Abstract
It is well known that there are strong genetic influences on Attention Deficit Hyperactivity Disorder (ADHD), with genetic association studies providing good evidence for the involvement of the dopamine neurotransmitter system in its aetiology. Developmental origins of ADHD represent an interesting area of research to understand the genetics that underlie early-appearing individual differences. However, understanding the molecular basis of ADHD requires accurate, unbiased, heritable measures that can be used for molecular genetic association analyses.
We take two approaches to examine the genetics of ADHD behaviours in infancy. Using quantitative genetic techniques we explore the relationship between objective measures of activity level (AL) in both home and laboratory environments as well as with parent-ratings of ADHD symptoms in a population sample of 2-year old twins. Molecular association analyses of these measures examine candidate genes previously associated with ADHD.
We find that ADHD symptoms, AL in the home and AL in the lab represent heritable phenotypes in 2-year-old infants. AL measured in the home has a strong genetic correlation with symptoms of ADHD, whereas AL in the lab correlates only modestly with the same ADHD measure. Genetic correlations suggest that AL in the home is more comparable than AL in the lab to ADHD behaviour and support the separation of all three for molecular analyses. There was modest evidence for association between DAT1, NET1 and ADHD symptom scores, as well as between DAT1 and AL in the lab.
Keywords: genetics, twins, activity level, actigraph, ADHD
INTRODUCTION
The current definition of ADHD defines age of onset of impairing symptoms as occurring before 7 years of age, although it is unusual to make the diagnosis in very young children. The developmental origins of ADHD can however be investigated through studies of related temperamental traits in preschool children. Both parent-rated measures of ADHD symptoms and temperament are highly heritable in children of ages 2 through 4 years (Price et al., 2005, Saudino, 2005) although problems of contrast effects in typically used inventories have been highlighted (Price et al., 2005, Saudino et al., 2004).
For quantitative trait locus (QTL) analyses, mechanical measures of AL represent an objective method, free from rater bias, that could potentially complement currently used ADHD rating scales. AL in twin samples proves to be heritable in infants and older children (Saudino and Eaton, 1991, Saudino and Zapfe, 2008, Wood et al., 2007) and shows significant phenotypic and genetic overlap with hyperactivity/impulsivity symptoms in 7-year olds (Wood et al., 2008). Mechanically assessed AL may therefore be useful in understanding the genetics of ADHD traits in infancy.
There is a paucity of literature regarding the molecular genetics of objectively measured AL, with studies of child temperament being limited to parental or observer ratings of related behavioural traits and a limited set of candidate genes (Auerbach et al., 1999, Auerbach et al., 2001, De Luca et al., 2003, Ebstein et al., 1998). The findings are difficult to interpret due to the varied use of age-specific temperament inventories, a mixture of parent and observer ratings and small sample size. Overall, there is evidence for effects of dopamine system genes, specifically the dopamine receptor D4 (DRD4) gene on motor organization in neonates (Ebstein et al., 1998) and AL in 12-month-old infants (Auerbach et al., 2001). Studies of objectively-measured AL are few, with just one study reporting association of DRD4 with actigraph scores in children with ADHD (Langley et al., 2004). Therefore, potential candidate genes come from molecular analysis in the ADHD field, where a number of neurotransmitter genes have been studied (Asherson et al., 2007, Barr et al., 2000, Bobb et al., 2005, Brookes et al., 2006a, Brookes et al., 2006b, Brophy et al., 2002, Cornish et al., 2005, Curran et al., 2005, Das et al., 2006, Domschke et al., 2005, Feng et al., 2005, Guan et al., 2008, Kustanovich et al., 2003, Lawson et al., 2003, Li et al., 2006, Mill et al., 2002, Mill et al., 2004, Mill et al., 2005, Sheehan et al., 2005, Xu et al., 2007, Xu et al., 2008, Xu et al., 2005). Of most interest for our work are findings of genetic association with ADHD symptoms in samples of a similar age, such as that of QTL association of the 10-repeat allele of a variable number tandem repeat (VNTR) in the 3’ untranslated region (UTR) of the dopamine transporter gene (DAT1) in young children (Mill et al., 2005).
Using quantitative genetic techniques in a population twin sample we have sought to determine the relationship between ADHD symptom scores and AL measures in both home and laboratory situations in infancy, hypothesising that there would be substantial genetic overlap between the measures. Although there have been few molecular studies in children of this age, we would expect (albeit from limited evidence) that the 10-repeat allele of DAT1 would be positively associated with ADHD symptoms and AL. In addition, an exploratory approach has been taken to look for genetic association with alleles previously implicated in the aetiology of clinically and quantitatively defined ADHD phenotypes.
METHOD
Sample
The Boston University Twin Project sample was recruited from birth records supplied by the Massachusetts Registry of Vital Records. Twins were selected preferentially for higher birth weight and gestational age. No twins with birth weights below 1750 grams or with gestational ages less than 34 weeks were included in the study. Twins were also excluded if one or both twins had a health problem that might affect motor activity (e.g., cerebral palsy, club foot) or had chromosomal abnormalities. The present analyses include 312 same-sex pairs of twins (144 MZ, 168 DZ), mean age 2.07 years (SD = .05). Although the sample was predominately Caucasian (85.4%), ethnicity was generally representative of the Massachusetts population (3.2% Black, 2% Asian, 7.3% Mixed, 2.2% Other). Socioeconomic status according to the Hollingshead Four Factor Index (1975) ranged from low to upper middle class (range = 20.5–66; M = 50.9, SD = 14.1).
