Abstract
Introduction
Coordinates of anatomical landmarks are captured using dynamic MRI to explore whether a proposed two-sling mechanism underlies hyolaryngeal elevation in pharyngeal swallowing. A principal components analysis (PCA) is applied to coordinates to determine the covariant function of the proposed mechanism.
Methods
Dynamic MRI (dMRI) data were acquired from eleven healthy subjects during a repeated swallows task. Coordinates mapping the proposed mechanism are collected from each dynamic (frame) of a dynamic MRI swallowing series of a randomly selected subject in order to demonstrate shape changes in a single subject. Coordinates representing minimum and maximum hyolaryngeal elevation of all 11 subjects were also mapped to demonstrate shape changes of the system among all subjects. MophoJ software was used to perform PCA and determine vectors of shape change (eigenvectors) for elements of the two-sling mechanism of hyolaryngeal elevation.
Results
For both single subject and group PCAs, hyolaryngeal elevation accounted for the first principal component of variation. For the single subject PCA, the first principal component accounted for 81.5% of the variance. For the between subjects PCA, the first principal component accounted for 58.5% of the variance. Eigenvectors and shape changes associated with this first principal component are reported.
Discussion
Eigenvectors indicate that two-muscle slings and associated skeletal elements function as components of a covariant mechanism to elevate the hyolaryngeal complex. Morphological analysis is useful to model shape changes in the two-sling mechanism of hyolaryngeal elevation.
Keywords: Imaging and Visualization in Biomechanics, Medical Imaging and Visualization, Deglutition, Morphometrics, Hyolaryngeal complex
1. Introduction
A primary movement in normal swallowing is hyolaryngeal elevation to help protect the airway and open a relaxed upper esophageal sphincter. Therefore, characterizing the movement of the hyoid and larynx is fundamental to the study of deglutition and dysphagia (Kim and McCullough 2008; Leonard and others 2000; Logemann and others 2000; Steele and others 2011). The hyolaryngeal complex is an interconnected set of structures including the hyoid bone, laryngeal cartilages, and associated muscles and ligaments that incorporate the trachea and esophagus (Figure 1a). The underlying mechanism of hyolaryngeal elevation is commonly described as the combined function of suprahyoid muscles aided by the thyrohyoid muscle (Cook and others 1989; Ertekin and Aydogdu 2003; Matsuo and Palmer 2008; Mepani and others 2009). Recent evidence shows that the long pharyngeal muscles also have mechanical advantage in elevating the hyolaryngeal complex (Pearson and others 2012b). This suggests a two-sling mechanism underlying hyolaryngeal elevation in the pharyngeal phase of swallowing. The suprahyoid muscles comprise the anterior muscular sling and the long pharyngeal muscles comprise the posterior sling (Figure 1b). Furthermore, physiological data show that muscles that comprise these muscular slings are active during swallowing (Pearson and others 2013; Van Daele and others 2005). The purpose of this study is to determine the action of the proposed two-sling mechanism of hyolaryngeal elevation by modeling hyolaryngeal mechanics using morphometric analysis of anatomical landmark coordinate data.
Figure 1.
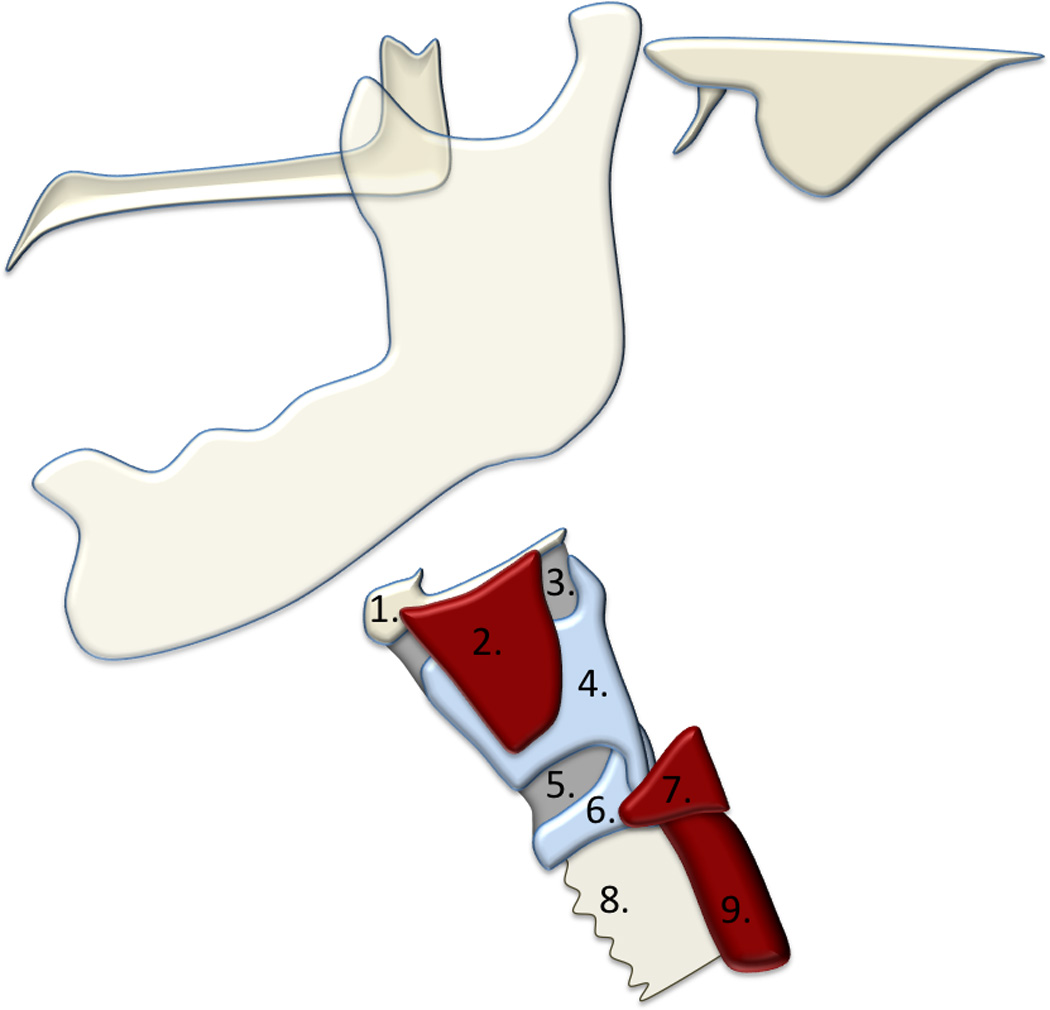
a. The hyolaryngeal complex includes the: 1.) hyoid, 2.) thyrohyoid muscle 3.) thyrohyoid membrane, 4.) thyroid cartilage, 5.) cricothyroid membrane, 6.) cricoid cartilage, and 7.) cricopharyngeus. The 8.) trachea and 9.) esophagus are incorporated into the hyolaryngeal complex. Elevation of this complex helps to protect the airway and open a relaxed upper esophageal sphincter.
