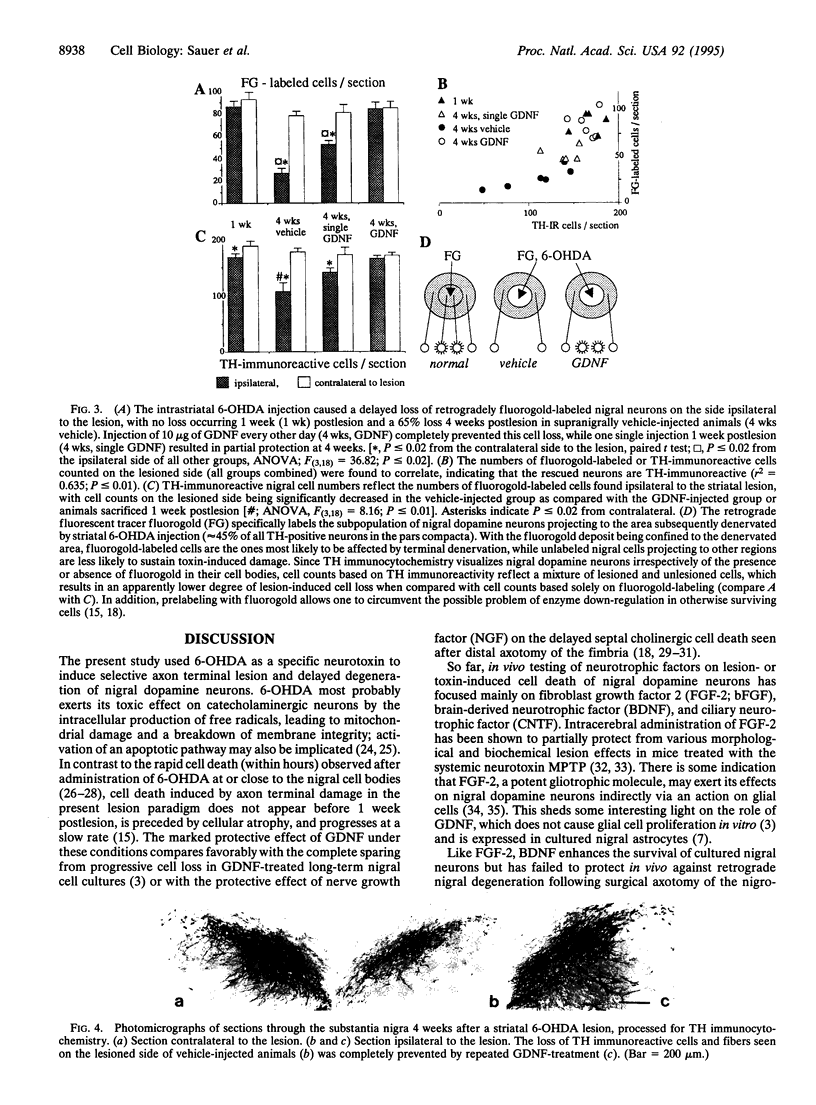
Abstract
Glial cell line-derived neurotrophic factor (GDNF) and transforming growth factor beta 3 (TGF-beta 3) are members of the TGF-beta superfamily with high neurotrophic activity on cultured nigral dopamine neurons. We investigated the effects of intracerebral administration of GDNF and TGF-beta 3 on the delayed cell death of the dopamine neurons in the rat substantia nigra following 6-hydroxydopamine lesions of dopaminergic terminals in the striatum. Fluorescent retrograde tracer injections and tyrosine hydroxylase immunocytochemistry demonstrated nigral degeneration with an onset 1 week after lesion, leading to extensive death of nigral neurons 4 weeks postlesion. Administration of recombinant human GDNF for 4 weeks over the substantia nigra at a cumulative dose of 140 micrograms, starting on the day of lesion, completely prevented nigral cell death and atrophy, while a single injection of 10 micrograms 1 week postlesion had a partially protective effect. Continuous administration of TGF-beta 3, starting on the day of lesion surgery, did not affect nigral cell death or atrophy. These findings support the notion that GDNF, but not TGF-beta 3, is a potent neurotrophic factor for nigral dopamine neurons in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck K. D., Valverde J., Alexi T., Poulsen K., Moffat B., Vandlen R. A., Rosenthal A., Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995 Jan 26;373(6512):339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Brecknell J. E., Dunnett S. B., Fawcett J. W. A quantitative study of cell death in the substantia nigra following a mechanical lesion of the medial forebrain bundle. Neuroscience. 1995 Jan;64(1):219–227. doi: 10.1016/0306-4522(94)00370-k. [DOI] [PubMed] [Google Scholar]
- Chadi G., Møller A., Rosén L., Janson A. M., Agnati L. A., Goldstein M., Ogren S. O., Pettersson R. F., Fuxe K. Protective actions of human recombinant basic fibroblast growth factor on MPTP-lesioned nigrostriatal dopamine neurons after intraventricular infusion. Exp Brain Res. 1993;97(1):145–158. doi: 10.1007/BF00228825. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg D. L., Bohn M. C. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995 Mar 16;85(1):80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Crews L. L., Wigston D. J. The dependence of motoneurons on their target muscle during postnatal development of the mouse. J Neurosci. 1990 May;10(5):1643–1653. doi: 10.1523/JNEUROSCI.10-05-01643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Björklund A. Loss of AChE- and NGFr-labeling precedes neuronal death of axotomized septal-diagonal band neurons: reversal by intraventricular NGF infusion. Exp Neurol. 1991 Aug;113(2):93–108. doi: 10.1016/0014-4886(91)90167-b. [DOI] [PubMed] [Google Scholar]
- Hagg T., Fass-Holmes B., Vahlsing H. L., Manthorpe M., Conner J. M., Varon S. Nerve growth factor (NGF) reverses axotomy-induced decreases in choline acetyltransferase, NGF receptor and size of medial septum cholinergic neurons. Brain Res. 1989 Dec 25;505(1):29–38. doi: 10.1016/0006-8993(89)90112-1. [DOI] [PubMed] [Google Scholar]
- Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. E., Phillips H. S., Pollock R. A., Davies A. M., Lemeulle C., Armanini M., Simmons L., Moffet B., Vandlen R. A., Simpson LC corrected to Simmons L. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994 Nov 11;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ungerstedt U. Specificity of 6-hydroxydopamine induced degeneration of central monoamine neurones: an electron and fluorescence microscopic study with special reference to intracerebral injection on the nigro-striatal dopamine system. Brain Res. 1973 Oct 12;60(2):269–297. doi: 10.1016/0006-8993(73)90791-9. [DOI] [PubMed] [Google Scholar]
- Ichitani Y., Okamura H., Matsumoto Y., Nagatsu I., Ibata Y. Degeneration of the nigral dopamine neurons after 6-hydroxydopamine injection into the rat striatum. Brain Res. 1991 May 24;549(2):350–353. doi: 10.1016/0006-8993(91)90481-a. [DOI] [PubMed] [Google Scholar]
- Ichitani Y., Okamura H., Nakahara D., Nagatsu I., Ibata Y. Biochemical and immunocytochemical changes induced by intrastriatal 6-hydroxydopamine injection in the rat nigrostriatal dopamine neuron system: evidence for cell death in the substantia nigra. Exp Neurol. 1994 Dec;130(2):269–278. doi: 10.1006/exnr.1994.1205. [DOI] [PubMed] [Google Scholar]
