Abstract
Resveratrol, a natural polyphenol, increases cellular antioxidant capacity by inducing the expression of a battery of cytoprotective genes through an antioxidant responsive element (ARE). However, upstream signaling events initiated by resveratrol leading to the activation of an ARE enhancer, particularly in immune cells, have not been fully elucidated. In this study, ARE-dependent transcriptional activation of ferritin heavy chain (ferritin H) gene by resveratrol was further investigated in Jurkat T cells and human peripheral blood mononuclear cells. We found that AMP-activated protein kinase (AMPK) plays a key role in the activation of nuclear factor E2-related factor (Nrf2) and subsequent ARE-dependent ferritin H gene transcription by resveratrol. Chromatin immunoprecipitation assay for Nrf2 after AMPKα knockdown with siRNA revealed that Nrf2 nuclear accumulation and subsequent binding to the ferritin H ARE induced by resveratrol was dependent on activation of AMPKα, but not PI3K/AKT. Furthermore, AMPKα knockdown blocked resveratrol-induced phosphorylation of glycogen synthase kinase 3β (GSK3β) at Ser9 as well as ARE-dependent transcriptional activation of the ferritin H and HO-1 genes, suggesting that AMPKα is an upstream kinase for GSK3β phosphorylation and activation of the Nrf2-ARE pathway. Consistently, GSK3β knockdown by siRNA enhanced resveratrol-mediated ferritin H mRNA induction, and the inhibition of AMPKα by compound C or siRNA diminished the protective effect of resveratrol against oxidative stress-induced cytotoxicity in CD3+ T cells. Collectively, these results suggest that AMPKα plays a significant role in ARE-dependent transcription of ferritin H genes by resveratrol and may influence the redox status in immune cells.
INTRODUCTION
Oxidative stress, induced by excessive levels of reactive oxygen species (ROS), is implicated in the pathogenesis of various human diseases and disorders such as cancer, neurodegeneration, and inflammation.1 A line of studies indicated that, in T cells, ROS-evoked signaling is required as a first step of T cell activation through T cell receptor (TCR) and CD28 co-stimulation.2,3 However, a dramatic increase in ROS levels is associated with T cell expansion, rendering them susceptible to oxidative damage.4,5 Under such prooxidative conditions, induction of antioxidant genes is an adaptive response to alleviate ROS toxicity and oxidant-induced cellular damage.1 Not only prooxidants, but also antioxidants with intrinsic radical scavenging properties can induce transcription of a set of antioxidant detoxification genes, such as heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1, glutathione-S-transferase, and an iron-storage protein, ferritin. Increased expression of these antioxidant proteins alleviates or prevents oxidative stress by enhancing the cellular antioxidant capacity. Transcription of these genes is regulated by the binding of the transcription factor, nuclear factor E2-related factor 2 (Nrf2), to the antioxidant responsive element (ARE) in response to various external stimuli.6,7
Resveratrol, a polyphenol found in the roots of white hellebore and Polygonum cuspidatum, has antioxidant properties.8 Since resveratrol was reported to possess anti-cancer activity,9 accumulating evidence has suggested its preventive effect on a wide variety of diseases including cancer10 and cardiovascular disorders.11 Given that oxidative stress is implicated in these diseases,1 it is not surprising, therefore, that resveratrol has been demonstrated to induce an antioxidant response10 ; however, the effects of resveratrol on cellular antioxidant gene expression in the immune system remain largely uncharacterized.
Ferritin, one of the ARE-regulated antioxidant/detoxification genes,12,13 plays a major role in intracellular iron storage.14 Although iron is an integral element that is required for many metabolic enzymes and cellular processes,15 excess levels of iron results in elevated ROS production through the Fenton reaction.14 Thus, excess intracellular iron must be detoxified and tightly regulated. Ferritin is composed of 24 subunits, consisting of heavy (H) and light (L) chains. The ferritin H subunit catalyzes the conversion of ferrous iron [Fe(II)] to ferric iron [Fe(III)] through intrinsic ferroxidase activity, while the ferritin L subunit is involved in the formation of the iron core; both subunits therefore contribute to the encapsulation of excess iron in the ferritin shell, which in turn prevents ROS production.14 The importance of ferritin H is demonstrated in that it is ubiquitously expressed in various tissues, and ferritin H knockout mice are embryonically lethal.16 With respect to the signaling pathways that regulate ferritin H gene transcription in response to external stimuli, we and others have demonstrated that the PI3K/AKT pathway regulates ARE-dependent transcription17,18 ; however, it still remains obscure whether PI3K/AKT plays a pivotal role in resveratrol-induced antioxidant gene activation, since resveratrol has been reported to exhibit an inhibitory effect on the PI3K/AKT pathway, resulting in repression of IL-17 expression in primary mouse cardiac fibroblasts19 and inhibition of cardiac hypertrophy.11
AMP-activated protein kinase (AMPK), a sensor of cellular energy and metabolic status, is a kinase regulated by the cellular AMP and ATP ratio.20 When AMPK is activated, energy-producing processes such as fatty acid oxidation and glucose uptake are facilitated, while energy consumption such as lipid and protein synthesis is suppressed.21 AMPK is composed of three subunits; the catalytic α-subunit, and the regulatory β- and γ- subunits. α-subunit phosphorylation at Thr172 enhances AMPK activity. A recent study demonstrating that AMPK activation attenuates T cell mediated autoimmune diseases and lymphoid migration has underscored the importance of AMPK in immune cells.22
In the present study, we defined AMPKα, but not PI3K/AKT, as a significant transcriptional activator of ARE-dependent ferritin H genes in response to stimulation by resveratrol. AMPKα activation by resveratrol led to the phosphorylation of glycogen synthase kinase 3β (GSK3β) at Ser9 and induced Nrf2/ARE-dependent antioxidant gene transcription such as ferritin H and HO-1. Our findings provide precise molecular insights into the cytoprotective features of resveratrol in human T cell.