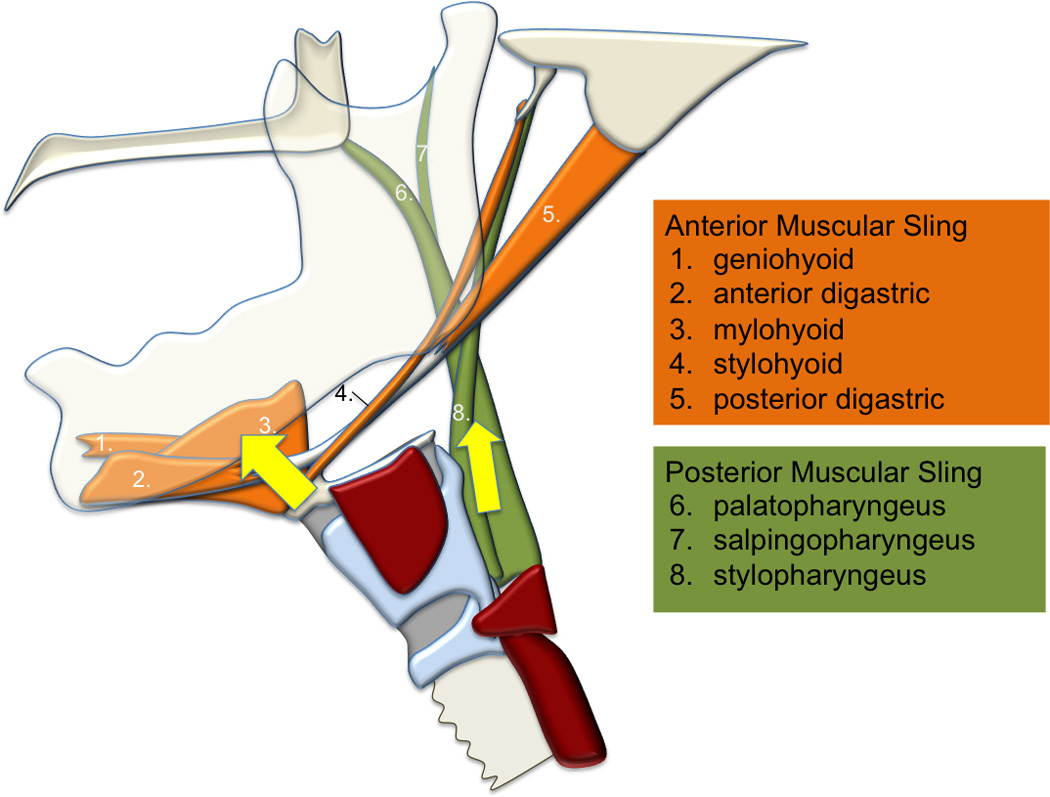
b. Proposed two-sling mechanism of hyolaryngeal elevation
Using kinematic measurements to characterize hyolaryngeal mechanics is confounded by the covariant elements of the swallowing mechanism. It is possible, for example, that the suprahyoid muscles alone are responsible for hyoid elevation and laryngeal elevation. Singular kinematic measurements cannot fully describe the covariant function of various muscle groups. However, multivariate morphological analysis of coordinates mapping features of a dynamic system over time can be used to characterize covariant shape changes associated with swallowing.
To characterize the morphology of the mechanism underlying hyolaryngeal elevation, we developed a method of tracking changes using nine anatomical landmarks (Figure 2). The first five coordinates track the relative position of three skeletal levers: the vertebrae, mandible and cranial base. The four remaining coordinates mark features of the hyolaryngeal complex including the hyoid, anterior larynx, posterior larynx, and upper esophageal sphincter. These coordinates approximate muscle attachments of the proposed two-sling mechanism (suprahyoid and long pharyngeal muscles)(Table 1).
Figure 2.
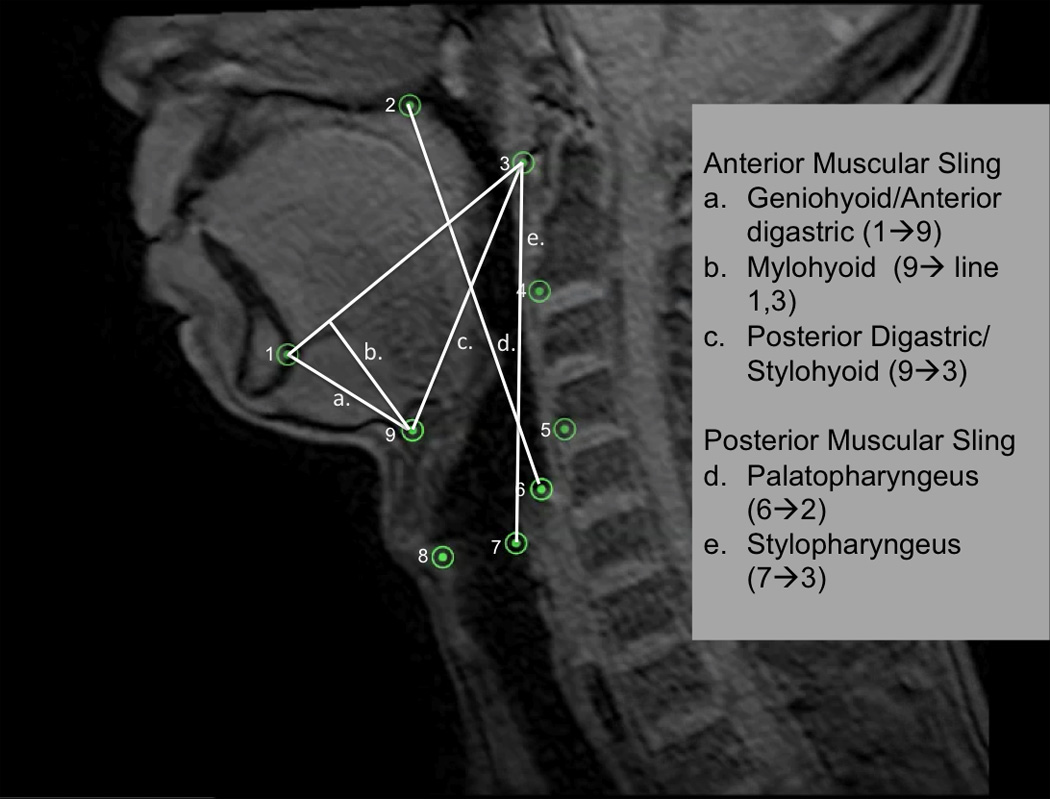
Nine coordinates mapping the features of the two-sling mechanism for hyolaryngeal elevation is mapped here on a T1 weighted sagittal plane dynamic MRI. Lines representing the two-sling mechanism are superimposed on the image and labeled.
Table 1.
Muscles are mapped by coordinates and organized into two functional groups hypothesized to elevate the hyolaryngeal complex
| Muscles | Representative Coordinates |
Functional Groups | Hypothesized Result |
|---|---|---|---|
| Geniohyoid | 1–9 | Anterior muscular sling (Suprahyoid muscles) | Eigenvector of 9 towards line 1–3 (mandible) |
| Stylohyoid | 3–9 | ||
| Digastric | 1-9-3 | ||
| Mylohyoid | 9→ line 1–3 (mandible) | ||
| Palatopharyngeus | 2–6 | Posterior muscular sling (Long pharyngeal muscles) | Eigenvector of 7 towards point 3 (cranial base) |
| Stylopharyngeus | 3–7 |
To investigate whether two muscular slings work together to elevate the hyolaryngeal complex in swallowing, a principal components analysis (PCA) was applied to the coordinate data. Morphometric PCA is a dimensionless multivariate analysis of a covariance matrix of coordinate data that has been fitted by Procrustean superimposition. The Procrustean fit adjusts for rotation of images and overall size differences between subjects. Each main source (principal component) of variance within the covariance matrix is determined such that the first principal component describes the greatest amount of covariance in the matrix, the second principal component describes the second greatest amount of covariance, and so on. These principal components are assigned eigenvalues. Eigenvalues, similar to squared correlation coefficients in bivariate data, describe what portion of the total variance is explained by each principal component. Within each principal component, vectors of shape change (eigenvectors) for each coordinate are determined and used to demonstrate shape changes associated with each principal component. These vectors are not to be interpreted as actual mean distance measurements but rather the magnitude and direction of covariance of a particular coordinate within a covariant system. Shape changes are represented by consistent vectors of change for each coordinate in the context of the entire system (Webster and others 2010).
