Abstract
Background
Low vitamin D intake and levels have been associated with increased joint symptoms in some observational studies but the findings are mixed and evidence from randomized trials sparse.
Objective
To evaluate the influence of supplemental calcium and vitamin D on joint symptoms in the Women's Health Initiative randomized, placebo-controlled, clinical trial.
Design
In post hoc analyses, the results of the WHI randomized clinical trial in which 36,282 postmenopausal women were randomized to calcium carbonate (1000 mg as elemental calcium) with vitamin D3 (400 IU) daily or placebo were examined in the 6% subgroup of 1911 participants, over-sampled for minorities, who had serial joint symptom assessment. Qualitative information on joint pain and joint swelling was collected by questionnaire before entry and 2 years after randomization. Logistic regression models were used to compare the occurrence and severity of joint symptoms across randomization groups.
Results
At baseline, total calcium and vitamin D intakes from diet and supplements were similar in the two randomization groups. In addition, both joint pain (reported by 73%) and joint swelling (reported by 34%) were commonly reported and comparable in the supplement and placebo groups. Two years after randomization, no statistically significant differences between supplement and placebo groups were seen for joint pain frequency (74.6% compared to 75.1%, [P=0.79] for supplement and placebo groups, respectively) or joint swelling frequency (34.6% compared to 32.4%, [P=0.29], respectively) or in severity scores for either outcome. Subgroup analyses suggested study participants also using non-protocol calcium supplements at study entry may have less joint pain with supplement group randomization (interaction P-value=0.02)..
Conclusions
Joint symptoms are relatively common in postmenopausal women. However, daily supplementation with 1000 mg of calcium carbonate and 400 IU of vitamin D3 in a randomized, placebo-controlled clinical trial setting did not reduce the self-reported frequency or severity of joint symptoms.
Severe vitamin D deficiency can lead to joint disorders (1) but reports on the influence of vitamin D intake and status on joint symptoms has been mixed. Both low vitamin D intake (2, 3, 4) and low 25-hydroxyvitamin D levels, (5, 6, 7) as measures of vitamin D status have been associated with increased joint pain. However, vitamin D status has been associated with knee osteoarthritis in only some (2, 5, 7) but not all (4, 8, 9, 10) observational study reports. In addition, reports from full-scale randomized trials are sparse (11). To address this issue, in post-hoc analyses we examined the influence of calcium and vitamin D supplementation on joint symptoms in a randomly identified subgroup of postmenopausal women participating in the Women's Health Initiative (WHI) calcium plus vitamin D supplement randomized, placebo-controlled clinical trial.
Methods
The WHI program includes 4 clinical trials (2 hormone therapy trials [HT], a dietary modification trial [DM], and a calcium plus vitamin D supplementation trial [CaD]) and an observational study. For the WHI clinical trials, postmenopausal women 50-79 years of age with life expectancy ≥ three years, no personal breast cancer history and no other cancer within 10 years were eligible. The HT and DM trials had additional eligibility requirements largely based on medical history. Women participating in the WHI hormone therapy trials (13, 14) or WHI dietary modification (15) trial were invited to enroll in an additional randomized, placebo-controlled trial evaluating calcium plus vitamin D supplementation at their first or second annual follow-up main trial visit (16, 17). For the calcium plus vitamin D trial, additional exclusions included hypercalcemia, history of kidney stones and current corticosteroid or calcitriol use. Personal use of calcium (no upper limit) and vitamin D was allowed while on study. The upper limit for allowed personal vitamin D initially was 600 International Units (IU) daily which was subsequently increased to 1,000 IU daily during the study course (17).
Eligible women who entered the calcium and vitamin D trial were randomly assigned, in a double-blind fashion, to receive active supplement or placebo stratified according to clinical center and calcium and vitamin D supplement containing calcium carbonate (500 mg as elemental calcium) with vitamin D3 (200 IU) or matching placebo was taken twice daily (GlaxoSmithKline Consumer Healthcare, Parsippany, NJ). Study pills were discontinued after development of kidney stones, hypercalcemia, kidney dialysis, and calcitriol use, which causes a greater hypercalcemia risk than other vitamin D compounds.