Zygosity was determined via DNA analysis using DNA obtained from cheek swab samples. In the cases where DNA was not available (n=3), zygosity was determined using parents’ responses on physical similarity questionnaires which have been shown to be more than 95% accurate when compared to DNA markers (Price et al., 2000). In our present sample we were able to assign zygosity with certainty to 99% of the twin pairs using the parent questionnaire, moreover agreement between questionnaire and DNA zygosity analyses was very high (kappa = .94).
Measures
Mechanical Assessment of Activity Level (AL)
The twins were assessed within approximately 2 weeks of their second birthday. The procedure consisted of two visits, 48-hours apart, to the laboratory. At the initial visit, informed consent from parents was obtained and the motion recorders were attached to each child. After attachment of the motion recorders, one twin was assessed within a standardised test situation, while the other twin was assessed within a laboratory play situation. The test situation involved administration of the Mental Scale of Bayley Scales of Infant Development–Second Edition (Bayley, 1993). The play situation comprised activity episodes from the Laboratory Temperament Assessment Battery–Preschool Version (Goldsmith et al., 1995). At the second visit, situations were reversed for each twin. Motion recorders were then removed and questionnaires and cheek scrapings collected. The twins were assessed by different testers, who were blind to any previously measured phenotypic status. However, for each twin, the tester was the same across the two laboratory situations.
During the two-day data collection period, activity level was assessed with the Minimitter actical (actigraph). This device is a miniature omni-directional accelerometer that has been designed to detect frequency and intensity of motion within the range of normal human movement. Actigraphs have been shown to be valid and reliable instruments for recording the activity of children (Pate et al., 2006). Four randomly selected actigraphs were assigned to each twin, one per limb, again in a random fashion. Actigraphs were attached by means of tyvek© adhesive wristbands. Arm attachment, at the wrist, was on the dorsal aspect of the forearm proximal to the radial carpal joint. Leg attachment, at the ankles, was superior to the lateral malleoli.
Parents were provided with instructions regarding the care of the motion recorders and a sheet for logging the times when the actigraphs were off a limb. To differentiate actigraphs within a twin pair, each actigraph was covered with white surgical tape on which the child's name was printed. Actigraphs were also color-coded and keyed to a parent record sheet indicating the color of actigraph that went on each limb. Thus, the parent was able to easily identify the set of actigraphs that belongs to each twin and, further, to match the instrument to the correct limb. Parents were encouraged to engage in normal activities and to maintain daily routines with their children during the two-day data collection period. The average length of time that the motion recorders were worn was 48.22 hours (SD = 3.22).
To adjust for variations in the total time that each instrument was worn within the home and lab situations, the number of activity units was converted to a rate per minute real time. Similar adjustments were made to the data from the test and play situations. Arm and leg activity counts were highly correlated (r = .69, p < .001), therefore, composite actigraph scores reflecting overall motor activity for each situation was calculated by averaging the four limb actigraph scores. Aggregating across motion records has been shown to increase both the reliability and validity of AL measures (Eaton, 1983, Melanson and Freedson, 1995). Consistent with prior research, actigraph scores were positively skewed (Saudino and Eaton, 1991, Wood et al., 2007) and were square-root transformed to create a more normal distribution.
Prior analysis of this data has revealed that co-variation between laboratory situations can be explained by shared genetic effects (Saudino and Zapfe, 2008) therefore, a mean was taken from the two laboratory AL scores. In this study, AL assessed during the two-day collection period in the home was analysed separately from the mean of the two lab-based measures.
Parent Reports of ADHD Behaviour
Parent ratings of hyperactivity were obtained using the hyperactivity subscales of the Child Behavior Checklist /1.5 – 5 years (CBCL) (Achenbach and Rescorla, 2000 ) and the Revised Rutter Parent Scale for Preschool Children (RRPSPC) (Hogg et al., 1997) which assess behaviors relating to overactivity, inattention, and impulsivity. In the present study reliabilities for the CBCL and the RRPSPC, as estimated by Cronbach’s alpha were .78 and .75, respectively.
Model Fitting Analysis
Because twin co-variances can be inflated by variance due to sex, all scores were residualised for sex effects. Residualised scores were used for all model-fitting procedures.
A Cholesky decompositon model was used to estimate the relative contributions of additive genetics (A), shared environment (C) and non-shared environment (E) to the phenotypic variances of ADHD and AL, as well as genetic and environmental contributions to the co-variation between measures. Models were fit to raw data using a maximum likelihood pedigree approach implemented in Mx structural equation modeling software (Neale et al., 2006). The overall fit of a model was assessed by calculating twice the difference between the negative log-likelihood (-2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated).
Genotyping
Polymorphisms were chosen based on previous association with ADHD in either clinical or QTL studies (see supplementary material Table 1). DNA was extracted from buccal swabs as described by Freeman et al. 2003. Both parents and offspring were genotyped. VNTR polymorphisms (DRD4 exon 3, DAT1 3’UTR, DAT1 intron 8, the 5-HTTLPR and MAOA promoter) were genotyped in-house. Protocols for genotyping the VNTRs are available on request from the authors. Single nucleotide polymorphisms (SNPs) were genotyped by Prevention Genetics (http://www.preventiongenetics.com/resgeno/researchgeno.htm).