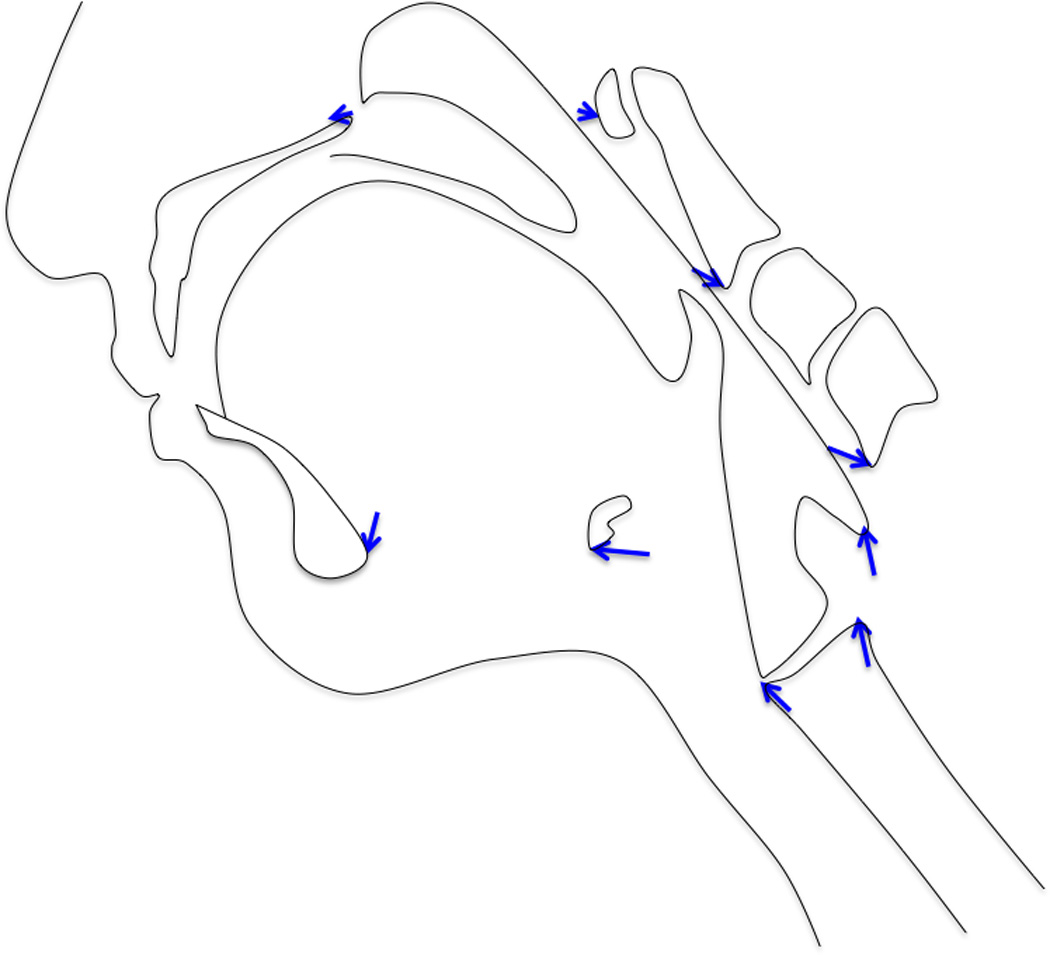
In this study we were interested in determining whether the anterior and posterior slings function to elevate the hyolaryngeal complex. By analyzing shape changes of the mechanism using PCA, the effect of the two-sling mechanism was evaluated. We hypothesized that if the effect of only one sling is observable in hyolaryngeal elevation, then all landmarks representing features of the hyolaryngeal complex should have eigenvectors directed towards the common attachment site for that sling (Figure 3). If both slings have an effect, then landmarks representing the distal anterior muscular sling attachments sites should approximate toward their proximal attachments and the same should be observed for the posterior muscular sling.
Figure 3.
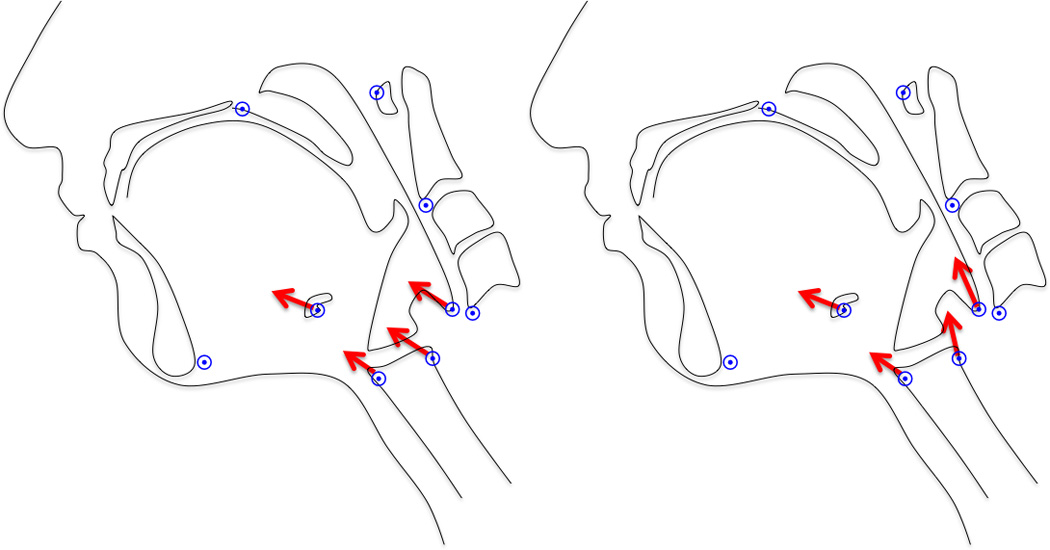
Testing the mechanism. If one sling underlies hyolaryngeal elevation, the eigenvectors of coordinates representing the hyolaryngeal complex should have a common direction as pictured on the left (in this case, the vectors for the anterior sling are depicted). If two slings underlie the system, eigenvectors of coordinates representing the distal attachment of the anterior and posterior muscular sling should diverge as pictured on the right.
We conducted two morphometric analyses to investigate this question and to demonstrate the capacity of this analysis to generate patient-specific and population data. We first analyzed coordinates of multiple swallows from one young healthy subject. In this way we control for the effects of individual differences (gender and size) and demonstrate shape changes attributable solely to movement of the hyolaryngeal complex. In the second study we recorded coordinates at minimum and maximum hyolaryngeal excursion from eleven young healthy subjects to demonstrate the vectors of shape change in a group of young healthy subjects.
2. Methods
2.1 Dynamic MRI
Six males and five females age 22–30 (mean age=25) were recruited for this study under a research protocol approved by the Boston University Medical Campus Institutional Review Board. All eleven subjects were judged to have normal swallowing ability by a speech-language pathologist. In a previous study we reported whole muscle T2 signal profiles derived from muscle functional MRI (mfMRI) acquired from these subjects (Pearson and others 2013). In this same series of acquisitions, two-planar (sagittal and coronal) T1-weighted dynamic MRI images were acquired from subjects during a repeated swallow task. Subjects drew the bolus from a tube connected to a container of 400ml thin liquid with contrast (magnesium). Acquisition parameters included: T1 weighted fast gradient echo sequence with TE/TR = 0.9/2.4 ms, 10 mm slice thickness, and a temporal resolution of 8.6 frames per second. In this study only the sagittal images were used, resulting in a 4.3 frame per second analysis.
