Abstract
The development of methods for capillary electrophoresis (CE) with on-line mass spectrometric detection (CE/MS) is driven by the need for accurate, robust and sensitive glycomics analysis for basic biomedicine, biomarker discovery, and analysis of recombinant protein therapeutics. One important capability is to profile glycan mixtures with respect to the patterns of substituents including sialic acids, acetate, sulfate, phosphate, and other groups. There is additional need for an MS-compatible separation system capable of resolving carbohydrate isomers. This review summarizes applications of CS/MS to analysis of carbohydrates, glycoproteins and glycopeptides that have appeared since 2008. Readers are referred to recent comprehensive reviews covering earlier publications.
2. Introduction
Protein glycosylation occurs through a series of biosynthetic events in the endoplasmic reticulum and Golgi apparatus that are under complex regulation. Structures of glycoproteins depend on numerous factors including glycosyltransferase enzyme and nucleotide donor concentrations and rates of passage through these compartments. As a result, glycosylation at a given protein site typically reflects a mixture of glycoforms that elaborate a core structure. With regard to function, glycoprotein glycans elaborate the physic-chemical properties of proteins and enable binding interactions with carbohydrate-binding protein domains. Key functions during biosynthesis include protein folding quality control and protein sorting. Cell surface glycans contain an array of antigenic epitopes (1) including those that form the ABO blood groups. These epitopes bind to carbohydrate binding domains enable the glycoprotein to bind lectin domains including the galectins, C-type, P-type and I-type lectins. Expression of carbohydrate epitopes is regulated according to cell phenotype during development and as expressed in adult physiology. Such interactions are key to cell-cell and cell-pathogen interactions as well as to development of aberrant cell growth including cancers.
As described in recent reviews, mass spectrometry is an enabling technology in glycomics (2–5). Precise measurement of a glycan mass combined with assumptions regarding the biosynthetic reactions determines the monosaccharide compositions of glycans present in the sample and their approximate relative abundances. Thus, mass spectral analysis of glycans released from a glycoconjugate defines the types of glycans present. Such analysis of glycopeptides produced by proteolytic digestion serves to map the glycosylated peptides. Because the mixtures are typically complex, there is a need to combine separations with mass spectrometry analysis in order to produce a comprehensive map of glycoconjugate glycans.
In order to meet the emerging needs for glycomics analysis, it is desirable to have separations methods that are (1) high in peak capacity, (2) high in dynamic range, and (3) robust. High peak capacity is necessary to separate glycan structural isomers. Sensitivity is necessary to enable detection of low abundance glycans of biological interest in the presence of high abundance glycans. Robustness is key to dissemination of any emerging mass spectrometric technology; it must be possible to use the technology on a routine basis. As summarized in recent reviews (6–10), on-line separations combined with mass spectrometry have become extremely useful for profiling of released glycans, but do not fill all three needs. Liquid chromatography based methods are based on hydrophilic interaction chromatography, reversed phase chromatography, reversed phase ion pairing chromatography, size exclusion chromatography or porous graphitized carbon chromatography. At the present time, the extent of isomeric separation of complex glycans available using any of these methods is quite limited.
Capillary electrophoresis (CE) separates analytes based on charge, size, and shape. The fact glycoconjugate glycans contain positional isomers makes CE attractive as a separations method. The topic of CE of glycans has been reviewed recently (11). While CE separations complement liquid chromatography profiling of glycans and glycoconjugates using a mass spectrometric detector, not all methods are appropriate for interfacing with a mass spectrometer. Capillary electrophoresis/mass spectrometry (CE/MS) for analysis of glycans, glycoproteins and other glycoconjugates has been the topic of recent comprehensive reviews (12–14). The intention here is to give an update on applications of CE/MS for glycans, glycopeptides and glycoproteins that have appeared since the most recent previous review (2009).