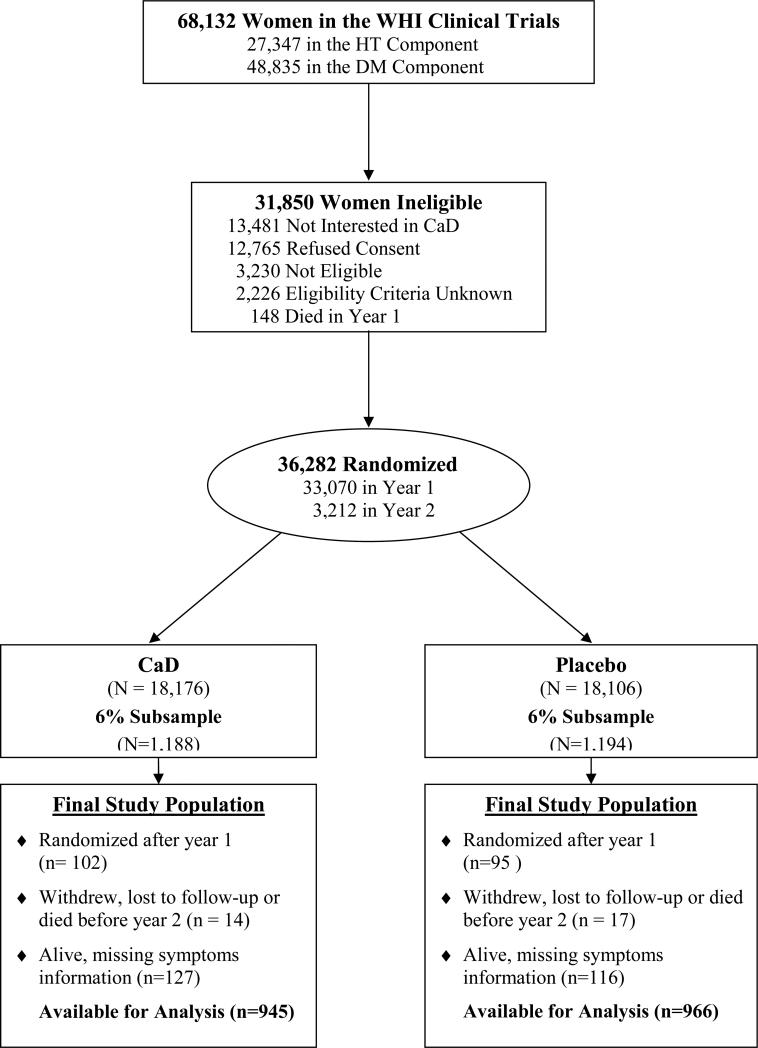
Details of the eligibility and conduct of the HT and DM trials have been reported (13, 14, 15). We now report on joint symptom outcomes in a subset of calcium and vitamin D trial participants. The participant flow through the WHI CT to arrive at the current study population with joint symptom assessment is outlined in Figure 1.
Figure 1.
Participant flow diagram of the Women's Health Initiative randomized trial of calcium and vitamin D to illustrate the identification of the randomized subset included in current analysis examining calcium and vitamin D supplement influence on joint symptoms.
From the 36,282 calcium and vitamin D supplementation clinical trial participants, a 6% sub-sample of 2185 participants for the current study was randomly identified from those who were randomized at their first annual visit for the main trial and who had information collected during follow-up on joint symptoms. The sampling was done on the entire clinical trial population (n= 68,132) with 6-fold higher odds of selection for non-White participants, and a sampling rate of 8.6% in the HT trials and 4.3% in the DM trial, resulting in a 6% overall sample. There was not a specific sampling target for the calcium and vitamin D supplementation trial since the women in that trial were also in a HT and/or DM trial (16). It was planned to assess joint symptoms at baseline and after 2 years in the identified subgroup. Among these 2185 women, 242 had missing information on joint symptoms or for other variables at year 2, 32 (1.5%) died or dropped out resulting in a study population of 1911.
Details of the eligibility and conduct of the calcium plus vitamin D trial have also been reported (12, 17). The trial completed the planned intervention duration of 7 years (mean) of follow-up and calcium and vitamin D supplement effects on hip fracture as primary study endpoint (17, 18) and colorectal cancer (18) and breast cancer (19, 20), as secondary endpoints, have been published.
