the VEGF in the DR development and neovascularization of the retina. PDR is associated with higher level of the VEGF in the ACL than NPDR. Moreover, NG due to PDR is associated with extremely high VEGF level. Several authors [22, 23] demonstrated the elevation VEGF value in the glaucomatous eyes and the benefits of the blockage of the VEGF in the eye [24, 25]. In this way, VEGF-A levels in ACL are associated with the severity of DR.
In the current study, cataract surgery and intraocular lens implantation enhanced the visual acuity in diabetic patients, but during follow-up final vision dramatically deteriorated in patients with PDR. Because the patients of this group had the highest HbA1C level, long DM duration about 16 years, more than half of them treated by panretinal RLC and high prevalence of the comorbid diseases, all these factors and high level of the VEGF-A can lead to progressive visual impairment. According to the results of this study, during 12-month follow-up for the operated diabetic patients the authors found an increase in the RR and OR with regard to complications due to DR progression in the groups of patients with high levels of VEGF-A (the upper quartile for the whole population studied, i.e. >137.4 pg/mL). Therefore, RR of NG development (and rubeosis iridis) following cataract extraction was 9.62, and OR was 12.3. This implies the high prognostic value of VEGF-A levels >137.4 pg/mL in evaluating the risk of complications and their onset times. Similar results were reported by Wakabayashi et al. [25]. They assessed the vitreous and ACL level of VEGF as a significant risk factor for the early post-operative hemorrhage and NVG development. Thus,medical approaches aimed to VEGF blockage may be helpful before cataract surgery among the diabetic patients with high-risk of NG development. For example in their study, Grover et al. [26] and Chalam et al. [27] demonstrated the effectiveness of the intracameral injection of the ranibizumab or bevacizumab in reversing iris neovascularization and decreasing intraocular pressure in cases of the NG. It was shown that intravitreal bevacizumab at the time of surgery was beneficial in reducing central macular thickness in short term [28–30]. Panretinal RLC also decreased ocular VEGF concentration and can prevent DR progression after phacoemulsification [31, 32].
In the present study, the authors aimed to find other risk factors which could be evaluated before surgery for better visual outcomes in diabetic patients. The authors revealed that decreasing of the GFR less than 60 mL/min, significantly increased the risk of NG development in post-operative period (by 5.9–7.0 times). It can be explained by expanded circulation of the waist product and, therefore, more severe retina hypoxia which resulted to the neo-vessels formation. There have been a number of studies [33, 34] aimed at investigating the relationship between DR stage and renal function which resulted in conflicting data. The authors used the surrogate markers of the renal insufficiency (albuminuria and estimated GFR) and DR (microaneurysms quantity). The current study showed that the intraocular VEGF as a direct marker of DR and cut-off limit of the GFR less than 60 mL/min correlated with visual outcomes and significantly increased neovascularization.
Most diabetic patients have many comorbidities, and the efficacy of treatment is often decreased. The literature suggests the increased frequency of complications in diabetic patients. Therefore, these patients should be closely monitored for the development of adverse events, maximum compensation of diabetes, main metabolic variables, and concurrent illnesses.
Conclusion
VEGF-A clearly plays a key role in the development and progression of DR, and this study has shown that its content in ACL increases with DR progression. On the other hand, VEGF-A may be of prognostic value in evaluating the potential risk of further neovascularization progression in diabetic patients.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novartis Pharma LLC. Dr. Kuzmin is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Anatoly Kuzmin, Dmitry Lipatov, Timofei Chistyakov, Olga Smirnova, Margarita Arbuzova, Alexander Ilin, Marina Shestakova and Ivan Dedov declare no conflict of interest.
Compliance with ethics guidelines
The study protocol was approved by local ethics committee and was conducted in accordance with the guidelines of the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.World Health Organization. Sight test and glasses could dramatically improve the lives of 150 million people with poor vision. Press release, 11 October 2006. http://www.who.int/mediacentre/news/releases/2006/pr55/en/index.html (Accessed 1 Dec 2012). [PubMed]
- 2.Dagher Z, Park YS, Asnaghi V, et al. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 3.Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.The diabetes control and complications trial research group The affect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 6.Simó R, Carrasco E, García-Ramírez M, Hernández C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 7.Noma H, Funatsu H, Mimura T, Harino S, Sone T, Hori S. Increase of vascular endothelial growth factor and interleukin-6 in the aqueous humour of patients with macular oedema and central retinal vein occlusion. Acta Ophthalmol. 2010;88:646–651. doi: 10.1111/j.1755-3768.2009.01524.x. [DOI] [PubMed] [Google Scholar]
