Abstract
Adjudin, also known as AF-2364 and an analog of lonidamine (LND), is a male contraceptive acting through the induction of premature sperm depletion from the seminiferous epithelium when orally administered to adult rats, rabbits or dogs. It is also known that LND can target mitochondria and block energy metabolism in tumor cells. However, whether Adjudin exhibits any anti-cancer activity remains to be elucidated. Herein we described the anti-proliferative activity of Adjudin on cancer cells in vitro and on lung and prostate tumors inoculated in nude mice. We found that Adjudin induced apoptosis in cancer cells through a Caspase-3-dependent pathway. Further experiments revealed that Adjudin could trigger mitochondrial dysfunction in cancer cells, apparently affecting the mitochondrial mass, inducing the loss of mitochondrial membrane potential and reducing cellular ATP levels. Intraperitoneal administration of Adjudin to tumor-bearing athymic nude mice also significantly suppressed the lung and prostate tumor growth. When used in combination with cisplatin, Adjudin enhances the sensitivity to cisplatin-induced cancer cell cytotoxicity. Taken together, these findings have demonstrated that Adjudin may be a potential drug for cancer therapy.
Keywords: Adjudin, Anti-cancer, Mitochondrial dysfunction
1. Introduction
Cancer is among the top three leading risks for mortality. For men, lung cancer is the most commonly diagnosed type and the leading cause of cancer death globally, whereas in the category of newly diagnosed cases prostate cancer ranks first in developed countries, and second worldwide [1]. Surgery, radiation therapy, and chemotherapies with various anti-cancer agents are still the most widely adopted methods for cancer treatment [2]. Despite the improvement in diagnostic screening and the discovery of new anti-cancer drugs, cancer patients’ mortality rate has remained more or less unchanged [1]. There remains a large gap between effective treatment of cancer and the currently available anticancer