The described clinical trial had institutional review board approval from all participating institutions and written informed consent was obtained from all participants. Statistical analyses and data management were conducted at the WHI Clinical Coordinating Center. Dietary supplement data was collected during in-person clinic visits. Women brought their supplement bottles to the baseline clinic visit and annually thereafter. A standardized interviewer-administered form was used to collect information on multivitamins and single supplements. Staff members directly transcribed the ingredients for each supplement and asked participants about frequency and duration of use. A validity study of these procedures found correlations with photocopied labels ranged from 0.8 to 1.0 (21, 22). Prescription medication use was similarly determined by in-person review of medication containers. All reported medications were matched to the Master Drug Data Base (MDDB; Medi-Span, Indianapolis, IN).
A self-assessment food frequency questionnaire (FFQ) specifically designed for WHI (23) was used to assess dietary intake over the previous 3 months at entry into the WHI HT or DM trials (23). DM trial participants also had the FFQ administered at year 1, coinciding with entry into the CaD trial. For non-DM participants, the baseline vitamin D intake at entry into the HT trials, which was 1 year before entry into the CaD trial, was used for baseline analyses. For DM participants, dietary vitamin D at baseline was correlated to year 1 values (correlation coefficient, P<0.0001). Total daily calcium intake at baseline was defined as the sum of the dietary intake (assessed with the use of a modified Block food frequency questionnaire) (23) and the average daily self-reported intake of elemental calcium from supplements and from prescription medications in the previous two weeks. Total vitamin D intake was similarly determined reflecting not only dietary vitamin D intake (largely from fortified dairy products and fatty fish) but also vitamins D supplement use. Information on physical activity was collected by questionnaire regarding walking outside the house and recreational physical activity including frequency, duration and intensity. This information was used to generate metabolic equivalent (MET) values (24). Measurements of height and weight were made in the clinic to permit body mass index (BMI) determinations.
Clinical outcomes were determined at annual clinic visits and semi-annual contacts. Annual clinic visits included counting or weighing returned pills as an adherence measure. Joint pain and swelling was assessed by questionnaire collected at initial WHI clinical trial entry (one year prior to the calcium vitamin D supplement trial randomization), one year after entry and again after 2 years on the calcium and vitamin D supplement study. Joint pain was assessed as: (yes/no), severity was assessed as none=0, mild=1, moderate=2, severe=3 and joint swelling was assessed similarly.
Statistical Analyses
The analysis of joint symptoms utilized results from the 1911 randomized participants with available baseline and follow-up information. Chi-square tests were used to compare the baseline characteristics between randomization groups. The frequency and severity of joint symptoms (pain and swelling) were compared by randomization group (active versus placebo) and logistic regression models were used to compare the occurrence of any symptoms versus none, both unadjusted and adjusted for age and race/ethnicity. Similarly, the average symptom score, where none = 0 and severe = 3, was compared in unadjusted and adjusted linear regression models. The difference in scores between follow-up and baseline were computed and analyzed the same way.
The influence of randomization to calcium and vitamin D supplementation or placebo on joint symptom was examined in 6 subgroups (BMI, physical activity, non-protocol calcium supplement use, non-protocol vitamin D supplement use, race/ethnicity, and hormone therapy use [randomization to the intervention group for HT trial participants and current HT use for women not in HT trials]). In these analysis, odds ratios and 95% confidence limits for effect of calcium and vitamin D supplementation on joint pain are estimated from a logistic regression model adjusted for linear age and race/ethnicity. P-values testing for interaction separately for each subgroup are from models including terms for the main effects for CaD supplementation and the subgroup, plus their interaction. For testing, age and BMI (log-transformed) were modeled as linear terms; physical activity was coded (0-4) representing the 5 quintile categories of physical activity. Less than one statistically significant interaction test (P<0.05) would be expected based on chance alone.
All analyses were performed using SAS statistical software, version 9.1.3 (SAS Institute Inc., Cary, North Carolina). All P-values are two-sided and P-values less than 0.05 were regarded as significant. The WHI study is registered with clinicaltrialsgov.NCT000000611.
Results
In the subgroup of 1911 clinical trial participants with serial joint symptom assessment, demographic characteristics, health behaviors, and medical history were well balanced between randomization groups. Considering all participants, mean age at entry was 62 years with a mean body mass index of 29. As a result of over-sampling for minorities, nearly half of participants were non-white (Table 1).