Genotyping quality control measures were implemented to assess the impact of genotyping error (details are available from first author). A total of 9 SNPs were excluded from further analysis due to failure at assay design or having a high level of Mendelian error. All markers included in the analysis conformed to Hardy-Weinberg equilibrium (p>0.01).
Association Analysis
Tests of allelic association were performed using the Quantitative Transmission Disequilibrium Test (QTDT) ((Abecasis et al., 2000). Overall association was tested using the ‘Total Association’ (AT) model which assesses both the within pair differences as well as between pair sums (i.e., the correlation between phenotypic and genotypic differences and sums for each twin pair) but is not robust to stratification.
The ‘Within Test’ (AW) of association assesses the within component only in a variance components framework and is therefore unaffected by stratification. The AP test of association in QTDT compares the within and between components of association and thus provides a test for stratification.
UNPHASED (http://www.mrc.bsu.cam.ac.uk/personal/frank/software/unphased/) was used to test X-linked markers (polymorphisms in MAOA) because QTDT cannot deal with such data. Because UNPHASED has no means for handling MZ twin data, mean phenotypic scores for MZ pairs were used in these analyses.
RESULTS
Quantitative Genetic Analysis
There was a strong phenotypic correlation between the RRPSPC and the CBCL ADHD measures (r = 0.60, p < 0.01); therefore, the two measures were averaged to form an ADHD composite measure, which was square root transformed for a more normal distribution.
Intraclass correlations inferred additive genetic effects for parent rated ADHD and AL in the lab, with DZ twin correlations being approximately half that of MZ twins. In contrast, AL scores in the home displayed high MZ correlations in conjunction with high DZ correlations, suggesting shared environmental effects on phenotypic variance for this measure (Table 1). These inferences were confirmed in the multivariate model (see supplementary material Table 2 for fit statistics).
Table I.
Intraclass correlations (95%confidence intervals) and variance component estimates.
| rMZ | RDZ | A | C | E | |
|---|---|---|---|---|---|
| ADHD Composite | 0.76 (0.68 – 0.82) | 0.36 (0.22 – 0.49) | 0.78 (0.61 – 0.83) | 0.01 (0.00 – 0.15) | 0.22 (0.17 – 0.28) |
| AL Home | 0.87 (0.83 – 0.91) | 0.70 (0.61 – 0.77) | 0.29 (0.16 – 0.44) | 0.57 (0.42 – 0.69) | 0.14 (0.11 – 0.18) |
| AL Lab | 0.75 (0.67 – 0.81) | 0.38 (0.24 – 0.51) | 0.68 (0.42 – 0.79) | 0.05 (0.00 – 0.29) | 0.26 (0.21 – 0.34) |
Predominantly additive genetic effects influence both the ADHD composite and AL in the lab (Table 2), with small but significant contributions from non-shared environment, which includes measurement error. In contrast, mainly shared environmental effects influence phenotypic variance of AL in the home yielding a relatively low, but significant heritability estimate of 0.29. Again there is a small but significant contribution of non-shared environment on phenotypic variance for this measure.
Table II.
Phenotypic (rP) and genetic (rG) correlations. 95% CI are given in parentheses
| rP | rG | |
|---|---|---|
| ADHD Composite/AL home | 0.20 (0.10 – 0.29) | 0.50 (0.25 – 0.76) |
| ADHD Composite/AL Lab | 0.22 (0.14 – 0.31) | 0.24 (0.03 – 0.42) |
| AL Home/AL Lab | 0.38 (0.30 – 0.46) | 0.60 (0.32 – 0.87) |
Table 2 presents phenotypic correlations between the measures under study. Correlations between the ADHD composite and AL in the home were modest (r = 0.20) with a similar finding for the ADHD composite and AL in the lab (r = 0.22). AL scores showed moderate correlations with each other (r = 0.38).
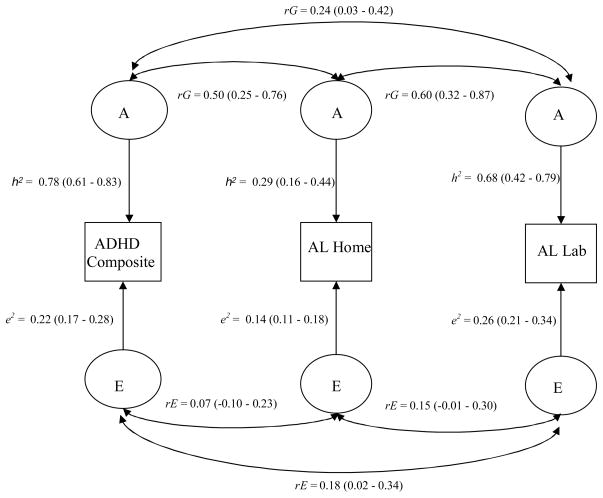
From the correlated factors solution (Figure I) we can estimate shared influences of A, C and E between variables. C has been omitted from the diagram due to the lack of significant C for either the ADHD composite or AL in the lab. The genetic correlation (rG) indicates the extent to which the genetic effects acting on one variable are the same as those acting on co-variables. The genetic correlations (Table 2) suggest that 50% of genetic effects on parent-rated ADHD are shared with AL in the home, whereas only 24% are shared with AL in the lab. Approximately 60% of genetic effects are shared between AL in the home and AL in the lab. Non-shared environmental variance is largely measure-specific with the only significant non-shared environmental correlation (rE) being between the ADHD composite and AL in the lab (rE = 0.18).
Figure I.