drugs; moreover, the clinically used chemotherapeutics have many side effects [3]. Researchers are ceaselessly in search for new drugs with more effective and targeted properties. One strategy is to examine the existing drugs that have not been evaluated as potential members in tumor therapeutics. This approach indeed has aroused greater interest than in the past [4]. For instance, Chloroquine, the effective anti-malarial and antirheumatoid drug, has emerged to be used in the clinical treatment of glioblastoma [5,6]. Moreover, using old drugs has the additional benefits of reducing the time for clinical trials.
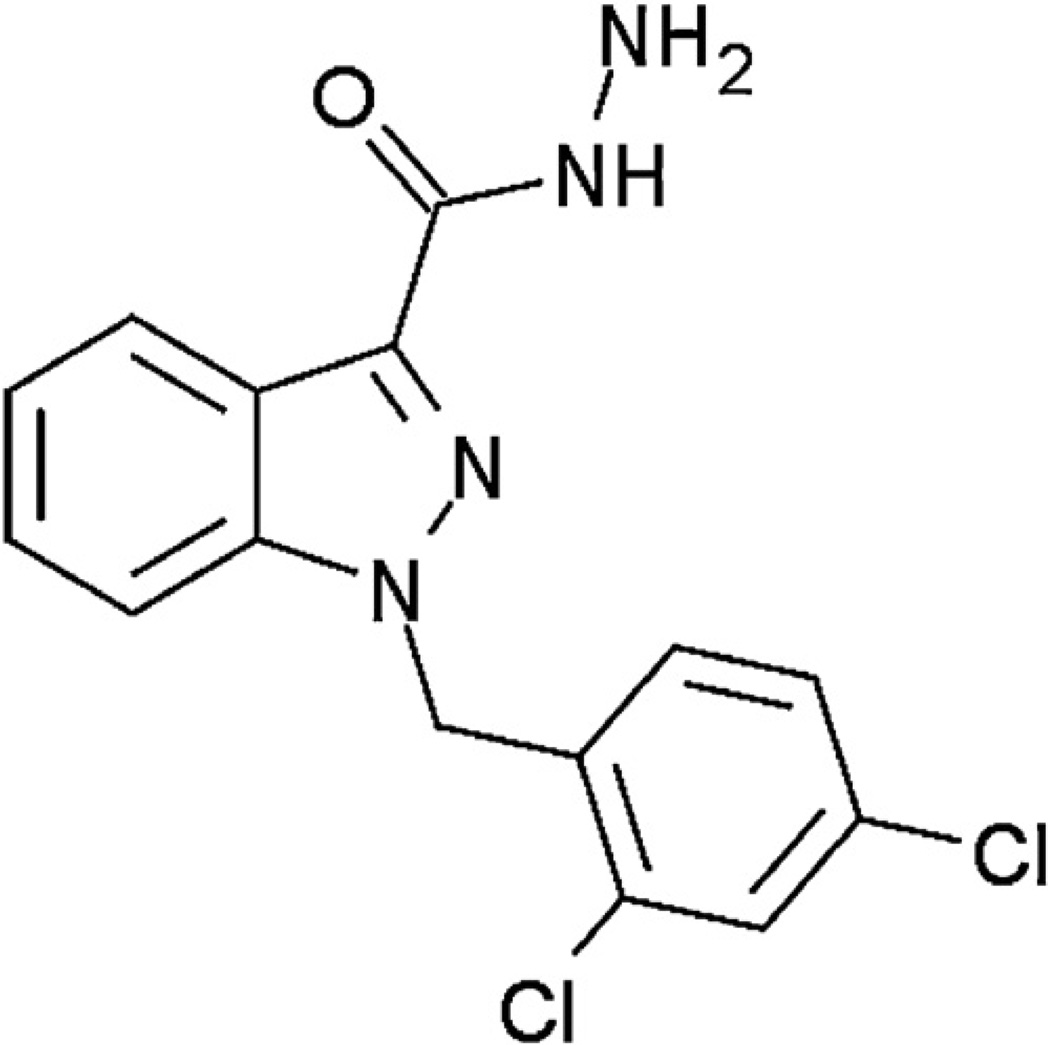
Adjudin, formerly known as AF-2364, is a molecule that mediates adherens junction disruption at the Sertoli-germ cell interface and consequently developed as a potential non-hormonal male contraceptive [7–11] (Fig. 1). As an analog of lonidamine (LND, an indazole-carboxylic acid), Adjudin was designed to show more potent anti-spermatogenic activity with less toxicity and without affecting testosterone production. LND was initially used as an anti-spermatogenic agent, but its anti-tumor properties were also soon recognized [12,13]. LND was characterized as a mitochondria-targeting drug that inhibited mitochondria-bound hexokinase, causing cancer cell apoptosis [14,15]. The clinical value of LND was clearly proved in phase II and III trials against a variety of solid tumors; however its major limitation was the poor clinical efficacy when used alone [16].
Fig. 1.
The chemical structure of 1-(2,4-dichlorobenzyl)-1H–indazole-3-carbohydrazide (Adjudin).
The aim of this study is to investigate whether Adjudin, like its analog LND, also possesses anti-cancer properties. We tested its anti-proliferative activity against a panel of cancer cell lines and in vivo lung and prostate tumor models on athymic nude mice. This study will be the first report to reveal Adjudin’s anti-cancer properties, and consequently, unveil its potential clinical utility as a chemotherapeutic.