Table 1.
Baseline Characteristics of Study Sample
| CaD (N = 945) | Placebo (N = 966) | P-value* | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age at screening, years | 0.57 | ||
| 50-59 | 373 (39.5) | 382 (39.5) | |
| 60-69 | 431 (45.6) | 424 (43.9) | |
| 70-79 | 141 (14.9) | 159 (16.6) | |
| Race/ethnicity | 0.72 | ||
| White | 483 (51.1) | 512 (53.0) | |
| Black | 217 (23.0) | 232 (24.0) | |
| Hispanic | 128 (13.5) | 118 (12.2) | |
| American Indian | 21 (2.2) | 22 (2.3) | |
| Asian/Pacific Islander | 80 (8.5) | 66 (6.8) | |
| Unknown | 16 (1.7) | 16 (1.7) | |
| Education | 0.84 | ||
| None - some high school | 78 (8.3) | 75 (7.8) | |
| High school diploma/GED | 181 (19.3) | 174 (18.1) | |
| School after high school | 352 (37.6) | 376 (39.1) | |
| College degree or higher | 326 (34.8) | 336 (35.0) | |
| Body mass index (BMI%), kg/m2 | 0.58 | ||
| <25 | 218 (23.2) | 242 (25.2) | |
| 25-<30 | 322 (34.3) | 325 (33.9) | |
| ≥30 | 399 (42.5) | 393 (40.9) | |
| Physical activity, METs/week | 0.23 | ||
| None | 189 (21.2) | 179 (19.5) | |
| >0 - 3.5 | 164 (18.4) | 138 (15.1) | |
| >3.5 - 8.0 | 186 (20.9) | 207 (22.6) | |
| >8.0 - 16.5 | 177 (19.9) | 201 (21.9) | |
| >16.5 | 174 (19.6) | 192 (20.9) | |
| Alcohol use | 0.66 | ||
| Non drinker | 126 (13.5) | 141 (14.8) | |
| Past drinker | 193 (20.7) | 203 (21.3) | |
| Current drinker | 613 (65.8) | 611 (64.0) | |
| Smoking | 0.75 | ||
| Never smoked | 503 (53.9) | 529 (55.6) | |
| Past smoker | 350 (37.5) | 342 (36.0) | |
| Current smoker | 80 (8.6) | 80 (8.4) | |
| NSAID medication use | 0.09 | ||
| No | 784 (83.0) | 829 (85.8) | |
| Yes | 161 (17.0) | 137 (14.2) | |
| Total vitamin D (supplements+diet), IU | |||
| Mean | 352.0 | 354.7 | 0.84 |
| < 200 | 394 (43.1) | 380 (40.6) | 0.70 |
| 200-<400 | 155 (17.0) | 164 (17.5) | |
| 400-<600 | 209 (22.9) | 219 (23.4) | |
| ≥ 600 | 156 (17.1) | 174 (18.6) | |
| Multivitamin use (w/or w/o minerals) | 0.17 | ||
| No | 626 (66.2) | 611 (63.3) | |
| Yes | 319 (33.8) | 355 (36.8) | |
| Total calcium (supplements+diet), mg | |||
| Mean | 1035.5 | 1070.2 | 0.21 |
| <800 | 394 (43.1) | 363 (38.7) | 0.16 |
| 800-<1200 | 217 (23.7) | 238 (25.4) | |
| ≥ 1200 | 303 (33.2) | 336 (35.9) | |
| Current hormone therapy** | 0.92 | ||
| None | 477 (50.6) | 489 (50.8) | |
| Estrogen | 216 (22.9) | 226 (23.4-) | |
| Estrogen plus progestin | 249 (26.4) | 247 (25.6) | |
From chi-square tests of independence for categorical variables, and t-tests for continuous total vitamin D and total calcium intake.
For women randomized to CaD at year 1 of the CT, who were in the 6% subsample, and had symptom information collected at CaD baseline and at CaD year 2.