3. General considerations for CE/MS of carbohydrates, glycopeptides and glycoproteins
Analysis of glycans using CE is carried out typically using reducing end derivatives that facilitate optical detection. Commercial kits are available for glycan reductive amination. Using these methods it is necessary to match observed migration times against those of standard compounds. As a result, peaks for which standards are not available cannot be identified. The use of MS as a detector for CE has the advantage that the mass dimension defines the glycan composition with respect to types of monosaccharides and substituents. This extra dimension of information provides a mass profile for the glycan migrating through the column; however, the use of a MS detector dictates that the electrolyte composition be compatible, specifically that all components be volatile (12, 13). This requirement eliminates many CE methods because of the presence of non-volatile electrolyte components. CE/MS is generally performed in free solution, rather than in gel filled capillaries because of compatibility issues. In order to maintain a sufficient flow of solution into the MS source, a co-axial sheath flow of a few μL/min is often used at the distal end of the capillary (for example see (15)). The problem is that this sheath flow necessarily dilutes the analytes migrating from the capillary. Another interface option employs a junction at the capillary tip to add sufficient flow of solution for the MS source (for recent examples see (16, 17)). Typically the flow for such a makeup flow is 0.2–0.4 nL/min, considerably lower than that used by the co-axial sheath flow.
4. Applications
a. CE/MS of lipopolysaccharides
Lipopolysaccharide (LPS) is found in the outer membrane of Gram-negative bacteria and acts as a strong stimulator of the innate immune system in host organisms. LPS from E. coli exists as a set of non-stoichiometric substituents on an inner core structure. The substituents include phosphate, ethanolamine, and acidic KDO monosaccharide residues. Because the presence of these substituents influences the LPS charge, CE is a useful means of profiling the variants. CE/MS using a sheath flow with ion trap MS detector has been used for this purpose.
Figure 1 shows the structure of E. coli LPS with positions of possible substituents labeled (18). Liquid chromatographic separation of de-acylated LPS has proven challenging because the compounds do not bind reversed phase columns. Successful analysis of the deacylated LPS required optimization of the sheath flow composition, and 50% methanol without additives was selected as the best choice. The running electrolyte for the CE separations was pH 9.0 ammonium acetate and the applied potential was 30 kV. The concentration of ammonium acetate for fully de-acylated LPS was 10 mM and that for partially de-acylated LPS was 50 mM. The latter electrolyte required use of lower separation potentials to minimize problems with capillary heating. The sheath flow solution was either methanol/water or methanol/water/formic acid depending on the analyte. Using this approach it was possible to separate LPS variants based on the number of KDO and phosphate groups. The CE conditions depended primarily on the presence or absence of acyl chains on the LPS.
Figure 1.
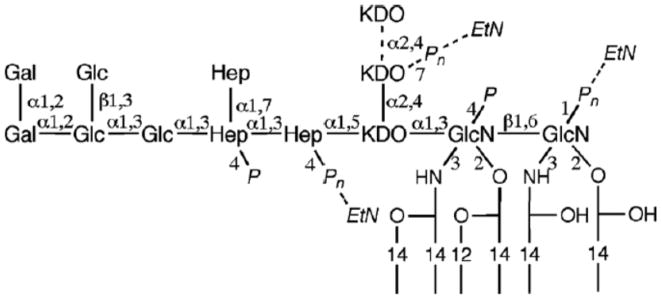
Chemical structure of LPS of E. coli C strain. Gal, d-galactose; Glc, d-glucose; Hep, l-glycero-d-manno-heptose; KDO, 3-deoxy-d-manno-octulosonic acid; GlcN, 2-amino-2-deoxy-d-glucose; EtN, ethanolamine; P, phosphate. Copyright 2009, Elsevier, used with permission.
b. CE/MS of N-glycans
The fact that many protein therapeutics are glycosylated drives the need for effective methods for rapid and precise testing of glycosylation in recombinant protein batches. Release of N-glycans using peptide N-glycosidase F (PNGase F) is often combined with reductive amination to add desired optical properties (19). Reductive amination using aminopyrene trisulfonate (APTS) has been used for CE/MS with on-line laser-induced fluorescence detection of N-glycans released from recombinant antibody preparations (15). Reductive amination using APTS adds a group with fluorescent properties appropriate for commercial laser-induced fluorescence detectors with excitation at 488 nm and emission at 520 nm. The sulfonate groups ensure that all glycans are negatively charged in the electrolyte, enabling use of coated neutral capillaries. The sulfonate groups also facilitate detection using on-line negative ion MS. The combination of the fluorescence detector and MS provides clear value in that it provides absolute quantification of glycans using fluorescent peak areas; the MS dimension determines the composition of the eluting APTS-glycans. The running electrolyte consisted of 40 mM aminocaproic acid, pH 4.5 and a sheath liquid of 50% isopropanol, 0.2% ammonia was used. This enabled migration of the APTS-labeled glycans with an applied potential of −30 kV and without the need to condition the capillary. The basic sheath liquid was appropriate for efficient negative ion electrospray ionization. This approach was used to assess the extent of sialylation of the recombinant antibody molecules. Sialylation affects clearance of antibody molecules in the blood stream and is thus important to measure. The authors’ use of a time-of-flight mass spectrometer to produce accurate masses provided confident determination of glycan compositions in the CE/MS data sets.