2. Materials and methods
2.1. Antibodies and reagents
The rabbit polyclonal antibody against cleaved Caspase-3 (#9664, 1:1000) and Cox IV (#4844, 1:1000) were purchased from Cell Signaling Technology (Danvers, MA, USA), the goat polyclonal antibody against β-actin (sc-1616, 1:1000) and Lamin A/C (sc-6215, 1:1000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), the rabbit polyclonal antibody against Bcl-2 (#1017-1, 1:500), Caspase-9 (#1023-1, 1:1000) and cytochrome C (#2119-1, 1:1000) were purchased from Epitomics Inc. (Burlingame, CA, USA) and the mouse polyclonal antibody against α-tubulin (T5168, 1:2000) was purchased from Sigma– Aldrich Inc. (St. Louis, MO, USA). The anti-rabbit, anti-goat and anti-mouse secondary antibodies were purchased from HuaAn Biotechnology (Hangzhou, China). DMSO used for dissolving Adjudin, Ac-DEVD-CHO(Caspase-3 inhibitor), Rotenone and Cisplatin were obtained from Sigma–Aldrich (St. Louis, MO, USA). Adjudin and lonidamine (LND) were synthesized at S.B.M. Srl (Rome, Italy) with a purity of >98% as described earlier [8,9]. All other non-mentioned reagents were obtained from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Cell culture
The human lung adenocarcinoma cell line A549, the human prostate cancer cell line PC3, the human lung fibroblast cells WI-38, the human benign prostatic hyperplasia epithelial cells BPH-1, the rat pheochromocytoma cells PC-12 and other cancer cell lines were all purchased from Cell Resources Center of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. The human endothelia progenitor cells (EPC) were isolated as described before [17]. A549 cells, WI-38 cells, BPH-1 cells, PC-12 cells and other cell lines were grown in Dulbecco’s Modified Eagle medium and PC3 cells in RPMI 1640 (Hyclone, Logan, UT, USA), all supplemented with 10% fetal bovine serum (PAA, Linz, Austria), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA). The EPC were grown in the endothelial cell growth medium (Lonza, Allendale, NJ, USA). These cell cultures were incubated in a CO2 incubator at 37 8C in a humidified atmosphere with 95% air/5% CO2. The use of the EPC was approved by the Ethical Committee of Shanghai Jiao Tong University.
2.3. Western blot
The Western blot analysis for Caspase-3, Caspase-9, cytochrome C, AIF and Bcl-2 was performed as previously described [18]. The mitochondrial, the nuclear and the cytosolic extracts were isolated from A549 cells using the mitochondrial and nuclear isolation kits (Thermo Scientific, Rockford, IL, USA) according to manufacturer’s instructions. The protein concentration was measured using the BCA assay kit (Thermo Scientific, Rockford, IL, USA). The extracts containing 25–50 µg of total protein were separated by 10–15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were electrotransferred onto nitrocellulose membranes using a semi-dry electrotransfer unit (Trans-Blot SD semi-dry transfer cell, BioRad, Hercules, CA, USA) according to the instrument protocol. The membrane was then blocked with TBST containing 5% skim milk, and hybridized with the corresponding primary antibody in TBST with gentle agitation overnight at 4 °C. The membrane was washed with TBST and hybridized with HRP-conjugated secondary antibody according to the primary antibody. After washing with TBST, protein bands specific for the antibody were visualized by enhanced chemiluminescence (ECL) (Thermo Scientific, Rockford, IL, USA) and images were captured using ChemiDoc XRS (BioRad, Hercules, CA, USA). The intensities of the bands were quantified using Gel-Pro Analyzer (Version 4.0) (Media Cybernetics, Silver Spring, MD, USA).
2.4. In vitro tumor cell growth assays
The 50% inhibitory concentration (IC50) of the tumor cell lines were determined using a modified MTT assay kit. IC50 was identified as a concentration of drug required to achieve a 50% growth inhibition relative to untreated controls. In brief, cells were seeded in 96-well plates at the density of 0.5 × 104/well in the complete growth medium and incubated for 24 h. Then the growth medium was replaced with a serial dilution of Adjudin (300 µM, 100 µM, 30 µM, 10 µM, 3 µM and 0) in growth medium (without serum). The cells were incubated for another 24 h followed by the addition of 10 µl of Cell Counting Kit-8 solution (Dojindo Laboratories, Kumamoto, Japan) to each well. After 4 h of incubation at 37 °C in the cell incubator, the absorbance at 450 nm was measured using a microplate reader (Synergy2, BioTek, Winooski, VT, USA). For each drug concentration group, the experiment involved six wells treated in parallel and was repeated at least three times. The average absorbance readings minus the cell-free baseline were normalized to the control cell group that received no drug treatment (×100%) and the plot was drawn. The concentration of the drug that caused 50% inhibition of cell growth, i.e., 50% reduction in the absorbance at 450 nm, was determined from the graph.
2.5. ATP assay
ATP was quantified using the Roche ATP Bioluminescence Assay Kit (HS II, Indianapolis, IN, USA) following the standard protocol provided by the vendor. In brief, cells were washed once with PBS and lysed with the Cell Lysis Reagent for 20 min. Then the homogenates (50 µl each) were mixed with the Luciferase Reagents (150 µl per sample), and the luminescence was detected using a plate reader (Synergy2, BioTek, Winooski, VT, USA). The protein concentrations of the samples were determined using the BCA assay. The ATP concentration of the sample was calculated using an ATP standard, and normalized against the total protein quantity of the same sample.