2.2 Anatomical Landmark Coordinates
Nine easily identifiable anatomical landmarks which map three skeletal levers and features of the hyolaryngeal complex were selected for this analysis based on prior anatomical research and a method originally developed for analyzing videofluoroscopy (Pearson and others 2012a)(Figure 4). The first five coordinates tracked the relative position of the cervical vertebrae, mandible and cranial base as follows: the anterior-inferior edge of C2 and C4 vertebrae; the mandible at the inferior genial tubercles; the posterior edge of the hard palate where the soft palate attaches; and the tubercle of the atlas representing a two-dimensional pivot for all three levers. The four remaining coordinates mark features of the hyolaryngeal complex as follows: the anterior inferior edge of the hyoid; the anterior and posterior commissures of the vocal folds indicating an anterior and posterior laryngeal coordinate; and the inferior point of the hypopharynx air column proximal to the upper esophageal sphincter. In the current study, these same coordinates are mapped on sagittal plane dynamic MRI scans.
Figure 4.
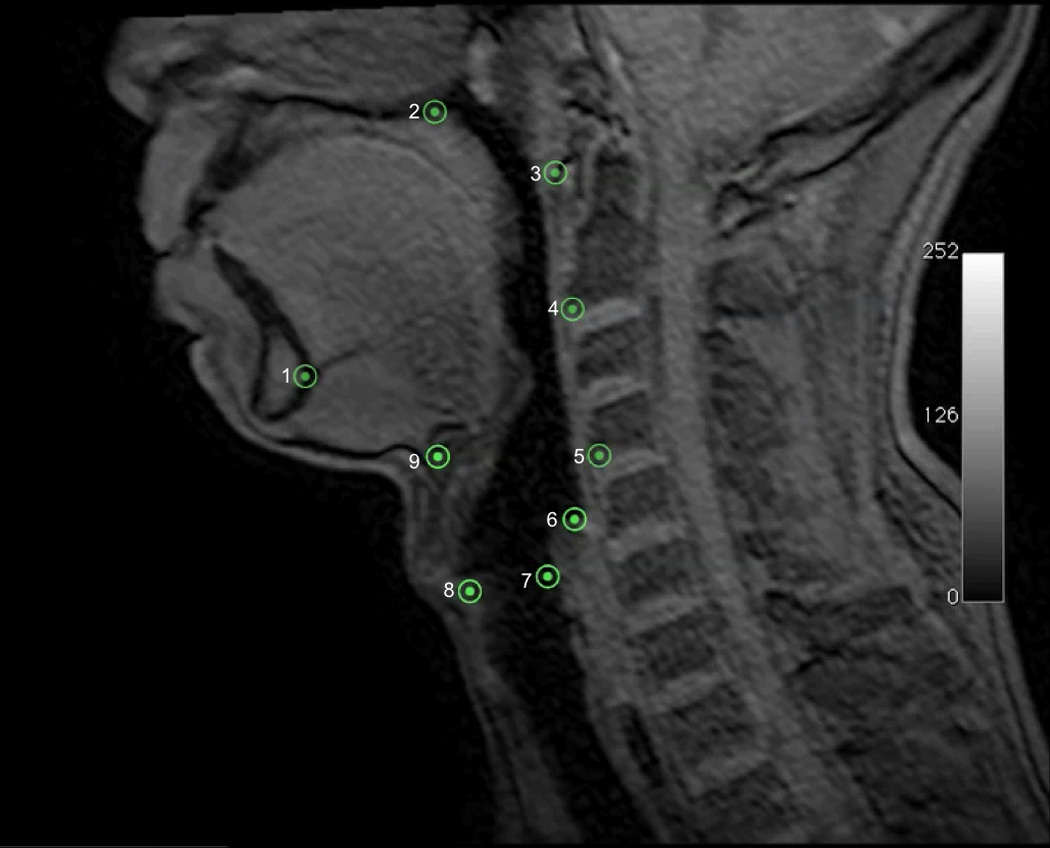
Nine coordinates mapping the features of the two-sling mechanism for hyolaryngeal elevation is mapped here on a T1 weighted sagittal plane dynamic MRI. Five coordinates(1=genial tubercles of the mandible, 2=posterior edge of the hard palate, 3=anterior tubercle of C1, 4=anterior inferior edge of C2, 5=anterior inferior edge of C4) map three skeletal levers: vertebrae (line 3→4→5), mandible (line 1→3), and cranial base continuous with the hard palate(line 2→3). The next four coordinates map interconnected features of the of the hyolaryngeal complex including: 6=inferior air column of hypopharynx proximal to the upper esophageal sphincter, 7=posterior commissure of the vocal folds (posterior larynx), 8=anterior commissure of the vocal folds (anterior larynx), 9=anterior inferior edge of the hyoid.
Coordinates were collected from MRI images using Osirix digital imaging and communication in medicine software (http://www.osirix-viewer.com). For the first morphometric analyses of the entire swallowing sequence of one subject, coordinates were collected from each frame of dynamic MRI studies of repeated swallows in a single randomly selected subject. For the second analysis, coordinates are collected from frames representing maximum and minimum hyolaryngeal elevation in the pharyngeal phase of swallowing.
2.3 PCA of a Single Subject Swallow
For the first PCA study, we evaluated shape changes within a single subject to control for between-subject differences (Okada and others 2011). As the temporal resolution of this dynamic MRI acquisition is much lower than videofluoroscopy, we used multiple repeated swallows in this study. In this series the subject swallowed 10 times. Landmark coordinates were collected from every frame, or dynamic, at 4.3 frames per second during a repeated swallows sequence (49 frames total). Each dynamic was classified according to bolus location and phase of oropharyngeal swallow. Oral Phase 1 was defined as the bolus in the anterior oral cavity, similar to the hold position in videofluoroscopic studies (Leonard and others 2000). Oral Phase 2 was defined as the bolus in the mid to posterior oral cavity, representing tongue propulsion prior to triggering pharyngeal swallowing. These operational definitions overlap with what has been called early and late stage II bolus transport in feeding (Hiiemae and Palmer 1999). Pharyngeal Phase 1 was defined as bolus in the hypopharynx during hyolaryngeal elevation. Pharyngeal Phase 2 was defined as pharyngeal clearance with the bolus passing through the upper esophageal sphincter. The breakdown for each classification was as follows: 15 dynamics (individual images in a series) for Oral Phase 1, 12 dynamics for Oral Phase 2, 12 dynamics for Pharyngeal Phase 1, and 10 dynamics for Pharyngeal Phase 2.
Morphometric analysis of coordinates was executed using MorphoJ software, an integrated software package for morphometric analysis including principal component analysis (PCA) (Klingenberg 2011). A covariance matrix of coordinates collected in each of the 49 dynamics was generated. Classifiers describing the phase of swallowing (Oral Phase 1 or 2, or Pharyngeal Phase 1 or 2) were assigned to each set of coordinates. A principal components analysis of this covariance matrix was executed. Results include eigenvalues of principal components, a scatter plot of the first two principal components coded by phase of bolus transport (to confirm the source of the first principal component), and shape change of the first principal component as modeled by eigenvectors of each landmark.
2.4 PCA of All Subjects
The second PCA study evaluated shape changes of all subjects pooled, comparing shape at minimum and maximum hyolaryngeal excursion (11 subjects; 2 frames per subject; 9 coordinates per frame) to demonstrate the effects of the two-sling mechanism in a group. Nine coordinates were mapped on an Oral Phase 1 and Pharyngeal Phase 1 frame from each subject to represent minimum and maximum excursion, respectively. Two investigators (an anatomist (WP) and a speech-language pathologist) independently charted all coordinates. Inter-judge reliability of all coordinates was r= 0.98–1.00.
After a Procrustean fit of all coordinates, a covariance matrix of Procrustean coordinates was generated using MorphoJ software. Classifiers for this study included gender and phase of swallowing; these were assigned to each set of nine coordinates. A principal components analysis of this covariance matrix was executed. Results of this part of the study include eigenvalues of all principal components, a scatter plot of the first two principal components coded by minimum and maximum hyolaryngeal excursion, and shape change of the first principal component as modeled by eigenvectors of each coordinate.