MATERIALS AND METHOD
Cells and reagents
K562 human erythroleukemia and Jurkat cells were purchased from the American Type Culture Collection. PBMC was obtained from healthy human volunteers. K562 cells were cultured in RPMI 1640 medium supplemented with 0.3 g/liter glutamine, 25 mM HEPES, and 10% FBS (Mediatech, Orlando, FL). Jurkat cells and PBMC were cultured in RPMI 1640 with 10% FBS, 0.45% glucose, and 1 mM sodium pyruvate. PBMC, K562 cells, and Jurkat cells were maintained in a humidified, 5% CO2 incubator at 37 °C. t-BHQ (Sigma–Aldrich, St. Louis, MO), Compound C (Calbiochem, Darmstadt, Germany), and resveratrol (Sigma-Aldrich) were dissolved in DMSO.
Plasmids and DNA transfection
pBluescriptSK(–) −4.5 kb, −4.4 kb, −4.0 kb, ARE, and mtARE human ferritin H-luciferase have been described elsewhere.13 Transient DNA transfection into cells was carried out by electroporation (Xcell; Bio-Rad, Hercules, CA). After electroporation of luciferase reporters with the transfection internal control pRL-null (Promega, Madison, WI), the cells were treated with various concentrations of resveratrol or t-BHQ for 24 h. Preparation of cell extracts and luciferase assays were performed using dual-luciferase assay reagents (Promega). Firefly luciferase expression driven by the ferritin H gene was normalized by Renilla luciferase activity.
Western blotting
Western blotting was performed using either whole cell lysates or cytoplasmic/nuclear fractions as described previously.13 Cell lysates were subjected to SDS/PAGE, and a primary antibody was incubated overnight at 4 °C. Antibody against AKT, phospho-AKT (Ser473), PTEN, AMPKα, phospho-AMPKα (Thr172), AMPKβ1/2, GAPDH, GSK3β, phospho-GSK3β (Ser9) (all from Cell Signaling, Danvers, MA), Lamin B, LDH, Nrf2 (all from Santa Cruz Biotechnology, Dallas, TX), and β-actin (Sigma-Aldrich) were used with a working dilution of 1:1,000 to 5,000 in TBS containing 0.1% Tween 20 and 5% (w/v) skim milk. Horseradish peroxidase-conjugated secondary antibodies were used at 1:5000 dilutions, and ECL® or ECL® Advance was used for Western blotting detection (Amersham–GE Healthcare, Piscataway, NJ). Rainbow molecular mass marker (Amersham–GE Healthcare) or Prosieve prestained protein marker (Cambrex, East Rutherford, NJ) was used for protein size markers for SDS/PAGE.
Northern blotting
Cells were treated for 24 h with various concentrations of resveratrol or t-BHQ in the presence or absence of the PI3K inhibitors, LY249002 or Wortmannin. 1–20 μg total RNA isolated with Trizol (Invitrogen, Carlsbad, CA) was applied to a 1.1% agarose formaldehyde-containing gel. The separated RNA was blotted onto a 0.45 mm nitrocellulose Protran BA85 membrane (Whatman Biosystems, Maidstone, UK), and ferritin H mRNA was hybridized with [α-32P]dCTP-labeled 0.9 kb fragment of the human ferritin H cDNA.
Quantitative real-time PCR
Total RNA was reverse-transcribed using 1 µM oligo-dT and Superscript reverse transcriptase (Invitrogen), according to the manufacturer's instructions. Quantitative real-time PCR was performed in the Mx3000P quantitative PCR system (Agilent Tech., Santa Clara, CA) using SYBR premix Ex taq (TAKARA Bio., Shiga, Japan). β-actin was used as an internal control
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was carried out as described previously.13 Briefly, 1 × 107 cells were cross-linked with 1% formaldehyde and cell lysates were prepared using a ChIP assay kit (Millipore, Billerica, MA). The cross-linked chromatin-DNA was sonicated with a Sonic Dismembrator for 12 cycles of pulses (10 s) and intervals (20 s). 1/10 aliquots of sonicated DNA were immunoprecipitated with 1 µg of rabbit IgG or anti-Nrf2 antibody (Santa Cruz Biotechnology), and semi-quantitative PCR for the ferritin H ARE was performed using a primer set (ARE primers: 5'-CCCTCCAGGTCTTATGACTGCTC-3' forward and 5'-GTTTCTGGAGGTTCAGCACGTC-3' reverse, non-ARE primer: 5'-CACACTGACTCCTCCAAATGAACTTTAG-3' forward, 5'-GTACCATATTCCCAAATGGTCGGTC-3' reverse) in the presence of 0.1 µCi of [α-32P]dCTP. Quantitative RT-PCR was carried out using ChIP DNA. % input means precipitated ChIP DNA normalized with input DNA.
siRNA transfection
PTEN, AMPKα, or GSK3β siRNA (Thermo Fisher, Waltham, MA) were transfected into cells by electroporation or the accell siRNA transfection method. Transfected cells were suspended in regular medium containing 10% FBS (electroporation) or low FBS (2%) conditions (accell siRNA) and incubated for 48 to 72 h. To detect expression of PTEN, AMPKα, and GSK3β, whole cell lysates were subjected to Western blotting with anti-PTEN, -AMPKα, -GSK3 β, -GAPDH and -β-actin antibodies. Northern blotting or quantitative RT-PCR was carried out for the detection of ferritin H or HO-1 mRNA.
Apoptosis staining with Annexin-V/PI/CD3
PBMCs were treated with resveratrol in the presence or absence of Compound C or AMPKα siRNA for 24 h. PBMCs were then treated with hydrogen peroxide for additional 24 h, followed by staining with Fluorescein isothiocyanate conjugated Annexin-V, propidium iodide (PI), and phycoerythrin conjugated anti-CD3 using the Apoptosis Detection kit (Becton-Dickinson, Franklin Lakes, NJ) according to the manufacturer’s instructions. After triple staining, the samples were immediately analyzed by flow cytometry.
Statistical analysis
All experiments were repeated at least three times. The results are represented as means ± SEM. The statistical significance was calculated by Student’s t-test, or Welch’s t-test from independent experiments. P value less than 0.05 was considered statistically significant.