At baseline, total calcium and vitamin D intakes, reflecting both dietary intake and supplement use, were similar in the two randomization groups with non-protocol vitamin D supplement use ≥ 400 IU daily reported by 42.0% of the placebo group and 40.0% of the supplement group, respectively (Table 1). During the course of the study, non-protocol calcium and vitamin D supplements were permitted and their use was similar between randomization groups. At two years, median non-protocol calcium use was 40 mg per day and non-protocol vitamin D supplement was being used by 48% of participants with mean dose of 199 IU and median dose of 0 IU per day. As a result, total vitamin D intake (diet plus non-protocol supplement plus protocol supplement) was 773 IU, mean and 724 IU, median in the supplement group and 367 IU, mean and 312 IU, median in the placebo group after 2 years. At that time, total calcium intake was 2031 mg, mean and 1877 mg, median in the supplement group and 1041 mg, mean and 920 mg, median in the placebo group. Adherence to the randomly assigned calcium and vitamin D supplement or placebo (defined as use of 80% or more of study medication) ranged from 60-63% during the first three years with an additional 13-21% taking at least half of their study pills with small difference between randomization groups.
Joint pain and swelling at baseline entry into the calcium plus vitamin D trial was closely comparable in the two randomization groups with over 70% of supplement and placebo group participants reporting joint pain and about a third reporting joint swelling. Joint symptoms at baseline and after two years on calcium plus vitamin D supplementation or placebo are shown by randomization group in Table 2. After two years, no statistically significant difference between supplement and placebo groups were seen for joint pain frequency (74.6% vs. 75.1%, for supplement and placebo groups, respectively, P=0.79) or joint swelling frequency (34.6% vs. 32.4%, respectively, P=0.29). The severity of joint pain or joint swelling also was similar in the supplement and placebo groups after 2 years (Table 2).
Table 2.
Joint Pain/Swelling by CaD Use (n=1911) in the Women's Health Initiative Study Sample
| CaD Trial Baseline | CaD Trial Year 2 | |||
|---|---|---|---|---|
| CaD % (N) | Placebo % (N) | CaD % (N) | Placebo % (N) | |
| Numbers* | 945 | 966 | 945 | 966 |
| Joint Pain | ||||
| None | 26.3% (247) | 28.1% (270) | 25.4% (239) | 24.9% (239) |
| Any | 73.7% (693) | 71.9% (691) | 74.6% (702) | 75.1% (722) |
| Severity | ||||
| Mild | 65.4% (453) | 64.8% (448) | 61.7% (433) | 63.2% (456) |
| Moderate | 27.6% (191) | 25.9% (179) | 29.5% (207) | 27.6% (199) |
| Severe | 7.1% (49) | 9.3% (64) | 8.8% (62) | 9.3% (67) |
| Change in Score at Year 2 | ||||
| Severity Score (mean ± SD) | 1.04 ± 0.82 | 1.04 ± 0.86 | 0.06 ± 0.84 | 0.09 ± 0.82 |
| Joint Swelling | ||||
| None | 65.7% (620) | 65.8% (630) | 65.4% (615) | 67.6% (648) |
| Any | 34.3% (324) | 34.2% (328) | 34.6% (326) | 32.4% (310) |
| Severity | ||||
| Mild | 72.2% (234) | 75.0% (246) | 72.1% (235) | 76.1% (236) |
| Moderate | 23.2% (75) | 20.4% (67) | 21.8% (71) | 18.7% (58) |
| Severe | 4.6% (15) | 4.6% (15) | 6.1% (20) | 5.2% (16) |
| Change in Score at Year 2 | ||||
| Severity Score (mean ± SD) | 0.45 ± 0.71 | 0.44 ± 0.69 | 0.02 ± 0.73 | 0.02 ± 0.72 |
For women randomized to CaD at year 1 of the CT, who were in the 6% subsample, and had symptom information collected at CaD baseline and at CaD year 2.
Statistical tests comparing CaD treatment randomization: p-values from either a logistic regression model (none/any) or linear regression model (average) demonstrated no statistically significant difference comparing CaD to placebo use at baseline, after two years of intervention or in the change from baseline to year 2. Tests were performed both unadjusted and adjusted for age, race/ethnicity.