2.6. JC-1 staining
Cells that had been treated with Adjudin at varying concentrations for overnight were washed with PBS and collected by trypsinization. The suspended cells were washed with PBS, and added with 10 µl of 200 µM JC-1 (2 µM final concentration Enzo, Farmingdale, NY USA) and incubated at 37 °C, 5% CO2, for 30 min. The suspend cells were pelleted by centrifugation at 2000 × g for 5 min, then resuspended by adding 500 µl PBS. Finally the cells were analyzed on a BD FACSAria II flow cytometer (San Jose, CA, USA) using 488 nm excitation with 530 nm and 585 nm bandpass emission filters.
2.7. Quantitative PCR (Q-PCR)
Total RNA was isolated from A549 cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNAs were synthesized from RNA using superscript first-strand synthesis system (Takara, Japan). Gene expression was quantified using probe-based SYBR Green PCR master mix kits (Takara, Japan), ABI 7900HT sequence detection system, and SDS software (Applied Biosystems, Foster City, CA, USA). A cycle threshold was determined for each gene of interest and normalized according to a housekeeping gene (GAPDH) determined in parallel. The Q-PCR primers used for detecting gene expression of BCL-2 (sense 5′-tgcgacaggagataggctg-3′ and antisense 5′-gccaaaatcacaagggttagctt-3′) were used. GAPDH gene expression was also quantified with primers (sense 5′-gcgacctggaagtccaactac-3′ and antisense 5′-atctgctgcatctgcttgg-3′) to serve as house-keeping gene control.
2.8. Immunofluorescent staining and quantification of mitochondrial morphology
Cells were cultured on coverslips and washed twice with cold PBS, fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature, and blocked for 1 h with 5% normal goat serum in PBS. Then cells were incubated with the specific primary antibody for AIF (apoptosis-inducing factor) in 1% normal goat serum for 1 h, washed, and incubated with secondary antibody (AlexaFluor 488 goat anti-mouse, Molecular Probes, Eugene, OR, USA). Cells were mounted with VectaShield Mounting media with DAPI nuclear stain (Vector Labs, Burlin-game, CA, USA). Fluorescence images were taken using a Leica SP5II Confocal Microscope (Leica Microsystem, Germany). To quantify the parameters of mitochondrial morphology, cells were stained with MitoTracker Red (200 nM, Molecular Probes, Eugene, OR, USA), then fixed, permeabilized and blocked as described above. And the images taken by the confocal microscope (30–50 cells per condition in each experiment and results were pooled from three independent experiments) were analyzed by employing a custom macro as described previously in NIH ImageJ software (version 1.45) [19]. The mean area/ perimeter ratio was employed as an index of mitochondrial interconnectivity and the inverse circularity used as a measure of mitochondrial elongation.
2.9. Apoptosis assays
Apoptosis was evaluated using the Annexin V/7-AAD Apoptosis Detection Kit (Southern Biotechnology, Birmingham, AL, USA) according to the manufacturer’s instructions. Briefly, cells were first resuspended in the binding buffer. Annexin V and 7-AAD were then added to the buffer and incubated at room temperature for 15 min in the dark, followed by flow cytometry.
2.10. Determination of mitochondrial mass
The fluorescent dye MitoTracker® mitochondrion-selective probes (Molecular Probes, Eugene, OR, USA) were used to detect the mitochondrial mass. In brief, when the cells reached 80% of confluency, prewarmed staining solution containing 200 nM MitoTracker probe was added to the cell culture. After incubation at 37 °C for 45 min, the staining solution was replaced with PBS and immediately transferred to a tube on ice for analysis by flow cytometry.
2.11. Colony-formation assay
A549 cells and PC3 cells were cultured for 2 weeks on the 35 µM plates, of which the top agar layer was made by mixing 5 × 103 A549 or PC3 cells with an equal volume of solution that contains 0.7% Agar, 2 × DMEM or 2 × 1640 medium, 20% FBS, 2% penicillin–streptomycin, and the different concentrations of Adjudin. The base agar was made by mixing 1% agar and an equal volume of 2 × DMEM or 2 × 1640 medium with 20% FBS and 2% penicillin–streptomycin. Colonies were stained with 0.05% crystal violet for 1 h and the colonies that contained >50 cells were counted.