As shown in Figure 2, PNGase F releases N-glycans from such glycoproteins as glycosyl amines (20). The glycosyl amine is rapidly hydrolyzed at slightly acidic pH but is more stable under basic conditions. Glycosyl amines may be derivatized with amine-reactive reagents to stabilize the glycan reducing ends and add a chromophore or fluorophore (21). The advantage to this approach is that the stereochemistry of the reducing end is preserved as a single anomer. Recently, fluorenylmethyloxycarbonyl chloride (Fmoc-Cl) has been used to derivatize N-glycans released using PNGase F (20). The authors analyzed purified glycoproteins including fetuin, α1 acid glycoprotein, immunoglobulin G and transferrin using to validate the method. The CE/MS mass electropherograms were acquired using a bare fused silica capillary, an electrolyte pH of 6.8 and an applied potential of 30 kV. A sheath liquid of water/methanol/formic acid was used with positive ion electrospray MS detection. The data showed FMOC-derivatized N-glycans migrating according to increasing degree of sialylation due to the electroosmotic flow (EOF). This enabled profiling of the N-glycans based on degree of sialylation. The method was applied to the analysis of Fmoc-labeled N-glycans from the therapeutic monoclonal antibody trastuzumab (Herceptin), rituximab (Rituxan) and palivizumab (Synagis) for which 10 μg aliquots were analyzed. The MS dimension provided clear value by facilitating detection of fucosylated N-glycans that are not easily resolved using CE alone. The authors also subjected 10 μg quantities of the glycoproteins to SDS-PAGE followed by PNGase F digestion of the excised bands and Fmoc derivatization. The N-glycans migrated in a diffuse pattern due to the presence of acrylamide polymer in the solution. Nonetheless, it was possible to extract mass spectra from the data showing the presence of the N-glycans from the glycoprotein samples.
Figure 2.
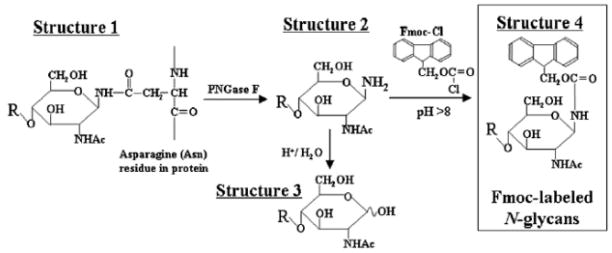
Flowchart for the preparation of Fmoc-labeled N-glycans after the release of N-glycans from protein. The N-glycan (structure 1) linked to the Asn-residue of the core protein/peptide is released with PNGase F as N-glycosylamine (structure 2). Structure 2 is hydrolyzed to yield free N-glycan (structure 3) under acidic conditions. However, at above pH 8, N-glycosylamine (structure 2) can be stabilized, therefore it can be directly reacted the amino groups with Fmoc-Cl. The Fmoc-labeled N-glycan (structure 4) was subjected to analysis by CE-ESI MS. Copyright 2008, Oxford University press, used with permission.