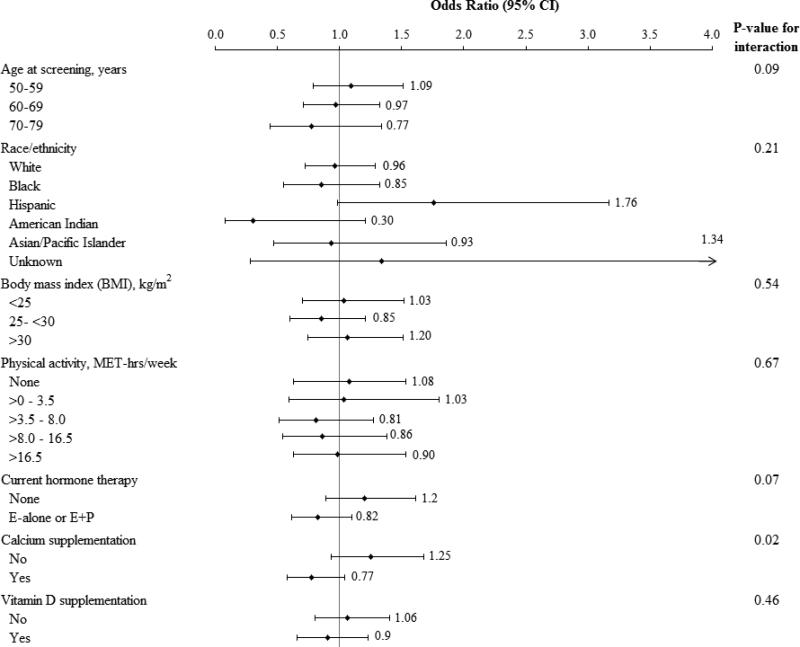
The potential for interaction with age, BMI, physical activity, non-protocol calcium and vitamin D use, race/ethnicity and hormone therapy on the association between joint pain and randomization group was examined (Figure 2). No interaction with age, BMI, race/ethnicity or physical activity was seen. The confidence intervals for all but White and Black women are very wide and are essentially non-informative. There was a suggestion of a favorable effect of calcium and vitamin D supplementation on joint pain in hormone therapy users and women 70-79 years old, but the interactions were not statistically significant (interaction P-values 0.07 and 0.09, respectively). Participants who were also using non-protocol calcium supplements at entry had less joint pain when randomized to the supplement compared to the placebo group (interaction P-value =.02) while no significant interaction was seen with non-protocol vitamin D supplement use at entry.
Figure 2.
Estimated effects of calcium and vitamin D supplement on the risk of joint pain according to selected baseline characteristics. In these analysis, odds ratios and 95% confience limits for effect of calcium and vitamin D supplementation on joint pain are estimated from a logistic regression model adjusted for linear age and race/ethnicity. P- values testing for interaction separately for each subgroup are from models including terms for the main effects for calcium and vitamin D supplementation and the subgroup, plus their interaction. For testing, age and BMI (log transformed) were modeled as linear terms; physical activity was coded (0-4) representing the 5 categories of physical activity.
Current hormone therapy reflects use at baseline if randomized to the Dietary Modification trial only or randomized to active versus placebo if randomized to one of the Hormone Therapy trials. Calcium supplementation and vitamin D supplementation reflects non- protocol use at study entry. E alone = estrogen alone, E+P = estrogen plus progestin.
Discussion
Joint symptoms were common in this population of postmenopausal women. However, in the setting of a randomized, placebo-controlled, clinical trial, calcium (1000 mg/d as elemental calcium) plus vitamin D supplementation (400 IU/d of D3) did not reduce the frequency or severity of joint symptoms of postmenopausal women compared to placebo. Thus, women using calcium plus vitamin D supplementation in this dose should not anticipate joint symptom relief.
Severe vitamin D deficiency can result in osteomalacia characterized by proximal muscle weakness and bone loss (1) but reports on the association between vitamin D intake and/or levels with joint symptoms have been mixed. While some observational studies suggest a threshold of 25 hydroxyvitamin D levels above 36 ng/ml is needed for lower osteoarthritis risk (2, 7), others find no association (4, 8, 9, 10). In previously reported analyses in WHI CT participants, women with low serum 25-hydroxyvitamin D levels did have statistically significantly higher joint pain scores compared to women with higher 25-hydroxyvitamin D levels but the threshold (seen in the lowest quintile) was a much lower 12 ng/ml (6) than found in some prior studies (2, 7) .The inconsistent observational study findings (25, 26, 27) support the need for randomized clinical trials to definitively address this issue.