RESULTS
Transcriptional activation of ferritin H gene via ARE by resveratrol in human T cells
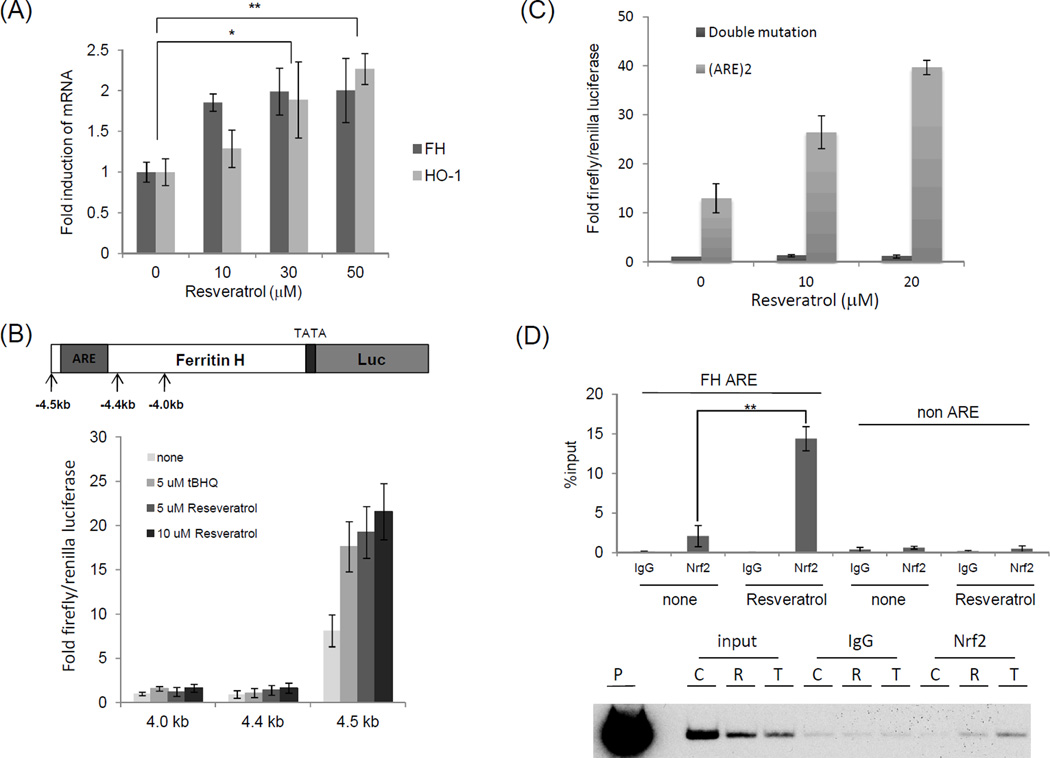
To investigate antioxidant gene expression in peripheral blood mononuclear cells (PBMCs), we first measured ferritin H and HO-1 mRNA levels following resveratrol treatment. Quantitative RT-PCR showed that ferritin H and HO-1 mRNA were induced by resveratrol in PBMCs at 24 h (Figure 1A). We next asked whether resveratrol transcriptionally activates the ferritin H gene in T cells. To answer this question, ferritin H promoter luciferase reporter constructs were employed. Ferritin H-luciferase reporter assay showed that resveratrol, as well as t-BHQ, a potent antioxidant that activates ARE-regulated gene transcription,17 activated expression of luciferase driven by the −4.5 kb, but not −4.4 kb or −4.0 kb human ferritin H 5' regulatory region in Jurkat cells (Figure 1B); as the ferritin H ARE is localized between the −4.4 kb and −4.5 kb region,12 this suggests the possibility that resveratrol activates the ferritin H gene through the ARE. To test this possibility, human ferritin H wild type-ARE or mutant-ARE luciferase reporters were transfected into Jurkat cells and treated with resveratrol for 24 h. Given that resveratrol activated luciferase expression driven by the wild type-ARE but not the mutant-ARE, the ARE is necessary for transcriptional activation of ferritin H by resveratrol (Figure 1C). ChIP assays showed 5–6 fold increases in Nrf2 binding to the ferritin H ARE by resveratrol within 4 h in Jurkat cells (Figure 1D top). Similar results were obtained in different cell types such as K562 human erythroleukemic cells (Fig. 1D, bottom). Collectively, these results suggest that resveratrol induces ferritin H transcription through the ARE.
Figure 1.
Transcriptional activation of human ferritin H ARE by resveratrol in human T cells (A) PBMCs obtained from healthy volunteers were treated with 10, 30, or 50 µM resveratrol for 24 h. Total RNA was purified and subjected with quantitative RT-PCR to measure ferritin H and HO-1 mRNA. Each RT-PCR product was normalized with β-actin mRNA, and the values were represented as a relative value of control (untreated cells). The mean and standard error were calculated from at least 3 independent experiments. (B, C) Jurkat cells were transfected via electroporation with 1 µg of −4.5 kb, −4.4 kb, or −4.0 kb human ferritin H-luciferase reporter (B), or 1 µg of human ferritin H wild-type ARE-, or double mutant ARE-luciferase plasmid (C) along with 10 ng of pRL-EF as an internal control. Cells were treated with indicated concentration of t-BHQ (t-BHQ was included as a positive control for ferritin H ARE activation17) or resveratrol for 24 h, and the resulting luciferase activity was assessed via luminometry. Induction was determined by setting –4.0 kb luciferase reporter (B) or double mutant ARE/control (C) at 1.0. (D) Top: Jurkat cells were untreated (none) or treated with 50 µM resveratrol for 4h. Ferritin H ARE and non ARE ChIP assays were performed with control rabbit IgG or Nrf2 antibody. qRT-PCR was carried out and the results of %input were shown. Bottom: Same experiment including t-BHQ was performed in K562 cells. Representative gel image was shown. C: untreated, R: 50 µM resveratrol, T: 50 µM t-BHQ, P: A plasmid DNA containing a 5.2 kb ferritin H 5’ region as a positive control for the PCR reaction as well as a marker of the ferritin H ARE 155 bp PCR band. The data represent means±SEM (n≧3). **P<0.01