- Javoy F., Sotelo C., Herbet A., Agid Y. Specificity of dopaminergic neuronal degeneration induced by intracerebral injection of 6-hydroxydopamine in the nigrostriatal dopamine system. Brain Res. 1976 Feb 6;102(2):201–215. doi: 10.1016/0006-8993(76)90877-5. [DOI] [PubMed] [Google Scholar]
- Kearns C. M., Gash D. M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995 Feb 20;672(1-2):104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Knusel B., Michel P. P., Schwaber J. S., Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990 Feb;10(2):558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B., Beck K. D., Winslow J. W., Rosenthal A., Burton L. E., Widmer H. R., Nikolics K., Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992 Nov;12(11):4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K., Suter-Crazzolara C., Fischer W. H., Unsicker K. TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995 Feb 15;14(4):736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K., Unsicker K. Transforming growth factor-beta promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience. 1994 Dec;63(4):1189–1196. doi: 10.1016/0306-4522(94)90583-5. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- McBride R. L., Feringa E. R., Smith B. E. The fate of prelabeled Clarke's column neurons after axotomy. Exp Neurol. 1988 Nov;102(2):236–243. doi: 10.1016/0014-4886(88)90099-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L. J., Johnson J. E., Lin L. F., Li L., Lo A. C., Newsome A. L., Prevette D. M., Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995 Jan 26;373(6512):344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Otto D., Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990 Jun;10(6):1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T. H., Mytilineou C. Protection from 1-methyl-4-phenylpyridinium (MPP+) toxicity and stimulation of regrowth of MPP(+)-damaged dopaminergic fibers by treatment of mesencephalic cultures with EGF and basic FGF. Brain Res. 1992 Dec 18;599(1):83–97. doi: 10.1016/0006-8993(92)90855-4. [DOI] [PubMed] [Google Scholar]
- Poulsen K. T., Armanini M. P., Klein R. D., Hynes M. A., Phillips H. S., Rosenthal A. TGF beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neurons. Neuron. 1994 Nov;13(5):1245–1252. doi: 10.1016/0896-6273(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Rinaman L., Milligan C. E., Levitt P. Persistence of fluoro-gold following degeneration of labeled motoneurons is due to phagocytosis by microglia and macrophages. Neuroscience. 1991;44(3):765–776. doi: 10.1016/0306-4522(91)90096-7. [DOI] [PubMed] [Google Scholar]
- Sauer H., Oertel W. H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994 Mar;59(2):401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schaar D. G., Sieber B. A., Dreyfus C. F., Black I. B. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993 Dec;124(2):368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Javoy F., Agid Y., Glowinski J. Injection of 6-hydroxydopamine in the substantia nigra of the rat. I. Morphological study. Brain Res. 1973 Aug 30;58(2):269–290. doi: 10.1016/0006-8993(73)90001-2. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Björklund L., Johansson M., Tomac A., Collins F., Olson L., Hoffer B., Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993 Dec;124(2):401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Thanos Solon. The Relationship of Microglial Cells to Dying Neurons During Natural Neuronal Cell Death and Axotomy-induced Degeneration of the Rat Retina. Eur J Neurosci. 1991;3(12):1189–1207. doi: 10.1111/j.1460-9568.1991.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Thompson L. Fetal transplants show promise. Science. 1992 Aug 14;257(5072):868–870. doi: 10.1126/science.1502548. [DOI] [PubMed] [Google Scholar]
- Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hoffer B. J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995 Jan 26;373(6512):335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Walkinshaw G., Waters C. M. Neurotoxin-induced cell death in neuronal PC12 cells is mediated by induction of apoptosis. Neuroscience. 1994 Dec;63(4):975–987. doi: 10.1016/0306-4522(94)90566-5. [DOI] [PubMed] [Google Scholar]
- Weiss R. Promising protein for Parkinson's. Science. 1993 May 21;260(5111):1072–1073. doi: 10.1126/science.8493547. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Matheson C., Lopez O. T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995 Jan 26;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]