Correlated factors solution. rG = Genetic correlation, rE = Non-shared environment correlation, h2 = Heritability estimate, e2 = Non-shared environment estimate. 95% confidence intervals for each estimate are given in parentheses.
Our analyses revealed high heritability for ADHD (78%) and AL in the lab (68%), rendering them useful for identifying genes at the molecular level. However, the low genetic correlation between the two (rG = 0.24) indicates largely unique additive genetic effects for each measure and should therefore be treated separately in molecular analyses. Phenotypic variance for AL in the home was largely driven by common environmental variance, potentially decreasing its utility for genetic association studies. Nonetheless, 50% of genetic effects are shared between parent-rated ADHD and AL in the home, suggesting that some genetic variants will be associated with both phenotypes. Given the variance components structure of the three variables as well as the genetic correlations between them, we considered them separately in our association analyses.
Molecular Genetic analysis
Total Test of Association (AT)
Nominal association was detected between the DAT1 3’UTR VNTR (p = 0.03, 10-repeat) and one NET1 SNP, rs11568324 (p = 0.04) with the ADHD composite. Two additional SNPs in NET1, rs3785157 (p = 0.06) and rs998424 (p = 0.07) and a SNP in 5-HTT, rs140701 (p = 0.09) provided weak evidence of association with the same measure (Table 3). One SNP in DAT1, rs11564750 was associated with AL in the lab at the nominal level (p = 0.05).
Table III.
QTDT analysis. Nominal p-values <0.05 are in bold, italicized numbers and those approaching this significance threshold are shown in italics. AT = Total Test of Association, AW = Within-Test of Association. AP test for stratification effects were not significant for any associated marker at p<0.05. NT = Not tested. X-linked markers tested using UNPHASED, with Chi2 referring to likelihood Chi2 ratios in these instances.
| ADHD Composite | AL Home | AL Lab | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Gene | Marker | AT | AW | AT | AW | AT | AW | ||||||||||||
| Chi2 | df | p | Chi2 | df | p | Chi2 | df | p | Chi2 | df | p | Chi2 | df | p | Chi2 | df | p | ||
| DAT1 | rs2550946 | 0.43 | 539.00 | 0.51 | 0.71 | 539.00 | 0.40 | 0.33 | 541.00 | 0.57 | 0.78 | 541.00 | 0.38 | 1.23 | 544.00 | 0.27 | 0.69 | 544.00 | 0.41 |
| 3'UTR VNTR | 7.00 | 610.00 | 0.03 | 5.09 | 610.00 | 0.08 | 2.83 | 612.00 | 0.24 | 1.56 | 612.00 | 0.46 | 1.72 | 615.00 | 0.42 | 0.03 | 615.00 | 0.86 | |
| Int8 VNTR | 3.42 | 606.00 | 0.18 | 2.84 | 606.00 | 0.24 | 2.09 | 608.00 | 0.35 | 0.26 | 608.00 | 0.88 | 0.90 | 611.00 | 0.64 | 0.30 | 611.00 | 0.58 | |
| rs11564750 | 0.06 | 557.00 | 0.80 | 0.75 | 557.00 | 0.39 | 0.03 | 559.00 | 0.87 | 0.21 | 559.00 | 0.65 | 3.98 | 562.00 | 0.05 | 3.38 | 562.00 | 0.07 | |
|
| |||||||||||||||||||
| DRD4 | VNTR | 4.74 | 592.00 | 0.19 | 3.26 | 592.00 | 0.35 | 2.85 | 594.00 | 0.42 | 3.38 | 594.00 | 0.34 | 3.62 | 597.00 | 0.31 | 0.77 | 597.00 | 0.38 |
|
| |||||||||||||||||||
| NET1 | rs11568324 | 4.38 | 548.00 | 0.04 | NT | NT | NT | 1.44 | 550.00 | 0.23 | NT | NT | NT | 0.04 | 553.00 | 0.85 | NT | NT | NT |
| rs3785157 | 3.68 | 573.00 | 0.06 | 4.65 | 573.00 | 0.03 | 0.16 | 575.00 | 0.69 | 0.00 | 575.00 | 0.97 | 0.24 | 578.00 | 0.63 | 1.03 | 578.00 | 0.31 | |
| rs998424 | 3.30 | 572.00 | 0.07 | 4.42 | 572.00 | 0.04 | 0.09 | 574.00 | 0.76 | 0.00 | 574.00 | 0.95 | 0.31 | 577.00 | 0.58 | 0.66 | 577.00 | 0.42 | |
| rs2242447 | 1.23 | 568.00 | 0.27 | 1.03 | 568.00 | 0.31 | 0.07 | 570.00 | 0.79 | 0.26 | 570.00 | 0.61 | 0.51 | 573.00 | 0.47 | 0.84 | 573.00 | 0.36 | |
|
| |||||||||||||||||||
| 5-HTT | rs140701 | 2.96 | 559.00 | 0.09 | 3.03 | 559.00 | 0.08 | 0.40 | 561.00 | 0.53 | 0.17 | 561.00 | 0.68 | 1.37 | 564.00 | 0.24 | 0.99 | 564.00 | 0.32 |
| rs11080121 | 2.16 | 577.00 | 0.14 | 2.71 | 577.00 | 0.10 | 0.25 | 579.00 | 0.62 | 0.31 | 579.00 | 0.58 | 2.35 | 582.00 | 0.13 | 2.23 | 582.00 | 0.14 | |
| rs2020936 | 1.24 | 559.00 | 0.27 | 1.92 | 559.00 | 0.17 | 0.07 | 561.00 | 0.80 | 0.01 | 561.00 | 0.91 | 0.23 | 564.00 | 0.63 | 0.15 | 564.00 | 0.70 | |
| rs2066713 | 0.04 | 561.00 | 0.84 | 0.00 | 561.00 | 1.00 | 0.28 | 563.00 | 0.59 | 0.40 | 563.00 | 0.53 | 0.56 | 566.00 | 0.45 | 0.50 | 566.00 | 0.48 | |
| VNTR | 0.31 | 592.00 | 0.57 | 0.03 | 592.00 | 0.87 | 0.00 | 596.00 | 0.98 | 0.04 | 596.00 | 0.84 | 0.78 | 599.00 | 0.38 | 0.16 | 599.00 | 0.69 | |
|
| |||||||||||||||||||
| SNAP25 | rs6039806 | 0.00 | 569.00 | 0.96 | 0.00 | 569.00 | 0.99 | 0.08 | 572.00 | 0.78 | 0.42 | 572.00 | 0.42 | 0.17 | 575.00 | 0.68 | 0.01 | 575.00 | 0.91 |