2.12. Subdermal tumor inoculation model
The male BALB/C nude mice (Silaike, Shanghai, China) weighing ~20g were equally implanted with A549 cells (0.5 × 107 cells) containing 3 mg/ml of matrigel (BD Biosciences, San Jose, CA, USA) and PC3 cells (1 × 106 cells) hypodermically. After 2 weeks, the mice with palpable tumors were divided into two groups (n = 4 per group in each experiment and repeated with a total of three experiments): i.p. injection of Adjudin which was dissolved in corn oil from a DMSO stock solution with final administered quantity at 100 mg/kg (~300 µM used in vitro); the equivalent vehicle control group were administered with the same amount of corn oil and DMSO via i.p. injection. Adjudin or vehicles were administered every three day in A549 and every other day in PC3 up to 2 weeks. Tumor volumes were determined and calculated using the formula 1/2 × LW2, where W is the smaller dimension. The use of the animals was approved by Shanghai Jiao Tong University Animal Care and Use Committee.
2.13. Statistical analysis
Each experiment was repeated at least three times. Results of a treated sample group were compared with the corresponding control group by ANOVA to be followed by Tukey’s Honest Significant Test using the GraphPad InStat (Version 3.05) (GraphPad Software Inc, La Jolla, CA, USA).
3. Results
3.1. Adjudin exhibited anti-cancer activity against a variety of cancer cell lines
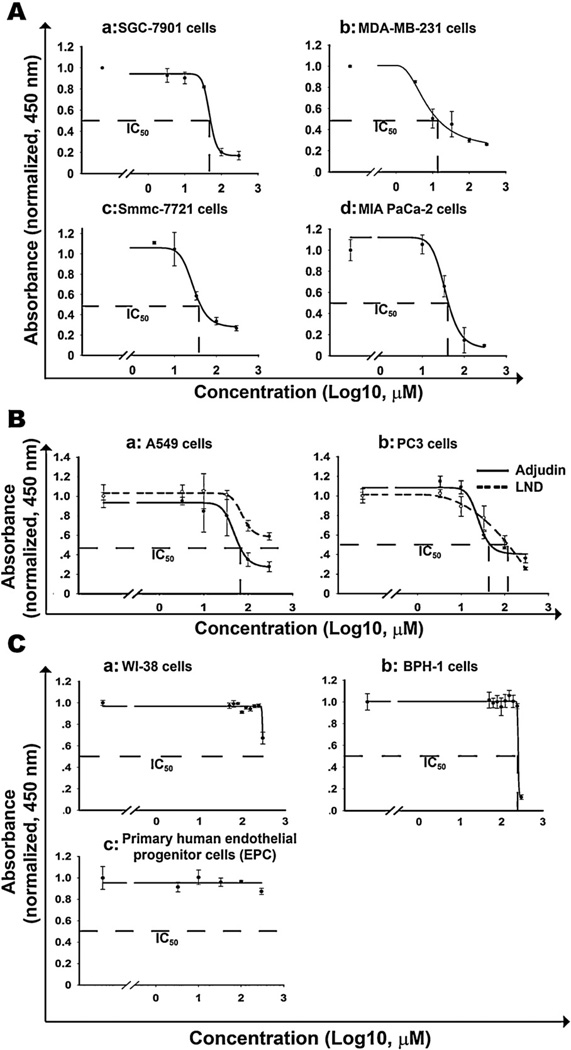
To investigate the effect of Adjudin on cancer cells, we treated more than ten different types of human or mice cancer cell lines with increasing concentrations of Adjudin and the cell proliferation was measured by the modified MTT assay. As a result, Adjudin inhibited cell proliferation in a dose dependent manner in SGC-7901 (human gastric adenocarcinoma cell), MDA-MB-231 (human breast adenocarcinoma cell), Smmc-7721 (human hepatoma cell) and MIA Paca-2 (human pancreatic adenocarcinoma cell) cells (Fig. 2A). The IC50 of Adjudin was determined to be 58.0 µM, 13.8 µM, 72.3 µM and 52.7 µM against SGC-7901, MDA-MB-231, Smmc-7721 and MIA Paca-2 cells, respectively, after treatment for 24 h. Similar results were obtained in other human and mice cancer cell lines (Table 1). A549 and PC3 cells, respectively, were routinely used cell lines for in vitro or in vivo lung and prostate cancer models in our lab. We chose these two cell lines for further analysis, even though these cells were not the most sensitive to Adjudin (Table 1). The results obtained in these two cell lines should at least be indicative of Adjudin’s chemotherapic function for other cancer cell types. To further study the anti-cancer effect of Adjudin compared with LND, the cell growth analysis was used to determine the value of IC50 after treatment of Adjudin and LND for 24 h. In both cell lines Adjudin was shown to be a more potent drug to reduce cell proliferation than LND. The IC50 of Adjudin in A549 cells and PC3 cells was 63.1 µM and 93.0 µM; however the value