- 8.Kerbel RobertS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shima D, Adamis AP, Yeo KT, Yeo TK, et al. Hypoxic regulation of vascular permeability factor (vascular endothelial growth factor) mRNA and protein secretion by human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:900. [Google Scholar]
- 10.Aiello LP, Ferrara N, King GL. Hypoxic regulation and bioactivity of vascular endothelial growth factor: characterization in retinal microvascular pericytes and pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:1868. [Google Scholar]
- 11.D’Amore PA. Vascular endothelial cell growth factor-A. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malecaze F, Clamens S, Simorre-Pinatel V, Mathis A, et al. Expression of angiogenic growth factors in diabetic neovascular membranes. Invest Ophthalmol Vis Sci. 1993;34:1039. [Google Scholar]
- 13.Adamis AP, Miller JW, O’Reilly M, Brown L, et al. Vascular permeability factor (vascular endothelial growth factor) is produced in the retina and elevated levels are present in the aqueous humor of eyes with iris neovascularization. Invest Ophthalmol Vis Sci. 1993;34:1440–1444. [Google Scholar]
- 14.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 16.Kohner EM, Porta M. Screening for diabetic retinopathy in Europe: a field guide-book. Copenhagen: WHO; 1992. [Google Scholar]
- 17.Selim KM, Sahan D, Muhittin T, Osman C, Mustafa O. Increased levels of vascular endothelial growth factor in the aqueous humor of patients with diabetic retinopathy. Indian J Ophthalmol. 2010;58:375–379. doi: 10.4103/0301-4738.67042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 19.Endo M, Yanagisawa K, Tsuchida K, et al. Increased levels of vascular endothelial growth factor and advanced glycation end products in aqueous humor of patients with diabetic retinopathy. Horm Metab Res. 2001;33:317–322. doi: 10.1055/s-2001-15122. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Sun H, Xu J, Kang J. Level of vascular endothelial growth factor and interleukin-6 in aqueous humor in diabetic retinopathy patients. Yan Ke Xue Bao. 2010;25:26–30. doi: 10.3969/g.issn.1000-4432.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Funatsu H, Yamashita H, Shimizu E, Kojima R, Hori S. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina. 2001;21:469–477. doi: 10.1097/00006982-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mizote M, Baba T, Hirooka K, Yamaji H, Shiraga F. Vascular endothelial growth factor concentrations in aqueous humor before and after subconjunctival injection of bevacizumab for neovascular glaucoma. Jpn J Ophthalmol. 2010;54:242–244. doi: 10.1007/s10384-009-0788-y. [DOI] [PubMed] [Google Scholar]
- 23.Kim YG, Hong S, Lee CS, et al. Level of vascular endothelial growth factor in aqueous humor and surgical results of ahmed glaucoma valve implantation in patients with neovascular glaucoma. J Glaucoma. 2009;18:443–447. doi: 10.1097/IJG.0b013e3181895e5c. [DOI] [PubMed] [Google Scholar]
- 24.Lim TH, Bae SH, Cho YJ, Lee JH, Kim HK, Sohn YH. Concentration of vascular endothelial growth factor after intracameral bevacizumab injection in eyes with neovascular glaucoma. Korean J Ophthalmol. 2009;23:188–192. doi: 10.3341/kjo.2009.23.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakabayashi Y, Usui Y, Okunuki Y, Ueda S, Kimura K, Muramatsu D, Kezuka T, Goto H. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:6403–6410. doi: 10.1167/iovs.12-10367. [DOI] [PubMed] [Google Scholar]
- 26.Grover S, Gupta S, Sharma R, Brar VS, Chalam KV. Intracameral bevacizumab effectively reduces aqueous vascular endothelial growth factor concentrations in neovascular glaucoma. Br J Ophthalmol. 2009;93:273–274. doi: 10.1136/bjo.2008.145714. [DOI] [PubMed] [Google Scholar]
- 27.Chalam KV, Gupta SK, Grover S, Brar VS, Agarwal S. Intracameral Avastin dramatically resolves iris neovascularization and reverses neovascular glaucoma. Eur J Ophthalmol. 2008;18:255–262. doi: 10.1177/112067210801800214. [DOI] [PubMed] [Google Scholar]
- 28.Fard MA, Yazdanei Abyane A, Malihi M. Prophylactic intravitreal bevacizumab for diabetic macular edema (thickening) after cataract surgery: prospective randomized study. Eur J Ophthalmol. 2011;21:276–281. doi: 10.5301/EJO.2010.1405. [DOI] [PubMed] [Google Scholar]
- 29.Lanzagorta-Aresti A, Palacios-Pozo E, Menezo Rozalen JL, Navea-Tejerina A. Prevention of vision loss after cataract surgery in diabetic macular edema with intravitreal bevacizumab: a pilot study. Retina. 2009;29:530–535. doi: 10.1097/IAE.0b013e31819c6302. [DOI] [PubMed] [Google Scholar]
- 30.Takamura Y, Kubo E, Akagi Y. Analysis of the effect of intravitreal bevacizumab injection on diabetic macular edema after cataract surgery. Ophthalmology. 2009;116:1151–1157. doi: 10.1016/j.ophtha.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Shah AS, Chen SH. Cataract surgery and diabetes. Curr Opin Ophthalmol. 2010;21:4–9. doi: 10.1097/ICU.0b013e328333e9c1. [DOI] [PubMed] [Google Scholar]
- 32.Suto C, Kitano S, Hori S. Optimal timing of cataract surgery and panretinal photocoagulation for diabetic retinopathy. Diabetes Care. 2011;34:e123. doi: 10.2337/dc11-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mottl AK, Kwon KS, Garg S, Mayer-Davis EJ, Klein R, Kshirsagar AV. The association of retinopathy and low GFR in type 2 diabetes. Diabetes Res Clin Pract. 2012;12:S0168. doi: 10.1016/j.diabres.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penno G, Solini A, Zoppini G, for the Renal Insufficiency And Cardiovascular Events (RIACE) Study Group et al. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:2317–2323. doi: 10.2337/dc12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]