Only one other full-scale, randomized clinical trial has addressed the issue of joint pain and vitamin D supplementation but studied a different population with a different intervention. McAlindon and colleagues (11) entered 146 men and women with symptomatic osteoarthritis of the knee with serial cartilage volume loss documented by magnetic resonance imaging. Participants were randomized to a higher dose vitamin D only intervention (daily oral vitamin D3, 2000 IU/d with escalation to target 25-hydroxyvitamin D serum levels >36 ng/ml) or placebo. After two years, no reduction in knee pain or cartilage volume loss was seen with the supplementation (11). As one possible explanation for the null findings, the authors suggested that the severity of the structural damage might have been too severe to expect reversal. Two smaller randomized trials, entering between only 50 to 60 participants, also evaluated higher dose vitamin D regimens (50,000 IU vitamin D2 weekly for three or four months) but reported no significant influence on musculoskeletal pain (28-29).
In the current study, we addressed, for the first time in a full-scale, randomized clinical trial setting, the clinically relevant question of whether postmenopausal women using calcium and vitamin D supplements in currently recommended dosage would experience any favorable effect on joint pain or swelling, common symptoms in postmenopausal women.
The vitamin D3 dose of 400 IU/d used in the WHI trial followed the Institute of Medicine recommendations which were available during the course of the trial (30). The Institute of Medicine recently updated their guideline and increased their recommended dietary allowance (RDA) for vitamin D intake moderately to 600 IU/d for those age ≤ 70 and 800 IU/d for those ages 71 and older (31). However, as about half of the WHI study participants were taking additional non-protocol vitamin D (19), many women in the supplement group had substantially greater vitamin D intakes. Based on the first comprehensive study of dose response to vitamin D supplementation in postmenopausal women in a recently reported randomized trial, Gallager and colleagues concluded that a vitamin D3 dosage of 600 IU per day would be sufficient to meet the nutritional requirements of nearly all (97.5%) healthy persons (32). Since the mean total vitamin D dose in the WHI supplement randomization group was 773 IU per day, including diet and protocol and non-protocol supplement use, we found no influence of the currently recommended vitamin D intake on joint symptoms in supplement group participants in this trial. While the influence of higher dose supplementation with calcium and vitamin D on joint symptoms are not known, current evidence does not support dosage exceeding the recent Institute of Medicine recommendation at this time (31).
The statistically significant, positive interaction which was seen between baseline non-protocol calcium use and joint pain benefit from protocol calcium and vitamin D supplementation was an unexpected finding. This result could reflect the play of chance or self-selection bias, especially since calcium has not historically been linked to joint symptoms. Alternatively, one could speculate that a calcium threshold level is required for vitamin D to favorably influence joint symptoms. In any case, the calcium result seen in a subgroup analyses clearly requires further study.
Study strengths include the size of the well characterized study population, the inclusion, by design, of a substantial minority population, joint symptom information collected within the context of a randomized clinical trial of calcium and vitamin D supplement use, and serial joint symptom assessment using a quantitative instrument which was prospectively applied. Study limitations include the fact that joint symptoms were not a prospectively identified study endpoints and, although randomly identified, only a subset of participants in the trial was included. Also, the joint symptom scale used has not been compared to other instruments or been formally validated. While joint symptoms in postmenopausal women have a range of etiologies (commonly osteoarthritis but also rheumatoid arthritis and other auto immune diseases as well as fibromyalgia and other causes), a quantitative, prospective serial assessment of the presence and severity of joint pain and swelling provides clinically relevant outcome information. Allowing non-protocol calcium and vitamin D use is another limitation. However, the difference in intakes between groups was sufficient to increase bone mineral density overall and decrease hip fracture in older participants and in adherent participants in the active intervention group (15). The influence of calcium and vitamin D supplementation individually on joint symptoms cannot be determined as both were provided combined in a single pill in this trial.
The findings from the current WHI randomized, placebo-controlled clinical trial evaluating calcium and vitamin D supplementation do not support use of calcium and vitamin D for joint symptom reduction at the dosage examined. These findings do not speak against current recommendations for vitamin D intakes for bone health and fracture risk reduction.