c. CE/MS of glucose ladders
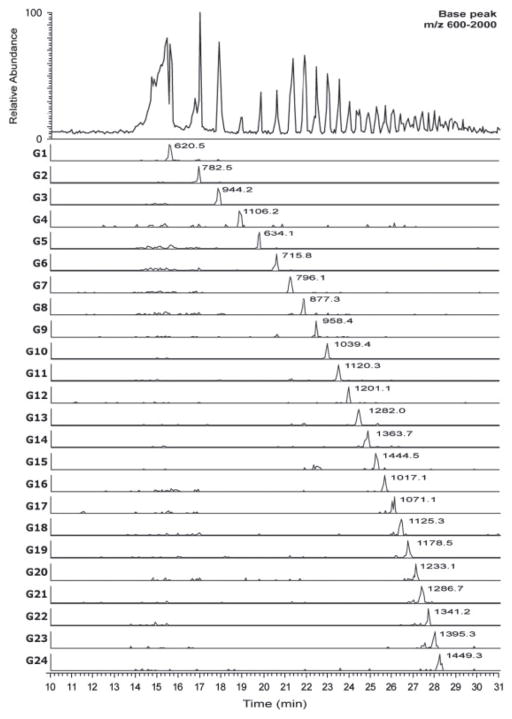
It is desirable to use the lowest flow rate of solution after the CE capillary that suffices to produce a stable electrospray. Given that reductive amination with APTS is a popular means of introducing both fluorophore and charge to glycans, researchers have optimized a junction interface that introduces a makeup flow of 0.3–0.4 μL/min of flow to supply the electrospray source (16). The makeup flow was composed of isopropanol/methanol/formic acid. The electrolyte solution was methanol/water/formic acid and the separation potential was −16 kV. This APTS-glycans were negatively charged in this acidic electrolyte, and migrated against the electroosmotic flow towards the LIF and MS detectors. The makeup flow also backfilled the capillary as electrolyte flowed toward the cathode due to the EOF. As shown in Figure 3, it was possible to analyze a mixture of glucose oligomers containing from 1–24 monosaccharides. Baseline resolution was obtained up to G15 (an oligomer of 15 monosaccharide residues). These results showed the potential for use of such APTS-labeled glucose ladders to calibrate CE/MS data acquired for glycans released from glycoproteins. Such glucose ladders have been used with great success for high performance liquid chromatography-based studies of the human N-glycome (22, 23). Such population-based studies require a rapid, reproducible and stable analytical platform. CE/MS using glucose ladders may enable increased information through the MS dimension to be produced for such studies.
Figure 3.
Base peak and extracted ion electropherograms for APTS labeled glucose ladder standard (G1–G24, referring to the number of glucose monosaccharides in each oligosaccharide) using optimized conditions (BGE 2.0% formic acid, 30% methanol; separation potential −16 kV). Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
d. CE/MS determination of sialic acids
Sialic acid residues cap many non-reducing end termini in glycoconjugate glycans and are present in many glycan epitopes (1). Their presence helps determine the carbohydrate-protein binding interactions that occur among mammalian cells and in host-pathogen interactions. Sialylation strongly influences the biological lifetime and antigenicity of proteins; the asialoglycoprotein receptor recognizes un-sialylated galactose residues and facilitates the removal of asialoglycoproteins from circulation. Humans lack the functional enzyme that converts Neu5Ac to Neu5Gc and in this respect differ from all other mammalian species including the closest primate relatives. As a result, the presence of Neu5Gc in a recombinant protein therapeutic has the potentially to cause undesirable immune reactions in humans (24). Most recombinant glycoproteins are expressed in animal cells and/or using animal derived serum or serum factors. Thus there is some level of incorporation of Neu5Gc into glycoproteins. As a result, there are strict standards on how much Neu5GFc can be present in a lot of a therapeutic glycoprotein. This drives the need for accurate and precise methods for analysis of sialic acids. The use of CE/MS has been demonstrated for this purpose (25). The authors released sialic acids from glycoconjugates using mild acid hydrolysis. Uncoated fused silica capillaries were used with an electrolyte solution of ammonium acetate pH 9.5. The sheath liquid was water/methanol/ammonium acetate and the separation potential was 30 kV. Peaks corresponding to Neu5Ac and Neu5Gc were well separated. The results showed that it was possible to detect Neu5Ac released from human serum in a 9 min CE/MS run.
e. CE/MS of glycopeptides
Analysis of glycopeptides provides information on the glycan composition and the peptide to which glycans are attached. There is an associated challenge, however, because glycopeptide physico-chemical properties reflect those of both the peptide and glycan portions of the molecule. As a result, glycopeptides elute as broad peaks using standard reversed phase columns in proteomics LC/MS workflows. It is therefore not surprising that use of CE/MS for profiling glycopeptides has been investigated in recent years. This application also drives the use of high resolution mass analyzers so as to define the peptide and glycan compositions with confidence.