Resveratrol-mediated transcriptional regulation of ferritin H mRNA is independent of the PI3K/AKT signaling pathway
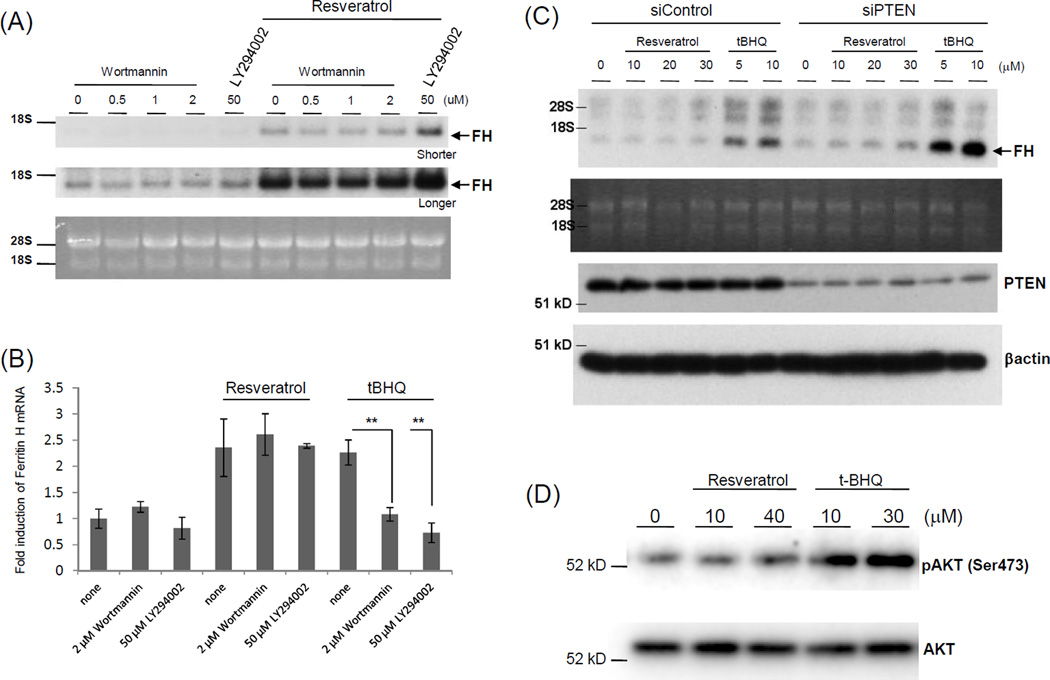
We previously reported that t-BHQ enhanced ferritin H mRNA induction via the ARE in Jurkat cells due to their PTEN deficiency, showing that the PI3K pathway is involved in t-BHQ-induced ferritin ARE activation.17 To elucidate the upstream signaling pathways by which resveratrol activates ferritin H mRNA transcription, we first investigated whether PI3K/AKT plays a significant role in ferritin H transcription in T cells. Treatment with two individual PI3K inhibitors Wortmannin or LY294002 did not affect ferritin H mRNA induction by 30 μM resveratrol, but inhibited its induction by 10μM t-BHQ for 24 h in Jurkat cells (Figure 2A and B), suggesting that PI3K is not involved in resveratrol-induced ferritin H transcription. To verify these results, we attempted knockdown of PTEN, the PI3K/AKT-negative regulator. Since PTEN is deficient in Jurkat cells due to the mutation of the PTEN gene,17 we used K562 erythroid leukemia cells, in which PTEN knockdown increased basal ferritin H mRNA expression as shown in our previous report17 ; however, it did not enhance ferritin H mRNA induction by resveratrol, contrasted with the enhancing effect of PTEN knockdown on ferritin H mRNA induction by t-BHQ (Figure 2C). Consistent with these results, t-BHQ, but not resveratrol, induced AKT phosphorylation at Ser473 (Figure 2D). These results suggest that t-BHQ activates the PI3K pathway leading to ferritin H transcriptional activation through the ARE in Jurkat cells, while resveratrol utilizes a different pathway from t-BHQ.
Figure 2.
Resveratrol-mediated ferritin H mRNA induction is independent of the PI3K/AKT pathway. (A) Jurkat cells were pretreated with 0.1% DMSO, 50 µM LY294002, or 0.5, 1, or 2 µM Wortmannin for 1 h, followed by 30 µM resveratrol treatments for 24 h, and ferritin H Northern blotting was carried out. RNA staining with ethidium bromide for comparative loading of RNA, and shorter (upper) or longer (lower) exposure results are shown. Representative results of three independent experiments are shown. (B) Jurkat cells were treated with 30 µM resveratrol and 10 µM t-BHQ with or without PI3K inhibitors for 24 h. Total RNA was isolated and subjected to quantitative RT-PCR to measure ferritin H mRNA, which was normalized with β-actin mRNA. The values were represented as a relative value of control (untreated cells). The mean and standard error were calculated from at least 3 independent experiments. P**<0.01 (C) K562 cells were transfected with non-targeting (siControl) or PTEN-targeting siRNA (siPTEN). After 60–70 h of transfection, cells were treated with 5 or 10 µM t-BHQ, or 10, 20, or 30 µM resveratrol for 24 h, and ferritin H Northern blot was carried out. RNA staining with ethidium bromide is shown for comparative loading of RNA. Whole cell lysates isolated in the same experiment were analyzed by Western blotting with anti-PTEN and anti-β-actin antibodies. Representative results of three independent experiments are shown. (D) Jurkat cells were treated with resveratrol (10 or 40 µM) or t-BHQ (10 or 30 µM) for 2 h, followed by Western blotting using anti-phospho AKT (Ser473) or anti-AKT antibodies.