| rs362987 | 0.04 | 572.00 | 0.84 | 0.07 | 572.00 | 0.79 | 0.01 | 574.00 | 0.93 | 0.18 | 574.00 | 0.18 | 0.35 | 577.00 | 0.55 | 0.26 | 577.00 | 0.61 | |
| rs3746544 | 0.05 | 558.00 | 0.83 | 0.18 | 558.00 | 0.67 | 0.31 | 560.00 | 0.58 | 0.34 | 560.00 | 0.34 | 0.08 | 563.00 | 0.77 | 0.09 | 563.00 | 0.76 | |
| rs1051312 | 0.63 | 555.00 | 0.43 | 0.04 | 555.00 | 0.85 | 0.54 | 557.00 | 0.46 | 0.38 | 557.00 | 0.54 | 1.06 | 560.00 | 0.30 | 0.89 | 560.00 | 0.34 | |
|
| |||||||||||||||||||
| MAOA | rs6323 | NT | NT | NT | 1.03 | 1.00 | 0.31 | NT | NT | NT | 0.61 | 1.00 | 0.43 | NT | NT | NT | 0.78 | 1.00 | 0.38 |
| VNTR | NT | NT | NT | 4.68 | 3.00 | 0.20 | NT | NT | NT | 1.14 | 3.00 | 0.77 | NT | NT | NT | 0.72 | 3.00 | 0.87 | |
Given the mix of ethnicities in this sample, markers found to be associated in the AT test were tested in a reduced sample where non-Caucasian subjects not from European ancestry (n = 45) were removed. The significance of the DAT1 3’UTR VNTR association was lowered in the reduced sample (p = 0.11) possibly due to the reduction in statistical power or the presence of stratification effects. However, rs11568324 and rs11564750 remained significantly associated (p = 0.05) at the nominal level.
Within pair Test of Association (AW)
We found no evidence for stratification effects (AP test, data not shown), although it cannot be ruled out due to low power to detect it in this sample. We therefore completed the AW test for all genetic markers, which is robust to stratification effects. Two SNPs in NET1, rs3785157 (p = 0.03) and rs998424 (p = 0.04) showed nominal significance in this test with the ADHD composite, although high linkage disequilibrium (LD) between these SNPs suggests non-independence. Further, the DAT1 3’UTR VNTR (p = 0.08) and rs140701 (p = 0.08) displayed an association trend with the same measure (Table 3). rs11568324 was not tested in the AW test due to low minor allele frequency (MAF = 0.01). rs11564750 displayed an association trend with AL in the lab (p = 0.07). None of the other markers were significantly associated with any measure.
DISCUSSION
This study examined the use of different methods for measuring behaviours related to ADHD in 2-year old infants. Both parent-ratings of ADHD symptoms and mechanically measured AL are independently heritable phenotypes in late infancy and are therefore potentially useful for study at the molecular level. Of particular interest in this study is the high genetic overlap between parent-rated ADHD and AL in the home (rG = 0.50), where shared genetic influences between parent-rated ADHD and AL in the lab is modest (rG = 0.24). From these analyses we cannot make assumptions on the structure of the genetic overlap between the measures but we can speculate on the reasons for the situational specificity of these results. One possibility is that there are fundamental differences in the behavioural responses to stimuli in the home and lab environments, related to novelty and salience of stimuli. The home setting is one that the children are familiar with and therefore show habituated (baseline) behavioural patterns, whereas in the lab environment the child is exposed to novel stimuli, which may also be more salient or stimulating to the child; behaviour in this situation could therefore reflect exploration of novel situations, may be modified by the salience of the stimuli or reflect other behaviours such as anxiety to an unfamiliar situation.
The known association between ADHD and novelty seeking (Anckarsater et al., 2006, Downey et al., 1997, Lynn et al., 2005) suggests that ADHD behaviours such as overactivity might manifest predominantly in those situations where stimulus is below a threshold level and may be more closely related to AL in habituated environments such as the home situation. This pattern of behavioural response is apparent in reports of stimulus-seeking behaviours in children with ADHD. In waiting situations where no stimulus is present, ADHD children show heightened activity compared to controls, whereas if stimulation is provided through the use of a video, ADHD children will display no significant differences in their activity compared to controls (Antrop et al., 2000). Theoretical research also indicates differences in performance during cognitive tasks depending on the nature of the stimulus. For example, although children with ADHD show slower and more variable responses during a simple reaction time task, differences from controls are abolished by increasing the presentation rate and reward value attached to the stimulus (Andreou et al., 2006; reviewed in (Kuntsi and Stevenson, 2000).