of LND was more than 200 µM and 120 µM respectively (Fig. 2B). We also tested the proliferation of human non-cancerous cell lines: lung fibroblast cells WI-38 and benign prostatic hyperplasia epithelial cells BPH-1 after treatment of Adjudin at various concentrations for 24 h. For WI-38 and BPH-1 cells, the IC50 of Adjudin could be observed at more than 300 µM and 200 µM, respectively, which is about 5 times and 2 times more than that for the cancer cell lines A549 and PC3 (Fig. 2C). We also tested the proliferation of primary human endothelia progenitor cells (EPC) after treatment of Adjudin at various concentrations for 24 h. The EPC growth was almost not affected by treatment of 300 µM Adjudin (Fig. 2C). Previous work on rat primary Sertoli cells [20] and current work on an organ culture model (data not shown) also showed Adjudin posed minimal toxicity to these normal cells. These results seemed to suggest a therapeutic window for Adjudin in cancer treatment.
Fig. 2.
IC50 analyses of Adjudin in different cancer cells, normal cells and comparison with its analog lonidamine (LND). The modified MTT assays were performed to test the viability of a panel of cancer cells that were incubated with increasing concentrations of Adjudin for 24 has described in Section 2. The Absorbance at 450 nm normalized to the level of control (indicative of the number of viable cells) were plotted against the Adjudin concentrations (in log 10, µM). The curve was obtained in SigmaPlot using 4-parameter logistic regression model. The 50% inhibitory concentration (IC50) was identified as a concentration of drug required to achieve a 50% growth inhibition. Similarly the IC50 of LND in the representative cancer cells were analyzed and compared with Adjudin in parallel. (A) IC50 analysis of human gastric adenocarcinoma cells SGC-7901 (a), human breast adenocarcinoma cells MDA-MB-231 (b), human hepatoma cells Smmc-7721 (c) and human pancreatic adenocarcinoma cells MIAPaCa-2 (d). (B) Comparison of IC50 of Adjudin and its analog lonidamine (LND) in A549 (a) and PC3 (b) cells. (C) IC50 analysis of human lung fibroblast cells WI-38 (a), human benign prostatic hyperplasia epithelial cells BPH-1 (b) and human endothelial progenitor cells (c) All experiments were performed in triplicate. Data are shown as mean ± SEM.
Table 1.
IC50 analyses of Adjudin and LND in a number of cancer cell lines Cells were plated at 5000/well in 96-well plates in replicates of 6, and treated with varying concentrations of Adjudin and LND the following day. After 24h treatment, the cell viability was assayed with a CCK8 kit. The absorbance at 450nm normalized to the level of control (indicative of the number of viable cells) were plotted against logarithmic value of the Adjudin and LND concentration (in log 10 µM) and the experiments were repeated three times at least. IC50 was determined from the graph in which the curve was obtained in SigmalPlot using 4-parameter logistic regression model; ND, not determined.