CE/MS with a time-of-flight mass analyzer has been used to map N- and O-linked glycopeptides from recombinant human erythropoietin (EPO) (26). The investigators used uncoated fused silica capillaries, electrolyte solution of acetic acid/formic acid pH 2.2, separation potential of 18 kV, and a sheath flow of 50% isopropanol modified with formic acid. The mass spectrometer was operated in the positive ion mode. The use of uncoated capillaries eliminates potential problems with bleeding of capillary coatings into the MS source. Glycopeptides may interact with the walls of uncoated capillaries, however, necessitating a washing procedure between each CE/MS sample injection. Glycopeptides were separated based on number of sialic acid groups and it was possible to identify variants in the number of acetyl modifications and the presence of N-glycolylneuraminic acid.
Glycoprotein glycans undergo sulfonylation in the Golgi apparatus mediated by specific sulfotransferases. Such modifications change the physic-chemical properties of the glycan and thereby create new protein binding activities. Examples include the sulfated Lewis glycan epitopes. Other complex glycan sulfated motifs include 4-sulfonylated-LDN and the HNK antigen (1). The sulfonate group increases the acidity of the glycoprotein; at the same time it poses particular analytical challenges. In particular, the sulfonylation may occur to only a small percentage of the population of glycoprotein molecules. Also, the MS ionization of sulfonylated molecules is likely to be suppressed in the positive ion mode. At the same time, ionization in the negative mode is likely to be enhanced by the increased acidity. For CE separations, it is not necessary to add charge to a sulfonylated glycan. Thus, CE methods can be designed to favor the migration of sulfonylated glycans over those of less acidic glycans. Investigators have employed an acidic electrolyte of ammonium formate pH 3.3, a sheath liquid of methanol/water/acetic acid, and an applied potential of −30 kV for this purpose (27). They applied their method to analysis of a proteinase K digest of thyroid stimulating hormone (TSH). This glycoprotein contains several sulfonylated N-glycans including the 4-sulfonylated-LDn type that contains sulfonylated GalNAc in the antennae. The CE/MS data, acquired using negative ion electrospray, showed abundant ions, demonstrating effective enrichment, from sulfonylated glycopeptides. The authors also showed that the glycopeptides could be analyzed using positive ion electrospray MS by including a basic peptide of sequence KKK in the sheath liquid.
f. CE/MS of intact glycoproteins
Glycoproteins exist as a population of molecules that are heterogeneous with respect to extent of glycosylation. It is desirable to analyze glycoproteins intact in order to assess the extent to which such populations are likely to display differences in function based on glycan structure. Such an analysis would require high resolution and mass accuracy in order to define the composition of the observed peaks accurately. It is also important that the method be reproducible so as to enable comparison of recombinant glycoprotein lots.
α1 Acid glycoprotein (AGP) levels are altered in blood plasma during inflammation. Its glycosylation microheterogeneity has been observed to vary according to disease state. As a result, it is a good candidate for development of methods for intact glycoprotein profiling. The 42 kDa protein consists of approximately 45% carbohydrate by mass and is highly heterogeneous (28). For CE/MS analysis, bare fused silica capillaries were coated with acrylamide–pyrrolidinemethacrylate copolymer (29, 30). The coating was repeated every two CE runs. The separation electrolyte was 6-aminocaproic acid/ammonia/methanol, the sheath liquid was isopropanol/water, and the separation potential was 28 kV. Analysis of deglycosylated AGP required use of a different capillary coating method. The CE/MS analysis was performed using a time-of-flight analyzer with moderate-high resolution. The method was used to compare AGP profiles from 16 serum samples. AGP was detected between 6 and 10 min as a broad peak envelope. Plots of the EIEs show an extremely complex series of peaks ranging from m/z 2000–3000. Despite the complexity, it was possible to deconvolute the data and determine the neutral masses of AGP glycol-variants. The data were used to make qualitative comparisons of the AGP molecular weights present among the sample set. A statistical analysis of the data was not presented.