AMPKα-dependent transcriptional activation of the ferritin H gene by resveratrol
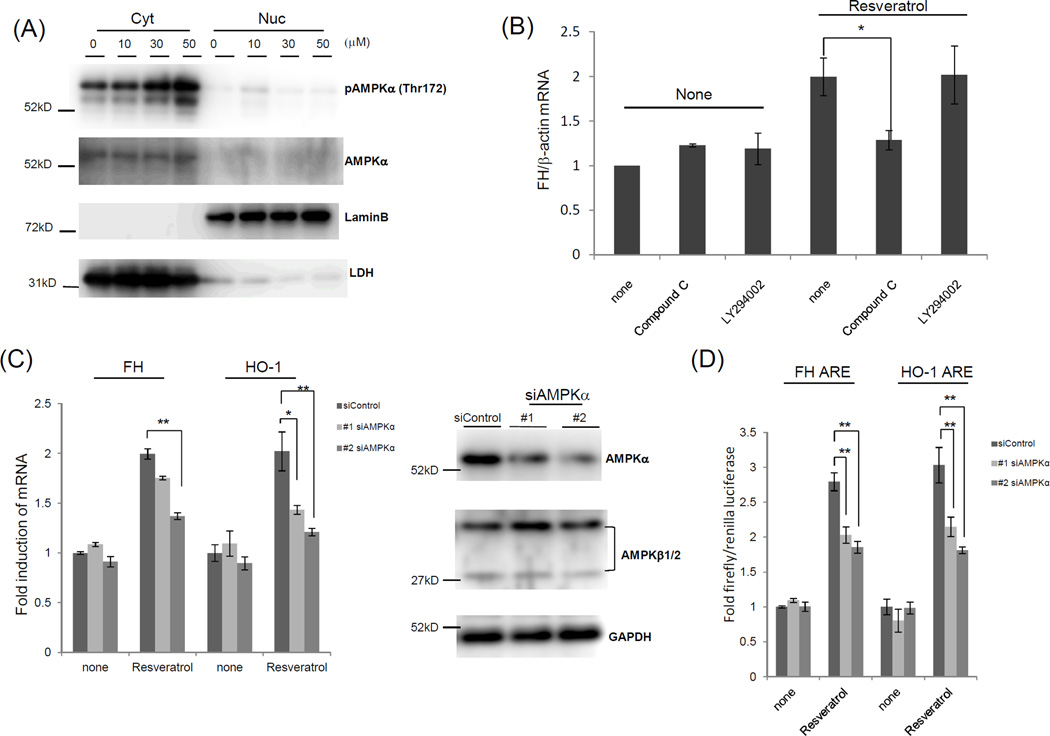
An array of recent studies revealed a number of proteins and pathways affected by resveratrol. Among these, AMPK has been particularly shown to mediate beneficial effects of resveratrol in several cell types and tissues.23 To test whether resveratrol activated AMPK in our system, Western blotting was conducted with a phospho-specific AMPKα antibody and showed that cytosolic AMPKα phosphorylation at Thr172 was induced following resveratrol treatment in Jurkat cells at 2 h (Figure 3A). To answer whether AMPK is involved in resveratrol-mediated ferritin H induction, Compound C, an AMPK inhibitor, was employed. Compound C inhibited ferritin H mRNA induction by resveratrol, while the PI3K inhibitor LY294002 did not (Figure 3B), suggesting the involvement of AMPK. To further investigate the role of AMPK in induction of ARE-regulated genes by resveratrol, Jurkat cells were transfected with two independent AMPKα siRNA and treated with resveratrol and the impact of AMPKα deficiency on ferritin H and HO-1 mRNA expression was assessed. Under AMPKα-specific knockdown, with no effect on AMPKβ1/2 and GAPDH expression levels (Figure 3C), quantitative real-time PCR showed that AMPKα deficiency blocked ferritin H and HO-1 mRNA induction by resveratrol (Figure 3C). Finally, ARE-luciferase assay revealed that AMPKα knockdown impaired ferritin H and HO-1-ARE-mediated transcriptional activity by resveratrol (Figure 3D). Collectively, these results suggest that activated AMPKα is involved in induction of the ferritin H and HO-1 genes in response to resveratrol.
Figure 3.
Resveratrol-mediated induction of ferritin H and HO-1 mRNA is associated with AMPKα. (A) Jurkat cells were treated with different amounts of resveratrol for 2 h. Nuclear and cytosolic fractions were subjected to Western blotting with antibodies against AMPKα, phospho-AMPKα (Thr172), lamin B (nuclear marker), and LDH (cytosol marker). (B) PBMCs were pre-treated with 10 µM Compound C and 10 µM LY294002 for 1 h, followed by 30 µM resveratrol treatment for another 24 h. Total RNA was purified and subjected to quantitative RT-PCR to measure ferritin H mRNA. RT-PCR product was normalized with β-actin mRNA, and the values are indicated as a relative value of control (untreated cells). (C) Jurkat cells were transfected with 2 different siRNA against AMPKα and incubated for 48 h. Cells were treated with 30 µM resveratrol and incubated for a further 24 h. Total RNA was purified and subjected to quantitative RT-PCR to measure ferritin H and HO-1 mRNA. RT-PCR product was normalized with β-actin mRNA, and the values are indicated as a relative value of control (untreated cells). Cells were lysed and subjected to Western blotting with antibodies against AMPKα, AMPKβ1/2, and GAPDH. (D) Jurkat cells were transfected with 1 µg of human ferritin H or HO-1 ARE-luciferase plasmid along with 10 ng of pRL-null with or without siRNA against AMPKα. Cells were treated with 10 µM resveratrol for 24 h, and the resulting luciferase activity was assessed via luminometry. Fold induction was determined by setting ARE/control as 1.0. The data represent means±SEM (n≧3). *P<0.05, **P<0.01
AMPKα enhances the GSK3β phosphorylation at Ser9 associated with Nrf2 nuclear translocation, and protection of T cells against oxidative stress in response to resveratrol
Since Nrf2 is involved in ferritin H mRNA induction by resveratrol (Figure 1D), we investigated the role of AMPKα in the activation of Nrf2. First, nuclear and cytoplasmic fractions were isolated from Jurkat cells treated with resveratrol for 4 h and 24 h, and Nrf2 protein levels were measured by Western blotting. Resveratrol induced Nrf2 nuclear accumulation within 4 h, and the accumulation was sustained until 24 h (Figure 4A). We next asked whether activation of AMPKα by resveratrol is essential for Nrf2 nuclear accumulation. To this end, the effect of AMPKα knockdown on Nrf2 nuclear accumulation was tested in Jurkat cells. Nrf2 Western blotting showed that AMPKα knockdown with two independent siRNA attenuated Nrf2 nuclear accumulation by resveratrol (Figure 4B). Consistent with this result, ChIP assay showed that AMPKα knockdown decreased Nrf2 binding to the ferritin H ARE (Figure 4C). These results suggest that resveratrol regulates Nrf2 nuclear accumulation and Nrf2 binding to the ARE through an AMPKα dependent pathway. Accumulating evidence has shown that GSK3β inhibits cellular antioxidant response through phosphorylation of multiple Serine residues in the Neh6 domain of Nrf2, resulting in nuclear exclusion or degradation of Nrf2.24,25 We therefore investigated whether GSK3β phosphorylation at Ser9, the inactive form of GSK3β,24 was regulated by resveratrol. Western blotting with Jurkat cells treated with 30 μM resveratrol for 0.5–6 h showed GSK3β phosphorylation at Ser9 (Figure 4D). Furthermore, resveratrol-induced GSK3β phosphorylation at Ser9 was inhibited by AMPKα knockdown (Figure 4E). In addition, GSK3β knockdown by siRNA enhanced resveratrol-mediated ferritin H mRNA induction in Jurkat cells (Figure 4F). Collectively, these results suggest that resveratrol-activated AMPKα induces phosphorylation of GSK3β at Ser9, which may inhibit GSK3β-mediated Nrf2 degradation, resulting in Nrf2 nuclear accumulation and transcriptional activation of the ferritin H gene through the ARE in T cells.