Eigenvectors associated with swallowing from the single subject swallow and of all subjects map hyolaryngeal mechanics. If eigenvectors of coordinates representing the distal attachments of the anterior muscular sling (suprahyoid muscles) and posterior muscular sling (long pharyngeal muscles) approximate anteriorly towards the mandible, then the suprahyoid muscles are indicated as the mechanism underlying hyolaryngeal elevation. If eigenvectors of coordinates representing the distal attachments of the anterior muscular sling (suprahyoid muscles) and posterior muscular sling (long pharyngeal muscles) approximate towards their respective proximal attachments, then a two-sling mechanism of hyolaryngeal elevation is indicated (Figure 3).
3. Results
3.1 PCA of a Single Subject Swallow
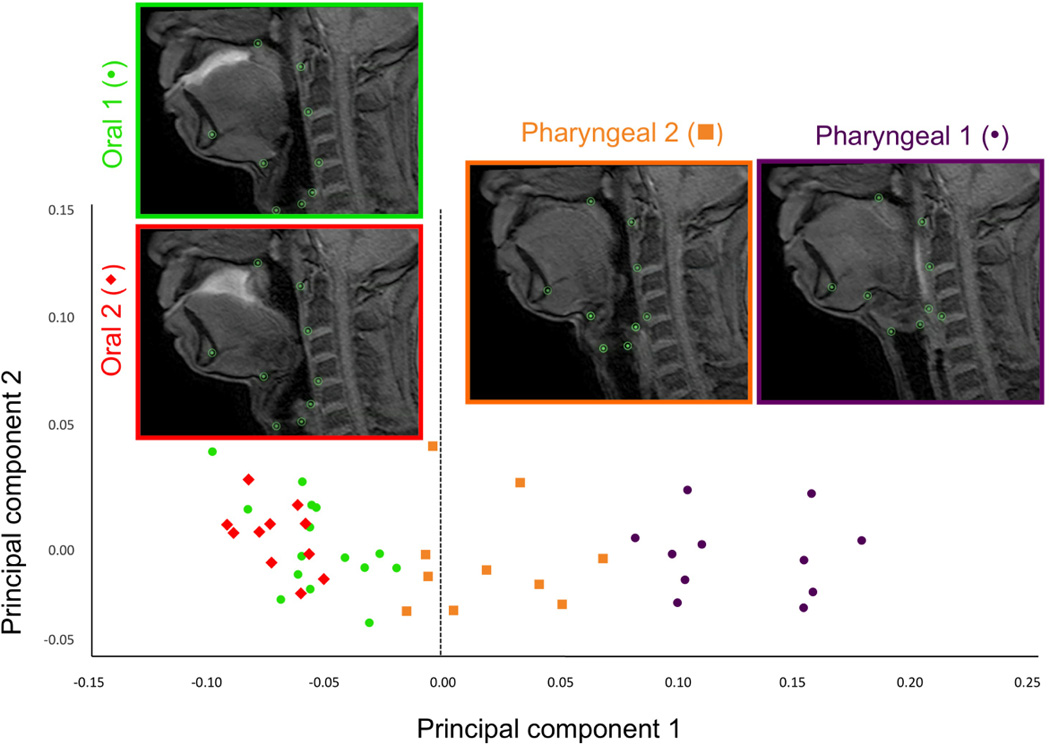
Principal component analysis of repeated swallows of a single subject including all phases of swallowing resulted in a first principal component (PC) with an eigenvalue representing 81.5% of the variance (Figure 5a). A scatter plot of PC1 and PC2 scores coded by phases of swallowing show that deviations of the grand mean are grouped by phase of swallowing on the PC1 axis, indicating that excursion of the hyolaryngeal complex underlies the variance of PC1 (Figure 5b). Predictably, maximum hyolaryngeal elevation, occurring at pharyngeal phase 1, is on one side of zero at the x-axis; and minimum hyolaryngeal elevation, occurring during the oral phases, is on the opposite side. These results indicate that these two excursion points explain the variance of PC1. Eigenvectors of the first PC for each coordinate are reported in Table 2. Shape changes, as determined by eigenvectors of each coordinate associated with hyolaryngeal excursion (principal component 1) are plotted and illustrated in Figure 5c.
Figure 5.
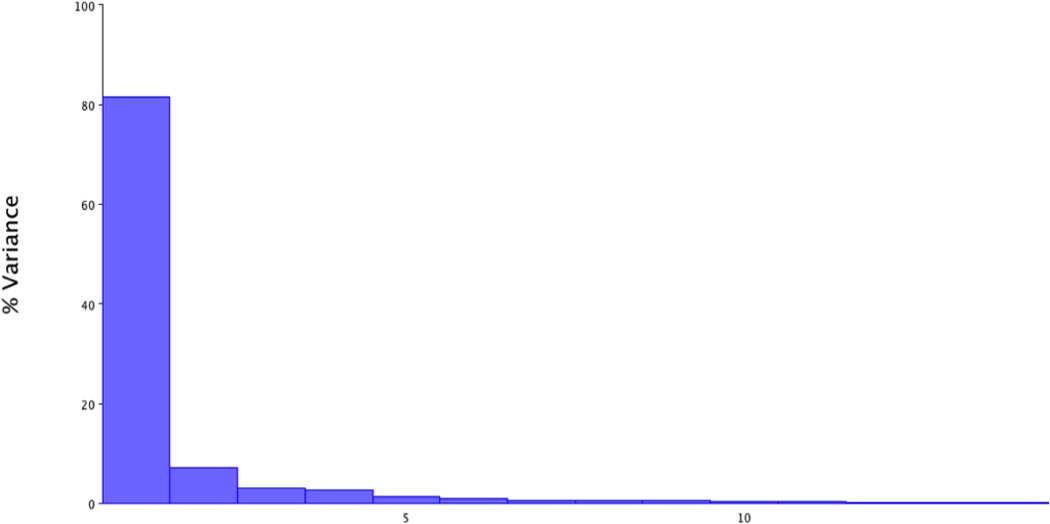
a. The first principal component of variance for one subject repeated swallows task accounts for 81.5% of the shape change variance
b. Plotted morphologies of one subject repeated swallows task. A scatter plot of PC scores (deviations of the grand mean) for each frame of swallowing coded by phase of swallowing shows that principal component 1 is largely defined by hyolaryngeal excursion. This scatter plot shows oral phase 1 and 2 at one end of the spectrum and pharyngeal phase 1 at the other indicating that these phases represent the extremes of hyolaryngeal excursion.
c. Eigenvectors of one subject repeated swallows task confirms two muscular slings underlie hyolaryngeal elevation
Table 2.