| Cell | Type | IC50 (µM)±SD |
|
|---|---|---|---|
| Adjudin | LND | ||
| A549 | Human lung adenocarcinoma cell |
63.1 ±1.7 | 205 ± 7.4 |
| H1299 | Human lung adenocarcinoma cell |
29 ± 2.4 | 266 ±4.2 |
| BGC-823 | Human gastric adenocarcinoma cell |
95.5 ±2.2 | ND |
| SGC-7901 | Human gastric adenocarcinoma cell |
58 ±1.4 | 117.2 ±4.8 |
| MKN45 | Human gastric adenocarcinoma cell |
64.6 ±1.6 | ND |
| MIA PaCa-2 | Human pancreatic adenocarcinoma cell |
52.7 ±2.2 | ND |
| MDA-MB-231 | Human breast adenocarcinoma cell |
13.8 ±3.4 | ND |
| U251 | Human glioma cell | 173.8 ±5.3 | ND |
| GL261 | Mouse glioma cell | 58.9 ±2.1 | 230.9 ±10 |
| Smmc-7721 | Human hepatoma cell | 72.3 3.4 | ND |
| HO-8910 | Human ovary adenocarcinoma cell |
55.8 ±1.8 | ND |
| PC-3 | Human prostate cancer cell | 93 ± 4.8 | 125.3 ±5 |
| Du145 | Human prostate cancer cell | 53.5 ±9.5 | >500 |
| L-929 | Mouse fibrosarcoma cell | 16.2 ±1 | ND |
| 4T1 | Mouse mammary tumor cell | 79.4 ± 2.4 | >300 |
3.2. Adjudin inhibited colony formation in A549 cells and PC3 cells
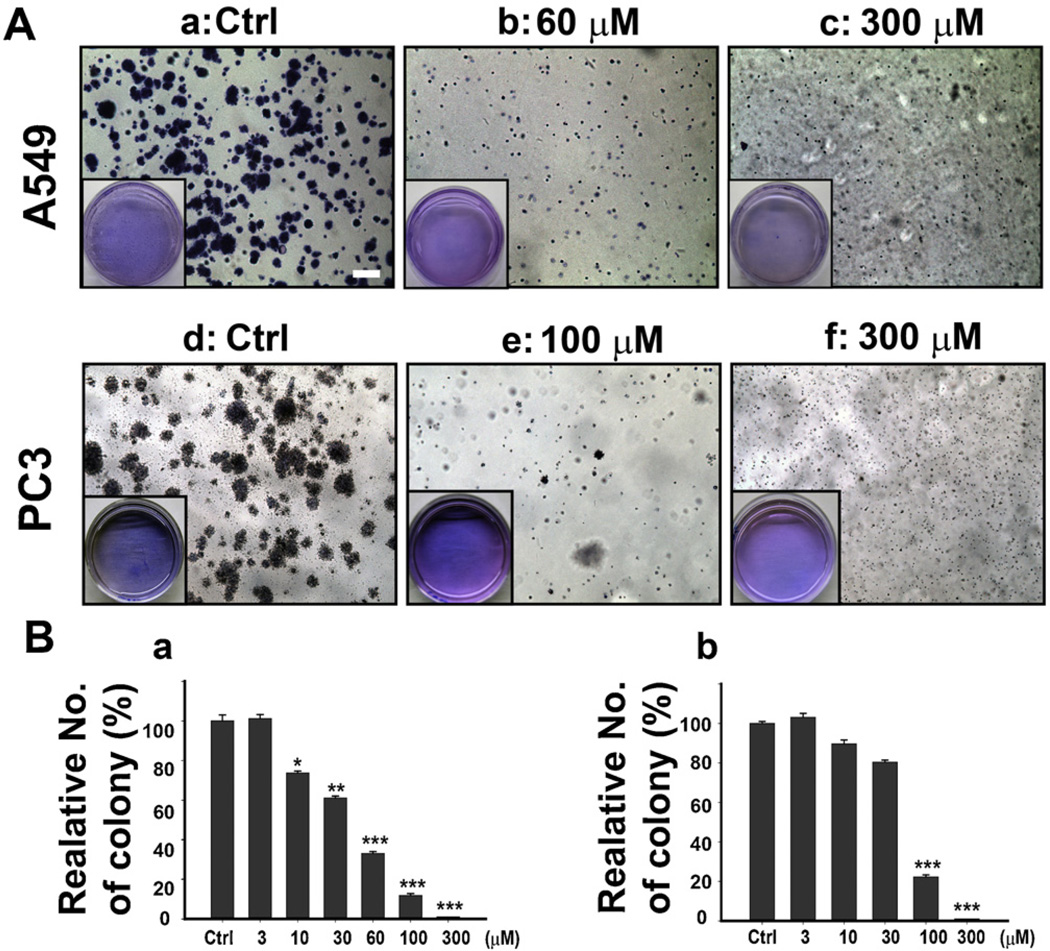
We further investigated the inhibitory effect of Adjudin in A549 cells and PC3 cells by the colony formation assay. The cells treated with increasing concentrations of Adjudin formed fewer and smaller clones than the control group (Fig. 3A). Treatment of both A549 (Fig. 3A–c and B-a) and PC3 cells (Fig. 3A–f and B-b) with 300 µM of Adjudin almost eradicated cancer cells, resulting in zero clone formation. These results indicate that Adjudin plays a role in A549 and PC3 cells’ anchorage-independent growth.