Figure 4.
AMPKα-dependent GSK3β phosphorylation at Ser9 is associated with Nrf2-ARE activation. (A, B) Jurkat cells were treated with 30 µM resveratrol for 4 h or 24 h (A). Transfected Jurkat cells with siRNA against AMPKα were treated with 30 µM resveratrol for 4 h (B). Nuclear and cytosolic fractions were subjected to Western blotting with anti-Nrf2, -AMPKα, -phospho-AMPKα (Thr172), -Lamin, and –LDH antibodies. (C) Jurkat T-cells were transfected with siRNA against AMPKα, and treated with 30 µM resveratrol for 4 h. ChIP assay was performed with control rabbit IgG or Nrf2 antibody. Precipitated DNA was subject to quantitative RT-PCR. %input means Ct values from precipitated DNA were normalized with Ct value from input DNA. Standard errors are shown from at least three independent experiments. (D) Jurkat cells were treated with 30 µM resveratrol for the indicated time periods. Cells were lysed and subjected to Western blotting with antibodies against GSK3β and phospho-GSK3β (Ser9). (E) Jurkat cells were treated with 30 µM resveratrol for 4 h after siRNA transfection against AMPKα for 48 h. Cells were lysed and subjected to Western blotting with anti-GSK3β and -phospho-GSK3β (Ser9) antibodies. (F) Jurkat cells transfected with control or GSK3β siRNA were treated with 30 µM resveratrol for 24 h. Total RNA and whole cell lysates were subjected to quantitative RT-PCR to measure ferritin H mRNA (left) and Western blotting with anti-GSK3β antibody (right). Each RT-PCR product was normalized to β-actin mRNA, and the values were represented as a relative value of control (untreated cells and siControl). The mean and standard error were calculated from at least 3 independent experiments. The data represent means±SEM (n=3). *P<0.05, **P<0.01
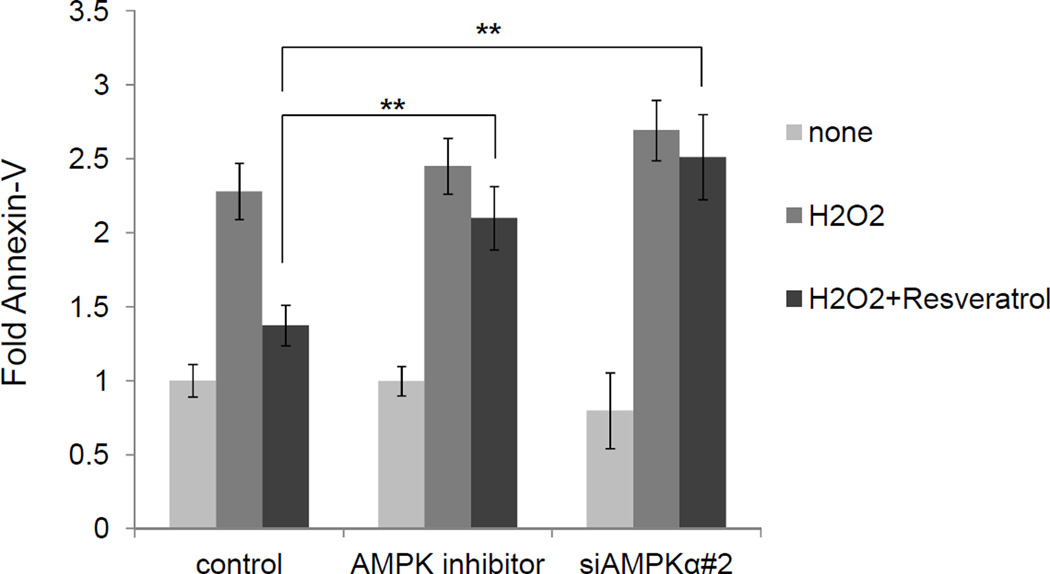
ARE-regulated antioxidant detoxification genes including ferritin have been shown to alleviate oxidative stress.14,26,27 To explore the involvement of AMPKα in cytoprotection against oxidative stress-mediated cytotoxicity in T cells, human PBMCs were pre-incubated with resveratrol for 24 h, followed by hydrogen peroxide challenge for an additional 24 h. Apoptosis assay measured by Annexin-V/PI/CD3 staining indicated that hydrogen peroxide increased Annexin-V positive CD3+ T cells (Figure 5), which was alleviated in T cells pre-treated with 30 µM resveratrol. In contrast, Annexin-V positive CD3+ T cells were significantly increased when AMPKα inhibitor (10 μM compound C) was added or AMPKα was knocked down before resveratrol pretreatment (Figure 5). These results suggest that the resveratrol-mediated antioxidant response to hydrogen peroxide-induced cytotoxicity is mediated by AMPKα in human T cells.
Figure 5.