Eigenvectors are here reported from the first principal component of the single subject repeated swallows and the between subject swallows. Eigenvectors indicate the direction of variance in the covariance matrix for each landmark with a common origin of (0,0) set for each eigenvector. A magnitude for each vector was calculated using Pythagoras’ theorem. The magnitude should not be construed as relative distance of shape change, rather a reflection of the distribution of covariance in shape change. Greater magnitude reflects a tighter distribution of covariance meaning that shape changes were less random.
| Anatomical Landmark |
Eigenvectors of single subject repeated swallows |
Eigenvectors of between subject hyolaryngeal excursion |
||||
|---|---|---|---|---|---|---|
| x- component |
y- component |
Magnitude of resulting vector |
x- component |
y- component |
Magnitude of resulting vector |
|
| 1-mandible | −0.09 | −0.34 | 0.35 | −0.17 | −0.24 | 0.29 |
| 2 -hard palate | −0.09 | −0.04 | 0.10 | −0.05 | −0.15 | 0.16 |
| 3-atlas | 0.15 | −0.03 | 0.15 | 0.13 | −0.20 | 0.24 |
| 4-C2 | 0.30 | −0.15 | 0.33 | 0.31 | −0.21 | 0.37 |
| 5-C4 | 0.48 | −0.23 | 0.53 | 0.37 | −0.17 | 0.41 |
| 6-hypopharynx/UES | −0.09 | 0.36 | 0.37 | 0.07 | 0.34 | 0.35 |
| 7-posterior larynx | −0.05 | 0.25 | 0.26 | −0.09 | 0.34 | 0.35 |
| 8-anterior larynx | −0.18 | 0.17 | 0.25 | −0.15 | 0.30 | 0.34 |
| 9-hyoid | −0.43 | 0.01 | 0.43 | −0.41 | 0.00 | 0.41 |
3.2 PCA of All Subjects
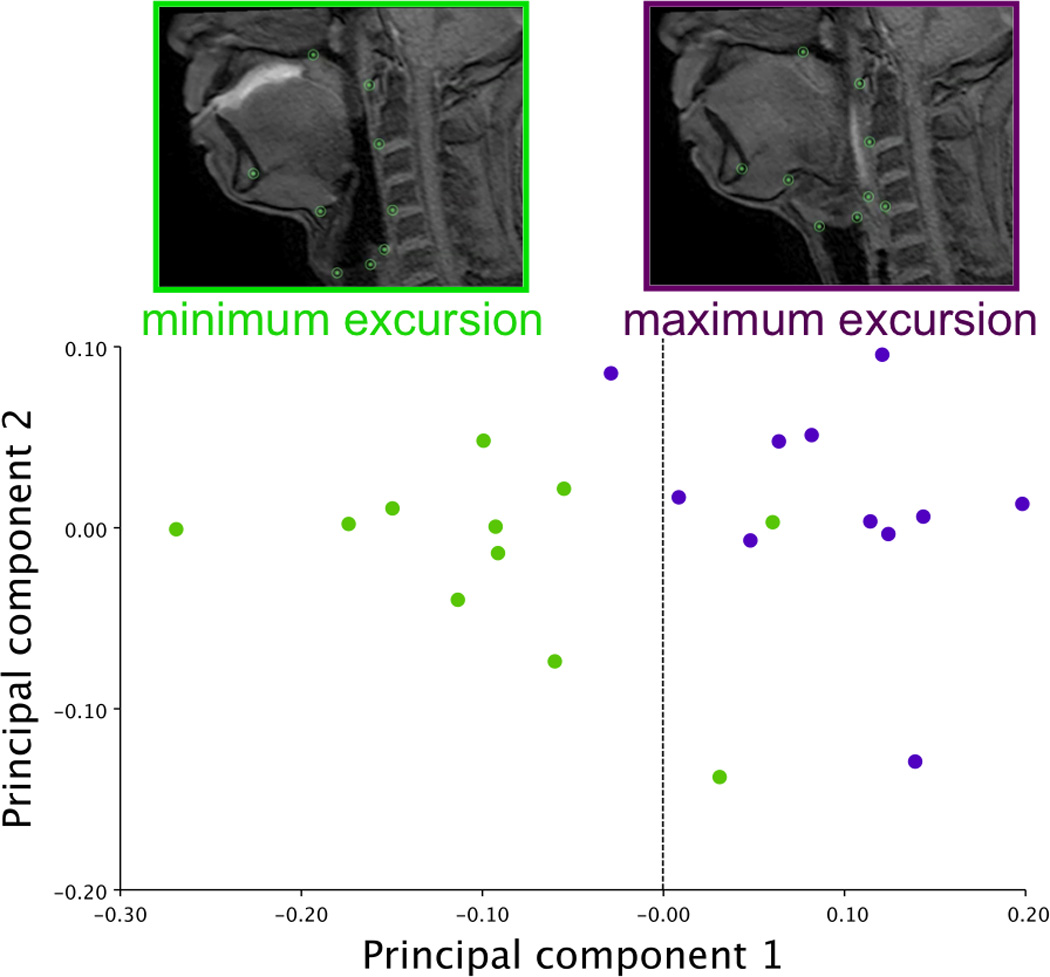
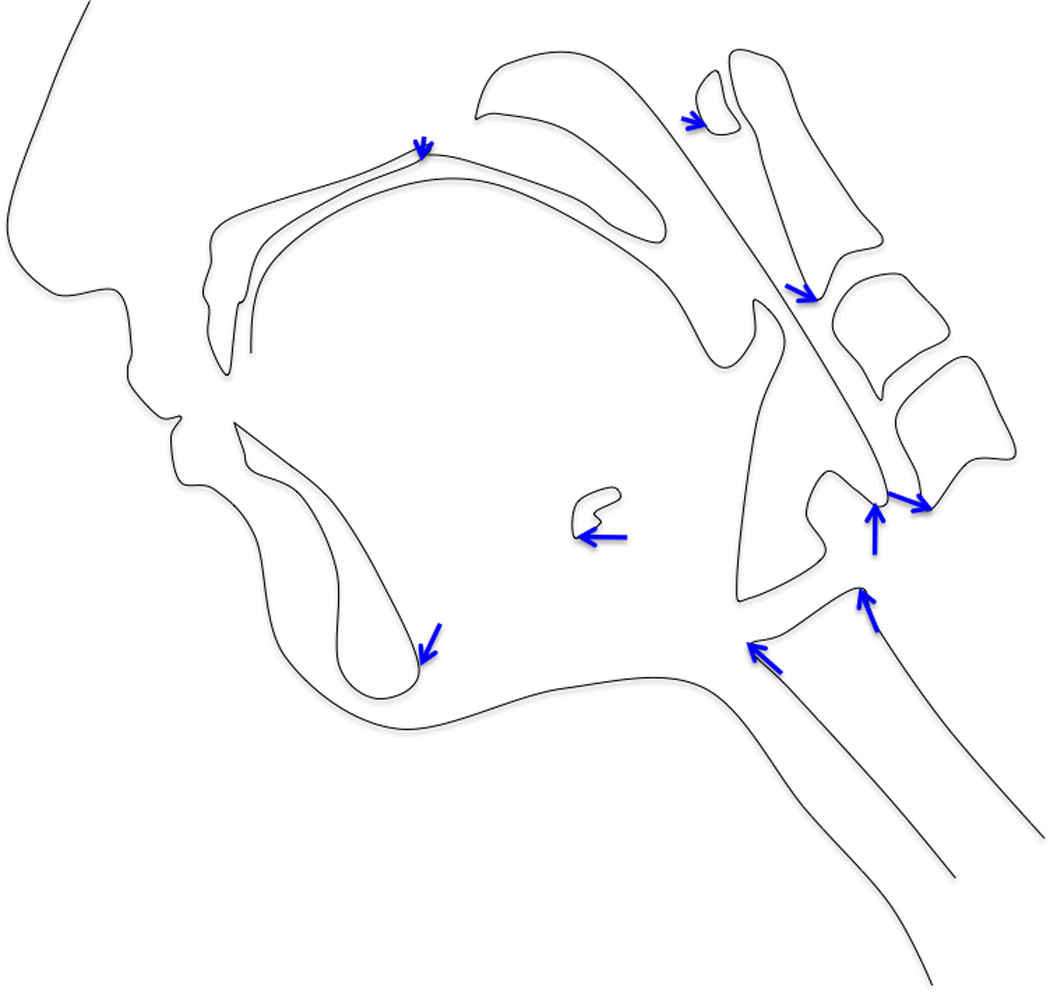
The PCA of minimum and maximum excursions of the hyolaryngeal complex in all subjects combined resulted in a first PC with an eigenvalue representing 58.8% of the variance (Figure 6a). A scatter plot of PC1 and PC2 scores coded by minimum (oral phase 1) and maximum (pharyngeal phase 1) positions show that excursion of the hyolaryngeal complex underlies the variance demonstrated in PC1 shape changes (Figure 6b). Eigenvectors of PC1 for each landmark range is reported in Table 2. Shape changes associated with these eigenvectors are plotted and illustrated in Figure 6c.