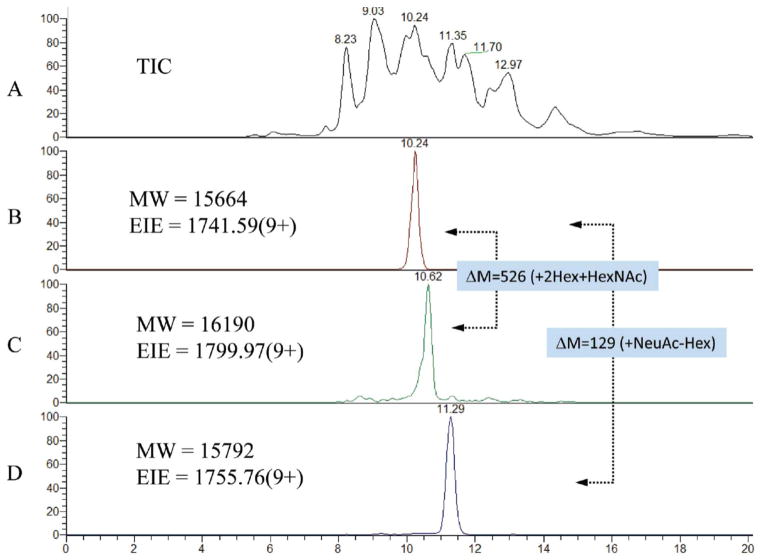
A Fourier transform MS system has been used for analysis of intact recombinant human chorionic gonadotropin (rHCG) (17). For this purpose a polyvinyl alcohol coated capillary was used with an electrolyte of 2% acetic acid (pH 2.5) and a separation potential of 8 kV. A pressurized electrolyte reservoir system was used to provide a post-capillary makeup flow of ~200 nL/min into the MS source. Figure 4 shows the total ion electropherogram for rHCG (A) and extracted ion electropherograms (EIEs) (B–D). The data were acquired using a limited m/z range (1400–2000) to maximize the scan speed of the MS instrument. The EIEs show peak widths of ~12 s, demonstrating impressive ability to resolve glycoforms. The data serve as a means for comparison of ion abundances among different process batches. It was not appropriate to compare the abundances of different rHCG ions to each other due to potential differences in ionization efficiencies. Coefficients of variation for replicate analyses on the same day were less than 10% for the 20 most abundant glycoforms of rHCG. This example demonstrates the potential power for high resolution MS for CE/MS of intact glycoproteins.
Figure 4.
Demonstration of the high resolution separation of intact r-RhCG derived from a murine cell line using CE-MS. (A) Total ion current and (B–D) selected extracted ion electropherograms (EIEs). The dotted arrows indicate mass shifts between pairs of masses. Copyright 2010, American Chemical Society, used with permission.
CE/MS using non-covalently coated capillaries was used to determine changes in recombinant glycoprotein structure caused by a variety of storage conditions (31). The glycoproteins analyzed were recombinant human growth hormone (HGH) and oxytocin. The non-covalently bound capillary coating was Polybrene-poly(vinyl sulfonic acid) or Polybrene-dextransulfate-Polybrene. The coating method, sheath flow composition, electrolyte, and applied potential differed according to the glycoprotein being analyzed. The method was useful for profiling recombinant glycoforms based on the extracted mass spectra. It was also possible to observe oxidations, sulfonate formation and deamidation occurring in heat exposed glycoprotein preparations.
5. Conclusions
Glycans may be analyzed using CE/MS as underivatized or reducing end derivatized forms. The analysis of underivatized glycans has the advantage of simplicity. For such separations, CE separations using forward (positive potential) polarity are often used so that the EOF sweeps all glycans toward the detector. The migration time increases with the number of acidic groups on the glycan. Thus, the most acidic glycans display long migration times and broad peak shapes. N-Glycans released using PNGase F exist as glycosyl amines that may be derivatized using amino reactive reagents. This approach preserves the stereochemistry of the reducing end as a single anomer and provides a chromophore/fluorophore for optical detection. Forward polarity CE allows separation of the derivatized glycans based on number of acidic groups. Sialic acids released from glycoproteins are readily analyzed using forward polarity CE/MS. Reductive amination of glycans using APTS has the advantage that it renders all glycans very acidic; they may therefore be separated using reversed polarity CE (negative applied potential). This enabled separation of glucose oligomers that are likely to be useful for standardizing CE/MS profiles of glycoconjugate glycans.