Acknowledgments
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Role of the Sponsor: The Women's Health Institute (WHI) Project Office at the National Heart, Lung, and Blood Institute (NHLBI), the Sponsor, reviewed and approved the final manuscript but had no other role in the preparation of this report. Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency: what a pain it is. Mayo Clin Proc. 2003;78:1457–9. doi: 10.4065/78.12.1457. [DOI] [PubMed] [Google Scholar]
- 2.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. 1996;125:353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, Pols HA. Vitamin d status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol. 2009;15:230–7. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 4.Muraki S, Dennison E, Jameson K, Boucher BJ, Akune T, Yoshimura N, Judge A, Arden NK, Javaid K, Cooper C. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2001;19:1301–6. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiently and knee osteoarthritis. Int Orthop. 2011;35:1627–31. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlebowski RT, Johnson KC, Lane D, Pettinger K, Kooperberg C, Wactawski-Wende J, Rohan T, O'Sullivan MJ, Yasmeen S, Hiatt R, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. 25-hydroxyvitamin D, vitamin D intake, and joint symptoms in postmenopausal women. Maturitas. 2010;68:73–78. doi: 10.1016/j.maturitas.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Williams EN, Nevitt MC. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42:854–60. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Niv J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth SL. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–36. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 9.Konstari S, Paananen M, Heliovaara M, Knekt P, Marniemi J, Impivaara O, Arokoski J, Karppinen J. Association of 25-hydroxyvtiamin D with the incidence of knee and hip osteoarthritis: a 22 year follow-up study. Scand J Rheumatol. 2012;41:124–31. doi: 10.3109/03009742.2011.617314. [DOI] [PubMed] [Google Scholar]
- 10.Al-Jarallah KF, Shehab D, Al-Awadhi A, Nahar I, Haider MZ, Moussa MA. Are 25(OH) D levels related to the severity of knee osteoarthritis and function? Med Princ Pract . 2012;21:74–8. doi: 10.1159/000330025. [DOI] [PubMed] [Google Scholar]
- 11.McAlindon T, LaValley M, Schneider E, Nuite T M, Lee JY, Price LL, Lo G, Dawson-Hughes B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309(2):155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang C, Stein E, Prentice R. Implementation of the Women's Health Initiative Study Design. Am Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 15.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 16.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(Suppl):S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium and vitamin D supplementation and the risk of fractures. N Engl J Med . 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 18.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Lane D, O'Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1604–1616. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT. Vitamin D and breast cancer: interpreting current evidence. Breast Cancer Res. 2011;13:217–24. doi: 10.1186/bcr2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Levy L, McLerran D, White E. Validity of methods used to assess vitamin and mineral supplement use. Am J Epidemiol. 1998;148(7):643–649. doi: 10.1093/aje/148.7.643. [DOI] [PubMed] [Google Scholar]
- 22.Patterson RE, Levy L, Tinker L, Kristal AR. Evaluation of a simplified vitamin supplement inventory developed for the Women's Health Initiative. Public Health Nutr. 1999;2(3):273–276. doi: 10.1017/s1368980099000361. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol . 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 24.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Ockene Jl. Recreational physical activity and the risk of breast cancer in postmenopausal women. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 25.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Howell A, Cuzick J. Wolfson Institute of Preventive Medicine. Vit D levels among patients with arthralgia: results from IBIS-II breast cancer prevention study. SABCS. 2006 (abstract 1068) [Google Scholar]
- 27.Hicks GE, Shardell M, Miller RR, Bandinelli S, Guralnik J, Cherubini A, Lauretani F, Ferrucci L. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56:785–91. doi: 10.1111/j.1532-5415.2008.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner AE, Arnspiger SA. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol. 2008;14:12–6. doi: 10.1097/RHU.0b013e31816356a9. [DOI] [PubMed] [Google Scholar]
- 29.Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ. Vitamin D and aromatase inhibitor-induced muscoloskelatal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat. 2011;129:107–16. doi: 10.1007/s10549-011-1644-6. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine . Dietary Reference Intakes for calcium, phosphorus magnesium, vitamin D and fluoride. National Academy Press; Washington, DC: 1997. [April 12, 2012]. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Vitamin D. Chapter 7 Available from: http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_subject=256&topic_id=1342&level14_id=10587. [Google Scholar]
- 31.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher JC, Sai A, Templin T, Smith L. Dose response to vitamin D supplementation in postmenopausal women. Ann Intern Med. 2012;156:425–437. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]