Figure 6.
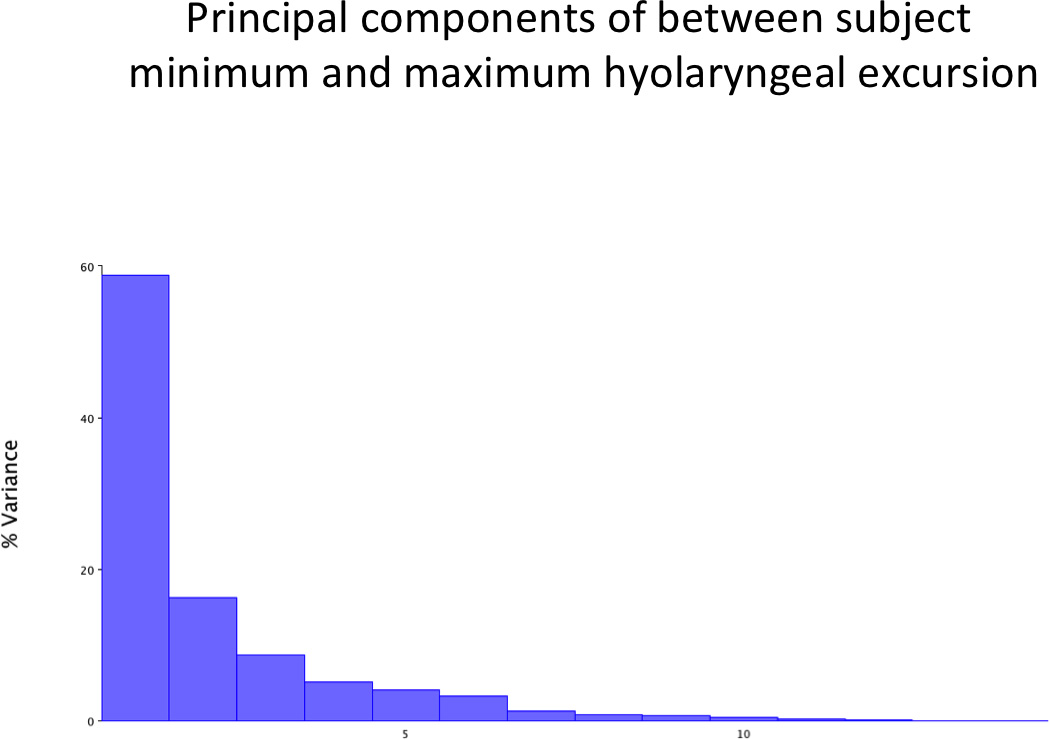
a. The first principal component of variance for all subjects swallow task accounts for 58.8% of the shape change variance
b. Plotted morphologies of all subjects swallow task. A scatter plot of summed shape change coded by maximum (pharyngeal 1) or minimum (oral 1) position of hyolaryngeal complex of the between subject analysis shows that principal component 1 is principally defined by hyolaryngeal excursion.
c. Eigenvectors of all subjects swallow task confirms two muscular slings underlie hyolaryngeal elevation
4. Discussion
This study documents two functional groups of muscles, and associated skeletal elements, working together to elevate the hyolaryngeal complex. Eigenvectors of landmarks representing the distal attachment of the anterior sling (hyoid) were oriented towards their proximal attachments (mandible), and the landmarks representing the distal attachment of the posterior sling (hypopharynx and posterior commissure of the vocal folds) were oriented towards their proximal attachments (hard palate the cranial base represented by the tubercle of the atlas) (Figs. 5c, 6c).
These morphometric findings indicate a two-sling mechanism underlying hyolaryngeal elevation in swallowing among young healthy adults; that is that the anterior and posterior muscular slings function not as individual muscle groups, but as components of a mechanism (Figure 7). These findings are consistent with shape changes anticipated by anatomical and functional studies of the anterior and posterior muscular slings (Pearson and others 2013; Pearson and others 2012b).
Figure 7.
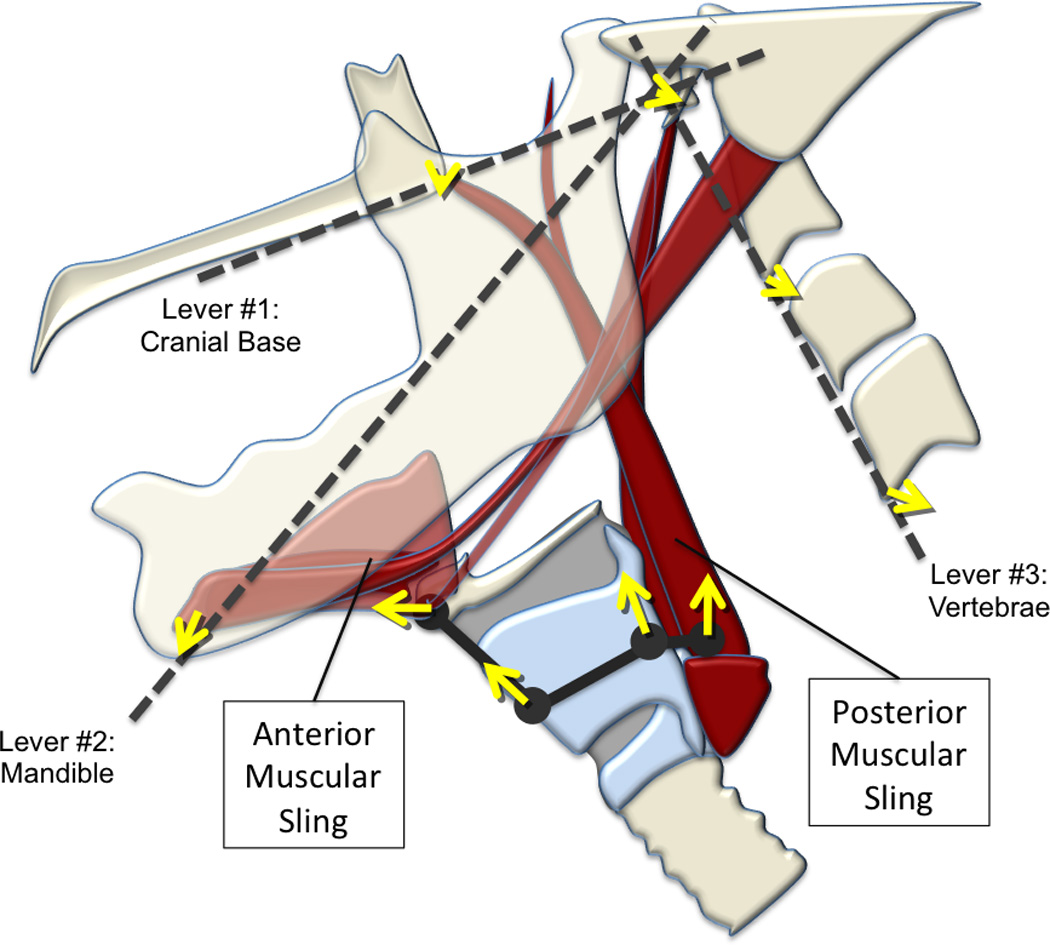
A diagram (in gray) of the two-sling mechanism, consisting of dashed lines and a solid line connecting large dots, is superimposed over an illustration of relevant anatomical structures. The three dashed lines represent skeletal levers including the cranial base (contiguous with the hard palate), mandible, and vertebrae mapped by 5 coordinates (mandible, posterior edge of hard palate, tubercle of the atlas, C2, C4). The solid gray line connecting large dots represents interconnected features of the hyolaryngeal complex mapped by four coordinates (hyoid, anterior commissure of the vocal folds, posterior commissure of the vocal folds, and the location of the upper esophageal sphincter). Each coordinate maps an element of the two-sling mechanism. Each of the yellow arrows indicates the relative magnitude and direction of covariation of each coordinate during swallowing in a cohort of young healthy normal subjects.