Further, although the degree to which AL measures are correlated to each other is greater than either is to ADHD, they do show a level of independence. This point is related to the aforementioned importance of situational specificity and reflects the fact that objective measurements reflect the environment in which they are measured (Saudino and Zapfe, 2008).
Exploration of genetic variants correlated with measures of ADHD and AL in infancy provided modest evidence of association with the behavioural measure. The DAT1 3’UTR association is consistent with reports by Mill et al. (2005) who completed a similar analysis with ADHD scores from 2, 3 and 4 year old twins, suggesting that DAT1 is a QTL for early ADHD symptoms. The direction of effect for rs3785157 and rs998424 in NET1 is consistent with the report from Xu et al (2005) who used a clinical sample of ADHD combined type cases. The high LD between these two markers (r2=0.94) suggests that these data are also consistent with the replication study from Bobb and colleagues (2005). The association with the allele of rs11568324 was found to be in the same direction as that previously reported in four independent studies of ADHD (Xu et al., 2008). Although rare, this allele appears to be associated with lower levels of ADHD symptoms in this study and a decreased risk for ADHD in previous studies. Finally, we found nominal evidence of association between rs11564750 in DAT1 and AL in the lab.
The results of our quantitative analysis suggested separation of the three measures for association testing. Subsequent exploratory association analysis of the risk variants with a composite measure of the three variables has provided additional evidence that these measures should be separated. Fewer associations were found in this analysis (data not shown), suggesting that aggregating the measures produces a variable that may be more related to the ‘activity’ domain of ADHD, which subsequently reduces the power to detect association with ADHD ‘risk genes’.
Clearly we have not yet accrued sufficient data to confirm or refute these associations with quantitative trait measures of ADHD or AL in young children and further data are required to reach convincing levels of evidence using genome wide levels of significance (around p<5 × 10−7; (The Welcome Trust Case Control Consortium, 2007). Nevertheless, it is of interest that with the exception of rs11564750 and AL in the lab, we only found evidence of association with the behavioural measure. This is predicted from the quantitative genetic findings since AL in the lab, although heritable, showed only limited genetic correlation with ADHD and would not therefore be predicted to show the same genetic associations as those for ADHD in most cases. AL in the home was influenced predominantly by non-genetic influences, suggesting low power of this measure to detect ADHD associated risk alleles.
A major limitation of this study is the power of the sample to detect genetic associations. Using the genetic power calculator (pngu.mgh.harvard.edu/~purcell/gpc/) we estimated that the sample had 47% power to detect a QTL affecting 1% of the phenotypic variance and 71% power to detect a 5% QTL. Despite this, we identified several nominal associations with the ADHD phenotype. Although none of these withstood correction for multiple testing at the experiment wide level, the number of tests performed for each of the three measures in this study would predict around 1 positive signal at the p <0.05 threshold, by chance alone; whereas we found three positive signals with the ADHD composite suggesting an above chance deviation. Therefore, these results are suggestive of true associations but should be interpreted with caution with the need for replication in a more powerful sample. It is interesting to consider the difference in findings between the AT and the AW tests of association, which may in part be explained by stratification effects introduced by different raters between twin pairs, an element of association that cannot be conclusively ruled out in this sample. This may go some way to explain the increase in significance for rs3785157 and rs998424 in the AW compared to the AT.
Although we started out with the hypothesis that mechanically assessed AL would overlap genetically with ADHD symptom scores in our sample, both quantitative and molecular genetic analyses have supported the separation of the measures in the study of infant ADHD traits. The results may differ, however, at other ages. Longitudinal analyses of observer-rated AL assessed at 14, 20, 24, and 36 months of age in the laboratory found new genetic effects emerged at 36 months (Saudino and Cherny, 2001) and future studies may find an effect of ADHD genes on AL in older children.
Supplementary Material
Acknowledgments
The BUTP is supported by grant MH062375 from the National Institute of Mental Health. The authors gratefully acknowledge the parents and twins in the BUTP. Special thanks to Dr. Jeff Zapfe for his help with the actical data.
Footnotes
The authors have no conflict of interest.