PBMCs were transfected with siRNA or treated with AMPKα inhibitor; control: non-target siRNA, AMPK inhibitor: non-target siRNA + 10 µM Compound C, siAMPKα: AMPKα-targeted siRNA. PBMCs were stimulated with 50 µM H2O2 for 24 h in the presence or absence of 30 µM resveratrol. PBMCs were gated with CD3 and % of Annexin-V positive cells were measured. Induction was determined by setting control/none as 1.0. The data represent means±SEM (n=3). **P<0.01
DISCUSSION
In this study we identified an AMPKα-Nrf2 axis as an important intracellular signaling pathway in resveratrol-treated human T cells leading to ARE-dependent transcriptional activation of the ferritin H and HO-1 genes (Figure 3). In addition, AMPKα activation by resveratrol reduced T cell apoptosis induced by hydrogen peroxide (Figure 5). Nrf2 translocation from cytosol into the nucleus following antioxidant treatment has been demonstrated as an activation mechanism of ARE-dependent gene transcription.6,7 Since AMPKα expression was almost exclusively in the cytosol (Figure 3A, Figure 4B) and AMPKα has been reported to directly phosphorylate and regulate several transcription factors such as MEF2, foxo3, and CREB,28 we considered the possibility of the direct phosphorylation of cytosolic Nrf2 by AMPKα. However, we did not find the AMPKα consensus phosphorylation site in the Nrf2 amino acid sequences, and preliminary results in our model system failed to show evidence of either direct interaction of AMPKα with Nrf2, or AMPKα-mediated Nrf2 phosphorylation. We therefore hypothesized that Nrf2 was indirectly activated and modified by a protein targeted by AMPKα. It was reported that GSK3β phosphorylates multiple Ser residues in the Neh6 domain of Nrf2, resulting in nuclear exclusion and/or degradation of Nrf2.24,25 In addition, active GSK3β was shown to impair both phase II gene expression and protection against oxidative stress.24 In human T cells, we observed that resveratrol induced inhibitory phosphorylation of GSK3β at Ser9 in an AMPKα-dependent manner (Figure 4) that may contribute to Nrf2 nuclear accumulation. In fact, GSK3β knockdown increased resveratrol-mediated ferritin H mRNA induction. Our results are consistent with the previous report by Shin et al., showing the involvement of AMPK in protection of hepatocyte mitochondria from arachidonic acid plus iron-induced apoptosis.29
We previously demonstrated that the genetic status of PTEN determines ferritin H transcription, in which PTEN deficiency enhanced ferritin H transcription through the ARE in response to oxidative stress.17 Consistent with this finding, several reports have demonstrated that the PI3K/AKT pathway activates phase II gene transcription through the ARE in concert with Nrf2 nuclear accumulation.30,31 Therefore, we hypothesized that inhibition of PI3K/AKT pathways might block resveratrol-mediated ferritin H mRNA induction. However, PI3K inhibitors, as well as PTEN knockdown had only marginal effects on ferritin H mRNA induction by resveratrol (Figure 2A). Thus, we concluded that resveratrol-mediated ferritin H ARE regulation is not mediated through activation of PI3K/AKT pathways, which appears to be consistent with reports that resveratrol inhibits PI3K32 and induces cell cycle arrest through AKT inhibition in certain cell types.33
AMPKα activation by resveratrol not only alleviates oxidative cell damage34 but also contributes to longevity. C. elegans life span is extended by treatment with metformin, a type II diabetes drug, via the cooperation of SKN-1 (C. elegans Nrf2 ortholog) and AMPK.35 Conversely, the Ferritin H homolog ftn-1 mutation in C. elegans reduced life span upon iron stress.36 Thus, AMPK-mediated ferritin H transcriptional regulation is important in understanding cellular senescence as well as iron homeostasis. Because of the beneficial effects of resveratrol such as the induction of antioxidant genes and anti-proliferative effects on cancer cells, various efforts have been made to employ resveratrol as a chemotherapeutic drug against cancer, type II diabetes, neurodegeneration, and organ rejection in liver transplantation.10 Resveratrol exhibits beneficial effects via activation of the histone/protein deacetylase sirtuin 1 (SIRT1) and subsequent deacetylation of foxo transcription factors, 20,28 both of which have been reported to be notable for association with longevity and activation by AMPK.28 Since resveratrol-induced ferritin H mRNA was dependent on AMPKα (Figure 3) and our sequence search hit a consensus binding site of FoxO transcription factors (FoxOs) in the 5’-regulatory region of the human ferritin H gene (Figure S1A of Supporting Information), we tried to investigate the role of FoxOs element and its activator SIRT1 in ferritin H transcription. Reporter assay revealed that the ARE-deleted 4.4kb-ferritin H promoter showed significantly decreased basal luciferase expression (Figure 1), but was slightly activated by resveratrol (less than 2-fold; Figure S1B of the Supporting Information). This activation might be independent of the FoxOs binding element, since 0.15kb was still activated by resveratrol. In addition, a SIRT1 inhibitor, Nicotinamide, failed to block resveratrol-mediated ferritin H mRNA induction, rather it slightly enhanced the induction (Figure S1C of the Supporting Information). Therefore, our results do not suggest the involvement of these proteins in the activation of the ferritin H ARE or FoxOs binding sites of the ferritin H promoter in response to resveratrol. Further experimental investigation will be needed to understand the detailed knowledge about the molecular mechanisms by which FoxOs and SIRT1 control ferritin H transcription.