Covariant shape changes of the hyolaryngeal mechanism cannot be characterized by conventional kinematic measurements. While elements of the hyolaryngeal complex are in motion, so are the elements of the three skeletal levers. Eigenvectors of coordinates representing the cervical vertebrae in this study indicate movement of the vertebrae during swallowing. Anecdotally, the number patients complaining of swallowing difficulty after cervical spine surgery may signify the importance of skeletal movement to swallowing function that can be appreciated by this modeling technique. To date, no studies have documented the importance of these levers in relationship to deglutition or dysphagia. Future studies of swallowing mobility could use this method to investigate if swallowing disorders have an impact on the skeletal elements, or if insults to the skeletal elements of the system impact swallowing.
Approximation of the thyroid cartilage to the hyoid has conventionally been attributed to activation of the thyrohyoid muscle alone (Cook and others 1989). However, in this cohort of subjects, the thyrohyoid did not achieve a significant effect size change in muscle use as determined by muscle functional MRI (Pearson and others 2013). Eigenvectors of the anterior and posterior commissures of the vocal folds diverge slightly and are not parallel in either of the PCAs (see the numerical eigenvectors of landmarks 7 and 8 in Table 2). The eigenvector of the posterior vocal fold towards the cranial base approximates the concentric contraction of the stylopharyngeus (a posterior sling muscle), which inserts on the posterior border of the thyroid. The eigenvector of the anterior vocal fold coordinate can be attributable to the suprahyoid muscles (anterior muscular sling) translating force to the thyroid via the thyrohyoid membrane. These data suggest that the posterior sling muscles may approximate the thyroid cartilage toward the hyoid independently of the thyrohyoid muscle.
Three main limitations of this study include low temporal resolution, translation of two-dimensional analysis of three-dimensional morphologies, and the experimental condition wherein subjects swallow in the supine position. Temporal resolution of this dynamic MRI sequence (4.3 fps) is much lower than videofluoroscopy (30 fps). To overcome this limitation a repeated swallows task was used in an attempt to capture the full range of motion in swallowing. A more robust investigation of the distinct elements of shape change in swallowing would be an analysis of three-dimensional coordinate data. Three-dimensional coordinate data could be collected with 320-detector-row multi-slice computed tomography (Inamoto and others 2011). However, the two-dimensional shape change analysis used in the current study is more immediately relatable to the results of other studies that use the much more common technique of videofluoroscopy. As for the postures of the subjects, manometry studies showed no difference between subjects swallowing in the supine versus upright position and may suggest that structure is largely unchanged (Barkmeier and others 2002). However, further studies are needed to understand how subject posture affects shape changes as measured by coordinate data of anatomical landmarks.
A major contribution of this study is the novel use of morphometrics to model hyolaryngeal mechanics in swallowing. Rather than measuring multiple kinematic variables of movement, this methodology analyzes covariant shape changes of a complex mechanism. Components of the system are modeled by linking various coordinates that represent underlying anatomical relationships. This method can be applied to dynamic MRI or videofluoroscopic imaging. By applying this methodology to one subject and a cohort of subjects, we demonstrate that this method can generate population or patient specific data. Applications could include analyzing a study cohort to model hyolaryngeal mechanics by etiology of dysphagia, or using patient-specific data to design or test therapies to improve particular elements of swallowing mechanics for an individual.
5. Conclusion
This study used morphometric analysis of multiple swallows in a single subject and hyolaryngeal excursion points in multiple subjects to demonstrate shape changes of the swallowing apparatus as modeled by coordinates of anatomical landmarks. These shape changes show the effect of a two-sling mechanism elevating the hyolaryngeal complex.
Acknowledgements
The authors would like to acknowledge the support and assistance of Ron Killiany, Ph.D. and staff of the Center for Biomedical Imaging, Boston University School of Medicine and to Keri Vasquez Milord, MS, CCC-SLP, BRS-S for serving as an inter-judge rater for this study. Completion of this project was supported in part by Grant Number F31DC011705 from the National Institute On Deafness And Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness And Other Communication Disorders or the National Institutes of Health.
Footnotes
Conflict of interest statement
We do not have any propriety, financial, professional or other personal interest of any nature in any product, service and/or company that could influence the position presented in this manuscript.
References
- Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Laryngeal activity during upright vs. supine swallowing. J Appl Physiol. 2002;93(2):740–745. doi: 10.1152/japplphysiol.00380.2001. [DOI] [PubMed] [Google Scholar]
- Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. American Journal of Physiology- Gastrointestinal and Liver Physiology. 1989;257(5):748–759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical Neurophysiology. 2003;114(12):2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM, Palmer JB. Food Transport and Bolus Formation during Complete Feeding Sequences on Foods of Different Initial Consistency. Dysphagia. 1999;14(1):31–42. doi: 10.1007/PL00009582. [DOI] [PubMed] [Google Scholar]
- Inamoto Y, Fujii N, Saitoh E, Baba M, Okada S, Katada K, Ozeki Y, Kanamori D, Palmer JB. Evaluation of swallowing using 320-detector-row multislice CT. Part II: Kinematic analysis of laryngeal closure during normal swallowing. Dysphagia. 2011;26(3):209–217. doi: 10.1007/s00455-010-9276-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23(3):274–279. doi: 10.1007/s00455-007-9135-y. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural Displacements in Normal Swallowing: A Videofluoroscopic Study. Dysphagia. 2000;15(3):146–152. doi: 10.1007/s004550010017. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and Biomechanical Characteristics of Oropharyngeal Swallow in Younger and Older Men. Journal of Speech, Language and Hearing Research. 2000;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- Okada S, Inamoto Y, Wattanaphun P, Saitoh E, Shibata S, Kagaya K, Fujii N, Ida Y, Kataoka Y, Katada K. Gender, age, height and weight effects on the volume and the shape of the pharynx and larynx--Measurement by using 320 detector row multislice CT. Dysphagia. 2011;26(4):469–470. (abs.). [Google Scholar]
- Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Physical Medicine & Rehabilitation Clinics of North America. 2008;19(4):691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mepani R, Antonik S, Massey B, Kern M, Logemann J, Pauloski B, Rademaker A, Easterling C, Shaker R. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia. 2009;24(1):26–31. doi: 10.1007/s00455-008-9167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG, Davidoff AA, Langmore SE. Reliability of a new approach to videofluoroscopic kinematic analysis. Dysphagia Research Society Annual Meeting; Dysphagiap; Toronto, CA. 2012a. p. 580. [Google Scholar]
- Pearson WG, Hindson DF, Langmore SE, Zumwalt AC. Evaluating Swallowing Muscles Essential for Hyolaryngeal Elevation by Using Muscle Functional Magnetic Resonance Imaging. International Journal of Radiation Oncology* Biology* Physics. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG, Langmore SE, Yu LB, Zumwalt AC. Structural Analysis of Muscles Elevating the Hyolaryngeal Complex. Dysphagia. 2012b;27(4):445–451. doi: 10.1007/s00455-011-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DCBH. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clinical Otolaryngology. 2011;36(1):30–36. doi: 10.1111/j.1749-4486.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Annals of Otology, Rhinology & Laryngology. 2005;114(6):478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- Webster M, Sheets HD, Alroy J, Hunt G. A practical introduction to landmark-based geometric morphometrics. Quantitative Methods in Paleobiology. Paleontological Society Papers. 2010;16:163–188. [Google Scholar]