References
- The Welcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–92. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: 2000. [Google Scholar]
- Anckarsater H, Stahlberg O, Larson T, Hakansson C, Jutblad SB, Niklasson L, Nyden A, Wentz E, Westergren S, Cloninger CR, Gillberg C, Rastam M. The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. Am J Psychiatry. 2006;163:1239–44. doi: 10.1176/ajp.2006.163.7.1239. [DOI] [PubMed] [Google Scholar]
- Antrop I, Roeyers H, Van Oost P, Buysse A. Stimulation seeking and hyperactivity in children with ADHD. Attention Deficit Hyperactivity Disorder. J Child Psychol Psychiatry. 2000;41:225–31. [PubMed] [Google Scholar]
- Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP, Buitelaar J, Banaschewski T, Sonuga-Barke E, Eisenberg J, Manor I, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Faraone SV. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. Am J Psychiatry. 2007;164:674–7. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, Ebstein R. Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Mol Psychiatry. 1999;4:369–73. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. J Child Psychol Psychiatry. 2001;42:777–83. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Barr CL, Feng Y, Wigg K, Bloom S, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL. Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder. Mol Psychiatry. 2000;5:405–9. doi: 10.1038/sj.mp.4000733. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of infant development. 2. San Antonio, TX: The psychological corporation; 1993. [Google Scholar]
- Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, Clasen LS, Sharp WS, Inoff-Germain G, Wavrant-De Vrieze F, Arcos-Burgos M, Straub RE, Hardy JA, Castellanos FX, Rapoport JL. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, Anney R, Franke B, Gill M, Ebstein R, Buitelaar J, Sham P, Campbell D, Knight J, Andreou P, Altink M, Arnold R, Boer F, Buschgens C, Butler L, Christiansen H, Feldman L, Fleischman K, Fliers E, Howe-Forbes R, Goldfarb A, Heise A, Gabriels I, Korn-Lubetzki I, Johansson L, Marco R, Medad S, Minderaa R, Mulas F, Muller U, Mulligan A, Rabin K, Rommelse N, Sethna V, Sorohan J, Uebel H, Psychogiou L, Weeks A, Barrett R, Craig I, Banaschewski T, Sonuga-Barke E, Eisenberg J, Kuntsi J, Manor I, Mcguffin P, Miranda A, Oades RD, Plomin R, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006a;11:934–53. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, Chen CK, Huang YS, Sethna V, Taylor E, Chen W, Breen G, Asherson P. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch Gen Psychiatry. 2006b;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- Brophy K, Hawi Z, Kirley A, Fitzgerald M, Gill M. Synaptosomal-associated protein 25 (SNAP-25) and attention deficit hyperactivity disorder (ADHD): evidence of linkage and association in the Irish population. Mol Psychiatry. 2002;7:913–7. doi: 10.1038/sj.mp.4001092. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–98. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Curran S, Purcell S, Craig I, Asherson P, Sham P. The serotonin transporter gene as a QTL for ADHD. Am J Med Genet B Neuropsychiatr Genet. 2005;134:42–7. doi: 10.1002/ajmg.b.30118. [DOI] [PubMed] [Google Scholar]
- Das M, Bhowmik AD, Sinha S, Chattopadhyay A, Chaudhuri K, Singh M, Mukhopadhyay K. MAOA promoter polymorphism and attention deficit hyperactivity disorder (ADHD) in indian children. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:637–42. doi: 10.1002/ajmg.b.30385. [DOI] [PubMed] [Google Scholar]
- De Luca A, Rizzardi M, Buccino A, Alessandroni R, Salvioli GP, Filograsso N, Novelli G, Dallapiccola B. Association of dopamine D4 receptor (DRD4) exon III repeat polymorphism with temperament in 3-year-old infants. Neurogenetics. 2003;4:207–12. doi: 10.1007/s10048-003-0146-z. [DOI] [PubMed] [Google Scholar]
- Domschke K, Sheehan K, Lowe N, Kirley A, Mullins C, O'sullivan R, Freitag C, Becker T, Conroy J, Fitzgerald M, Gill M, Hawi Z. Association analysis of the monoamine oxidase A and B genes with attention deficit hyperactivity disorder (ADHD) in an Irish sample: preferential transmission of the MAO-A 941G allele to affected children. Am J Med Genet B Neuropsychiatr Genet. 2005;134:110–4. doi: 10.1002/ajmg.b.30158. [DOI] [PubMed] [Google Scholar]
- Downey KK, Stelson FW, Pomerleau OF, Giordani B. Adult attention deficit hyperactivity disorder: psychological test profiles in a clinical population. J Nerv Ment Dis. 1997;185:32–8. doi: 10.1097/00005053-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Eaton WO. Measuring activity level with actometers: reliability, validity, and arm length. Child Development. 1983:720–726. [Google Scholar]
- Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH. Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Mol Psychiatry. 1998;3:238–46. doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Feng Y, Crosbie J, Wigg K, Pathare T, Ickowicz A, Schachar R, Tannock R, Roberts W, Malone M, Swanson J, Kennedy JL, Barr CL. The SNAP25 gene as a susceptibility gene contributing to attention-deficit hyperactivity disorder. Mol Psychiatry. 2005;10:998–1005. 973. doi: 10.1038/sj.mp.4001722. [DOI] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. The laboratory temperament assessment battery-preschool version:Description of procedures. Madison WI: University of Wisconsin; 1995. [Google Scholar]
- Guan L, Wang B, Chen Y, Yang L, Li J, Qian Q, Wang Z, Faraone SV, Wang Y. A high-density single-nucleotide polymorphism screen of 23 candidate genes in attention deficit hyperactivity disorder: suggesting multiple susceptibility genes among Chinese Han population. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002139. [DOI] [PubMed] [Google Scholar]