CE/MS has been used form mapping glycopeptides. For this purpose, the need for high accuracy mass analysis increases. This is because there is need to define the compositions of both the glycan and peptide portions of the molecule. The number of possible compositions for an observed glycopeptide m/z is the multiple of the possible peptide and glycan variants. Thus, the higher the mass accuracy, the greater the confidence in the interpretation of glycopeptide mass spectra. One approach for glycopeptide separation is to use an acidic electrolyte and forward polarity with uncoated capillaries and positive ion MS detection. This approach has been shown for analysis of N-glycopeptides, some of which are sialylated. Use of reversed polarity and acidic electrolyte enriches for sulfonylated glycopeptides, the MS detection of which is facilitated with negative ion electrospray.
Methods for CE/MS of intact glycoproteins have advanced in the period covered by this review. There is a clear need for the highest possible mass spectrometric resolution and mass accuracy so as to assign the glycoprotein composition with the greatest confidence possible. In addition, there is a need to minimize interactions between the glycoprotein analytes and the capillary wall. One approach is to use non-covalently coated capillaries and forward polarity CE. Another approach is to use covalently coated neutral capillaries at acidic pH and forward polarity CE.
With regard to CE/MS interfaces, the majority of articles published in the period of this review used a coaxial sheath to provide 2–5 μL/min flow to the MS source. The use of junction-type interfaces that provide 0.2–0.5 μL/min makeup flow dilute the CE analytes to a lesser degree but is not as commonly reported for glycan or glycoconjugate CE/MS in the published literature.
Acknowledgments
The author’s effort is supported by US National Institute of Health grants P41RR10888 and R01HL098950.
References
- 1.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 2.Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrom Reviews. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- 3.Zaia J. Mass spectrometry and glycomics. OMICS. 2010;14:401–418. doi: 10.1089/omi.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielik AM, Zaia J. Historical overview of glycoanalysis. Methods Mol Biol. 2010;600:9–30. doi: 10.1007/978-1-60761-454-8_2. [DOI] [PubMed] [Google Scholar]
- 5.Zaia J. Mass spectrometry and the emerging field of glycomics. Chem Biol. 2008;15:881–892. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaia J. On-line separations combined with MS for analysis of glycosaminoglycans. Mass Spectrom Rev. 2009;28:254–272. doi: 10.1002/mas.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuhrer M, de Boer AR, Deelder AM. Structural glycomics using hydrophilic interaction chromatography (HILIC) with mass spectrometry. Mass Spectrom Rev. 2009;28:192–206. doi: 10.1002/mas.20195. [DOI] [PubMed] [Google Scholar]
- 8.Mechref Y, Novotny MV. Editorial: Glycomics through hyphenated techniques. Mass Spectrom Rev. 2009;28:191–191. doi: 10.1002/mas.20209. [DOI] [PubMed] [Google Scholar]
- 9.Mechref Y, Novotny MV. Miniaturized separation techniques in glycomic investigations. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 doi: 10.1016/j.jchromb.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 10.Wuhrer M, Deelder AM, Hokke CH. Protein glycosylation analysis by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:124–133. doi: 10.1016/j.jchromb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Volpi N, Maccari F, Linhardt RJ. Capillary electrophoresis of complex natural polysaccharides. Electrophoresis. 2008;29:3095–3106. doi: 10.1002/elps.200800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mechref Y, Novotny MV. Glycomic analysis by capillary electrophoresis-mass spectrometry. Mass Spectrom Rev. 2009;28:207–222. doi: 10.1002/mas.20196. [DOI] [PubMed] [Google Scholar]
- 13.Campa C, Coslovi A, Flamigni A, Rossi M. Overview on advances in capillary electrophoresis-mass spectrometry of carbohydrates: a tabulated review. Electrophoresis. 2006;27:2027–2050. doi: 10.1002/elps.200500960. [DOI] [PubMed] [Google Scholar]
- 14.Amon S, Zamfir AD, Rizzi A. Glycosylation analysis of glycoproteins and proteoglycans using capillary electrophoresis-mass spectrometry strategies. Electrophoresis. 2008;29:2485–2507. doi: 10.1002/elps.200800105. [DOI] [PubMed] [Google Scholar]