While AMPK function has been recognized as part of an evolutionarily conserved energy-sensing pathway, some of reports have shown that the role of AMPK contains diverse aspect of T cell biology beyond metabolisms. TCR-mediated gene regulation depends on two major signaling pathways, H2O2 and Ca2+ signaling.38 with the latter activating NFAT transcription factors by which cytokines required for T cell proliferation such as IL-2 is induced. Immediate AMPKα phosphorylation is observed after TCR stimulation.39 TCR-activated AMPK in Jurkat and PBMCs through a PKC-theta dependent pathway activated NFAT, resulting in IL-2 mRNA induction.40 Other reports indicated that AMPK had multiple features at different stages of T cell lineage.41 In this study, we showed that resveratrol effectively alleviated H2O2-induced T cell apoptosis, at least in part through AMPKα activation (Figure 5). The innate immune system requires ROS to maintain an effective biological defense system; LPS-induced ROS generation is involved in the maturation of dendritic cells,42 and ROS generated in activated T cells through TCR serve as second messengers to trigger T cell proliferation,4 while exposure to excess oxidative stress repressed TCR-induced T cell activation.43 We and others demonstrated that increased expression of ferritin H protects cells from oxidative cell damage.27,44–46 The concentration of resveratrol used in this study showed no change in AMP and ROS levels in PBMCs at 4h time point (Figure S2 of the Supporting Information), which revealed that the Nrf2 activation by resveratrol is independent of ROS. Although the AMP was not increased by resveratrol, the AMP/ATP ratio is important for AMPKα activation.21 Further investigation will be needed to elucidate the mechanisms through which resveratrol activates AMPKα. Collectively, these results suggest that resveratrol increases the cellular antioxidant capacity by inducing ferritin H and possibly other ARE-regulated genes, in which AMPKα activation plays a pivotal role in maintaining the redox status during T cell proliferation and development.
Supplementary Material
ACKNOWLEDGEMENTS
Funding
This work was supported in part by the NIH research grants R01GM088392 and R01GM095550 from National Institute of General Medical Sciences to Y. Tsuji, and a Grant-in-Aid for Young Scientists (Start-up) 20890098 to K. Iwasaki. Paul Ray was supported by the NIH training grant T32ES007046 from National Institute of Environmental Health Sciences.
We thank Dr. Itoh for kindly providing us with the HO-1 ARE-luciferase plasmid DNA.
ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- ARE
antioxidant-responsive element
- ChIP
chromatin immunoprecipitation
- HO-1
Heme oxygenase-1
- Nrf2
nuclear factor E2-related factor
- PBMC
peripheral blood mononuclear cell
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- ROS
reactive oxygen species
- t-BHQ
tert-butylhydroquinone
- GSK3β
glycogen synthase kinase 3β
Footnotes
The authors declare that they have no conflict of interest.
ASSOCIATED CONTENT
*S Supporting Information
Candidate FoxOs binding element in the 5’-regulatory region of the human ferritin H gene and SIRT1 is not important for resveratrol-mediated ferritin H gene activation (Figure S1). Resveratrol does not increase either AMP or ROS levels in PBMCs (Figure S2). Supplemental materials may be accessed free of charge online at http://pubs.acs.org.
REFERENCES
- 1.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;9:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaminski MM, Sauer SW, Klemke C-D, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 3.Larbi A, Kempf J, Pawelec G. Oxidative stress modulation and T cell activation. Exp. Gerontol. 2007;42:852–858. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Rad. Biol. Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Hildeman DA, Mitchell T, Teague TK, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 6.Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nuc. Aci. Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JY, Ohta T, Maruyama A, et al. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol. Cell. Biol. 2006;26:7942–7952. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang MS, Cai EN, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 11.Chan AYM, Dolinsky VW, Soltys CLM, et al. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J. Biol. Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji Y JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24:7567–7578. doi: 10.1038/sj.onc.1208901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki K, MacKenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol.Cell. Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Red. Signal. 2008;10:997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puig S, Askeland E, Thiele DJ Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira C, Bucchini D, Martin ME, et al. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol.Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto K, Iwasaki K, Sugiyama H, Tsuji Y. Role of the Tumor Suppressor PTEN in Antioxidant Responsive Element-mediated Transcription and Associated Histone Modifications. Mol. Biol. Cell. 2009;20:1606–1617. doi: 10.1091/mbc.E08-07-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papaiahgari S, Zhang Q, Yleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid. Red. Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2078–H2087. doi: 10.1152/ajpheart.01363.2007. [DOI] [PubMed] [Google Scholar]
- 20.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 22.Nath N, Khan M, Paintlia MK, Hoda MN, Giri S. Metformin Attenuated the Autoimmune Disease of the Central Nervous System in Animal Models of Multiple Sclerosis. J. Immunol. 2009;182:8005–8014. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid. Red. Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 24.Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3 beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 25.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/beta-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKenzie EL, Ray PD, Tsuji Y. Role and regulation of ferritin H in rotenone-mediated mitochondrial oxidative stress. Free Radic. Biol. Med. 2008;44:1762–1771. doi: 10.1016/j.freeradbiomed.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canto C, Auwerx J AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3 beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem. Pharmacol. 2010;79:1352–1362. doi: 10.1016/j.bcp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Martin D, Rojo AI, Salinas M, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki K, Miwa Y, Haneda M, Uchida K, Nakao A, Kobayashi T. Significance of HLA class I antibody-induced antioxidant gene expression for endothelial cell protection against complement attack. Biochem. Biophys. Res. Commun. 2010;391:1210–1215. doi: 10.1016/j.bbrc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 32.Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem. J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301:168–176. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa I, Trindade LS. Dietary restriction and aging in rodents: a current view on its molecular mechanisms. Aging. dis. 2010;1:89–107. [PMC free article] [PubMed] [Google Scholar]
- 35.Onken B, Driscoll M. Metformin Induces a Dietary Restriction-Like State and the Oxidative Stress Response to Extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YI, Cho JH, Yoo OJ, Ahnn J. Transcriptional regulation and life-span modulation of cytosolic aconitase and ferritin genes in C-elegans. J. Mol. Biol. 2004;342:421–433. doi: 10.1016/j.jmb.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 37.Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat. Rev. Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: Selective regulation of mitogen-activated protein kinase activation and Fas ligand expression. J. Exp. Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JY, Choi AY, Oh YT, et al. AMP-activated protein kinase mediates T cell activation-induced expression of FasL and COX-2 via protein kinase C theta-dependent pathway in human Jurkat T leukemia cells. Cell. Signal. 2012;24:1195–1207. doi: 10.1016/j.cellsig.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T Cells Are Metabolically Anergic. J. Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauveau C, Remy S, Royer PJ, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 43.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 44.Kaur D, Yantiri F, Rajagopalan S, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 45.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem. J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham CG, Bubici C, Zazzeroni F, et al. Ferritin heavy chain upregulation by NF-kappa B inhibits TNF alpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.