- Hogg C, Rutter M, Richman N. Emotional and Behavioural Problems in Children. Windsor, Berkshire, UK: NFER–NELSON; 1997. [Google Scholar]
- Kuntsi J, Stevenson J. Hyperactivity in children: a focus on genetic research and psychological theories. Clin Child Fam Psychol Rev. 2000;3:1–23. doi: 10.1023/a:1009580718281. [DOI] [PubMed] [Google Scholar]
- Kustanovich V, Merriman B, Mcgough J, Mccracken JT, Smalley SL, Nelson SF. Biased paternal transmission of SNAP-25 risk alleles in attention-deficit hyperactivity disorder. Mol Psychiatry. 2003;8:309–15. doi: 10.1038/sj.mp.4001247. [DOI] [PubMed] [Google Scholar]
- Langley K, Marshall L, Van Den Bree M, Thomas H, Owen M, O'donovan M, Thapar A. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161:133–8. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- Lawson DC, Turic D, Langley K, Pay HM, Govan CF, Norton N, Hamshere ML, Owen MJ, O'donovan MC, Thapar A. Association analysis of monoamine oxidase A and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:84–9. doi: 10.1002/ajmg.b.10002. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–84. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Lynn DE, Lubke G, Yang M, Mccracken JT, Mcgough JJ, Ishii J, Loo SK, Nelson SF, Smalley SL. Temperament and character profiles and the dopamine D4 receptor gene in ADHD. Am J Psychiatry. 2005;162:906–13. doi: 10.1176/appi.ajp.162.5.906. [DOI] [PubMed] [Google Scholar]
- Melanson E, Freedson PS. Validity of the Computer Science and Applications, Inc. activity monitor. Medicine & Science in Sports & Exercise. 1995:934–940. [PubMed] [Google Scholar]
- Mill J, Curran S, Kent L, Gould A, Huckett L, Richards S, Taylor E, Asherson P. Association study of a SNAP-25 microsatellite and attention deficit hyperactivity disorder. Am J Med Genet. 2002;114:269–71. doi: 10.1002/ajmg.10253. [DOI] [PubMed] [Google Scholar]
- Mill J, Richards S, Knight J, Curran S, Taylor E, Asherson P. Haplotype analysis of SNAP-25 suggests a role in the aetiology of ADHD. Mol Psychiatry. 2004;9:801–10. doi: 10.1038/sj.mp.4001482. [DOI] [PubMed] [Google Scholar]
- Mill J, Xu X, Ronald A, Curran S, Price T, Knight J, Craig I, Sham P, Plomin R, Asherson P. Quantitative trait locus analysis of candidate gene alleles associated with attention deficit hyperactivity disorder (ADHD) in five genes: DRD4, DAT1, DRD5, SNAP-25, and 5HT1B. Am J Med Genet B Neuropsychiatr Genet. 2005;133:68–73. doi: 10.1002/ajmg.b.30107. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7. Richmond, VA: Virgina Commonwealth University; 2006. [Google Scholar]
- Pate RR, Almeida MJ, Mciver KL, Pfeiffer KA, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14:2000–6. doi: 10.1038/oby.2006.234. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research. 2000;3:129. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Price TS, Simonoff E, Asherson P, Curran S, Kuntsi J, Waldman I, Plomin R. Continuity and change in preschool ADHD symptoms: longitudinal genetic analysis with contrast effects. Behav Genet. 2005;35:121–32. doi: 10.1007/s10519-004-1013-x. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. J Dev Behav Pediatr. 2005;26:214–23. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Cherny SS. Sources of continuity and change in observed temperament. In: Emde RN, Hewitt JK, editors. The transition from infancy to early childhood: Genetic and environmental influences in the MacArthur Longitudinal Twin Study. New York: Oxford University Press; 2001. pp. 89–110. [Google Scholar]
- Saudino KJ, Eaton WO. Infant temperament and genetics: an objective twin study of motor activity level. Child Dev. 1991;62:1167–74. [PubMed] [Google Scholar]
- Saudino KJ, Wertz AE, Gagne JR, Chawla S. Night and Day: Are Siblings as Different in Temperament as Parents Say They Are? Journal of Personality and Social Psychology. 2004;87:698–706. doi: 10.1037/0022-3514.87.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Zapfe JA. Genetic influences on activity level in early childhood: do situations matter? Child Dev. 2008;79:930–43. doi: 10.1111/j.1467-8624.2008.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K, Lowe N, Kirley A, Mullins C, Fitzgerald M, Gill M, Hawi Z. Tryptophan hydroxylase 2 (TPH2) gene variants associated with ADHD. Mol Psychiatry. 2005;10:944–9. doi: 10.1038/sj.mp.4001698. [DOI] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Saudino KJ, Asherson P, Kuntsi J. High Heritability for a Composite Index of Children's Activity Level Measures. Behav Genet. 2008 doi: 10.1007/s10519-008-9196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Saudino KJ, Rogers H, Asherson P, Kuntsi J. Genetic influences on mechanically-assessed activity level in children. J Child Psychol Psychiatry. 2007;48:695–702. doi: 10.1111/j.1469-7610.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Brookes K, Chen CK, Huang YS, Wu YY, Asherson P. Association study between the monoamine oxidase A gene and attention deficit hyperactivity disorder in Taiwanese samples. BMC Psychiatry. 2007;7:10. doi: 10.1186/1471-244X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hawi Z, Brookes KJ, Anney R, Bellgrove M, Franke B, Barry E, Chen W, Kuntsi J, Banaschewski T, Buitelaar J, Ebstein R, Fitzgerald M, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Faraone SV, Gill M, Asherson P. Replication of a rare protective allele in the noradrenaline transporter gene and ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1564–7. doi: 10.1002/ajmg.b.30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Knight J, Brookes K, Mill J, Sham P, Craig I, Taylor E, Asherson P. DNA pooling analysis of 21 norepinephrine transporter gene SNPs with attention deficit hyperactivity disorder: no evidence for association. Am J Med Genet B Neuropsychiatr Genet. 2005;134:115–8. doi: 10.1002/ajmg.b.30160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.