- 15.Gennaro LA, Salas-Solano O. On-line CE-LIF-MS technology for the direct characterization of N-linked glycans from therapeutic antibodies. Anal Chem. 2008;80:3838–3845. doi: 10.1021/ac800152h. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell EJ, Ratnayake C, Jayo R, Zhong X, Chen DD. A promising capillary electrophoresis-electrospray ionization-mass spectrometry method for carbohydrate analysis. Electrophoresis. 2011 doi: 10.1002/elps.201100027. [DOI] [PubMed] [Google Scholar]
- 17.Thakur D, Rejtar T, Karger BL, Washburn NJ, Bosques CJ, Gunay NS, Shriver Z, Venkataraman G. Profiling the glycoforms of the intact alpha subunit of recombinant human chorionic gonadotropin by high-resolution capillary electrophoresis-mass spectrometry. Anal Chem. 2009;81:8900–8907. doi: 10.1021/ac901506p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima H, Inagaki M, Tomita T, Watanabe T, Uchida S. Separation and characterization of lipopolysaccharide related compounds by HPLC/post-column fluorescence derivatization (HPLC/FLD) and capillary zone electrophoresis/mass spectrometry (CZE/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1537–1542. doi: 10.1016/j.jchromb.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Anumula KR. Advances in fluorescence derivatization methods for high-performance liquid chromatographic analysis of glycoprotein carbohydrates. Anal Biochem. 2006;350:1–23. doi: 10.1016/j.ab.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Nakano M, Higo D, Arai E, Nakagawa T, Kakehi K, Taniguchi N, Kondo A. Capillary electrophoresis-electrospray ionization mass spectrometry for rapid and sensitive N-glycan analysis of glycoproteins as 9-fluorenylmethyl derivatives. Glycobiology. 2009;19:135–143. doi: 10.1093/glycob/cwn115. [DOI] [PubMed] [Google Scholar]
- 21.Hase S. High-performance liquid chromatography of pyridylaminated saccharides. Methods Enzymol. 1994;230:225–237. doi: 10.1016/0076-6879(94)30015-1. [DOI] [PubMed] [Google Scholar]
- 22.Lauc G, Essafi A, Huffman JE, Hayward C, Knezevic A, Kattla JJ, Polasek O, Gornik O, Vitart V, Abrahams JL, Pucic M, Novokmet M, Redzic I, Campbell S, Wild SH, Borovecki F, Wang W, Kolcic I, Zgaga L, Gyllensten U, Wilson JF, Wright AF, Hastie ND, Campbell H, Rudd PM, Rudan I. Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1alpha as a master regulator of plasma protein fucosylation. PLoS Genet. 2010;6:e1001256. doi: 10.1371/journal.pgen.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, Wright A, Kolcic I, O’Donoghue N, Bones J, Rudd PM, Lauc G. Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res. 2009;8:694–701. doi: 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- 24.Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotech. 2010;28:863–867. doi: 10.1038/nbt.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortner K, Buchberger W. Determination of sialic acids released from glycoproteins using capillary zone electrophoresis/electrospray ionization mass spectrometry. Electrophoresis. 2008;29:2233–2237. doi: 10.1002/elps.200700801. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez E, Ramos-Hernan R, Benavente F, Barbosa J, Sanz-Nebot V. Capillary electrophoresis time-of-flight mass spectrometry for a confident elucidation of a glycopeptide map of recombinant human erythropoietin. Rapid Commun Mass Spectrom. 2011;25:2307–2316. doi: 10.1002/rcm.5114. [DOI] [PubMed] [Google Scholar]
- 27.Imami K, Ishihama Y, Terabe S. On-line selective enrichment and ion-pair reaction for structural determination of sulfated glycopeptides by capillary electrophoresis-mass spectrometry. J Chromatogr A. 2008;1194:237–242. doi: 10.1016/j.chroma.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochimica et biophysica acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 29.Ongay S, Neususs C. Isoform differentiation of intact AGP from human serum by capillary electrophoresis-mass spectrometry. Anal Bioanal Chem. 2010;398:845–855. doi: 10.1007/s00216-010-3948-5. [DOI] [PubMed] [Google Scholar]
- 30.Neususs C, Pelzing M. Capillary zone electrophoresis-mass spectrometry for the characterization of isoforms of intact glycoproteins. Methods Mol Biol. 2009;492:201–213. doi: 10.1007/978-1-59745-493-3_11. [DOI] [PubMed] [Google Scholar]
- 31.Haselberg R, Brinks V, Hawe A, de Jong GJ, Somsen GW. Capillary electrophoresis-mass spectrometry using noncovalently coated capillaries for the analysis of biopharmaceuticals. Anal Bioanal Chem. 2011;400:295–303. doi: 10.1007/s00216-011-4738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]