Fig. 3.
Adjudin inhibited colony formation in A549 and PC3 cells. (A) PC3 Cells and A549 cells were treated as indicated in Section 2 and were cultured for 2 weeks, then stained with crystal violet, and photographed with a light microscope. The boxed areas (inset) are the typical view of the dishes taken by a digital camera. The scale bar shown in (a) represents 200 µM. (B) The quantification of colony formation in A549 cells (a) and PC3 cells (b). The numbers of colony were normalized against the control. All experiments were performed in triplicate. Data are shown as mean ± SEM.
*P < 0.05, **P < 0.01 and ***P < 0.001 indicate significant differences from the respective control groups compared by one-way ANOVA to be followed Tukey’s Honest Significant Test.
3.3. Adjudin induced cancer cell apoptosis through the Caspase-3 dependent pathway
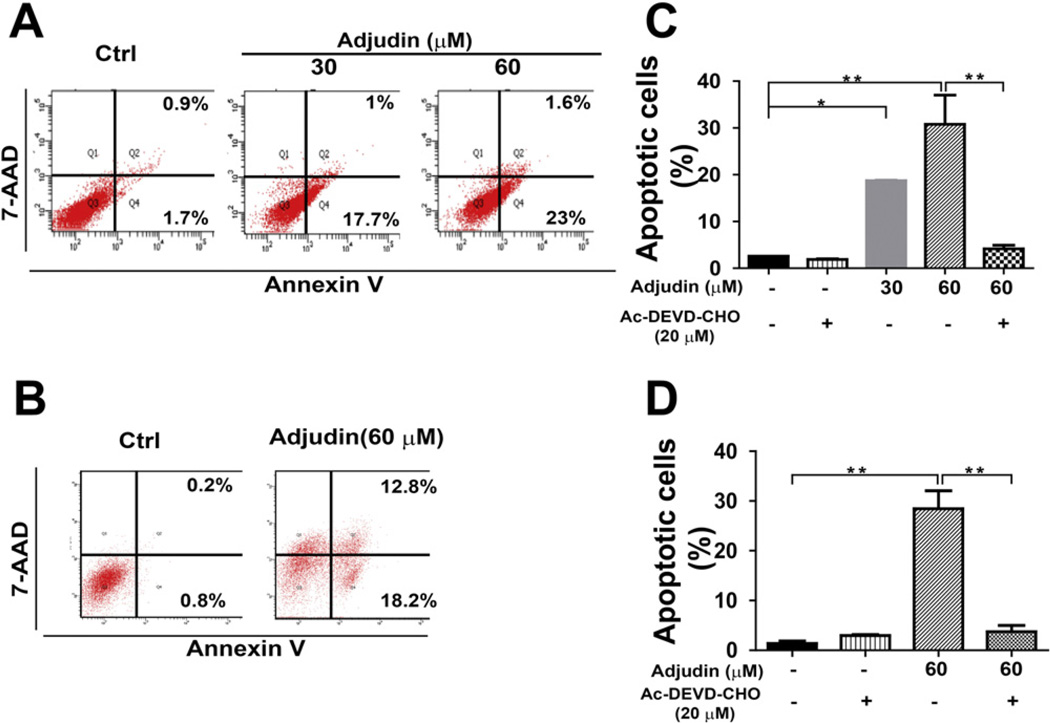
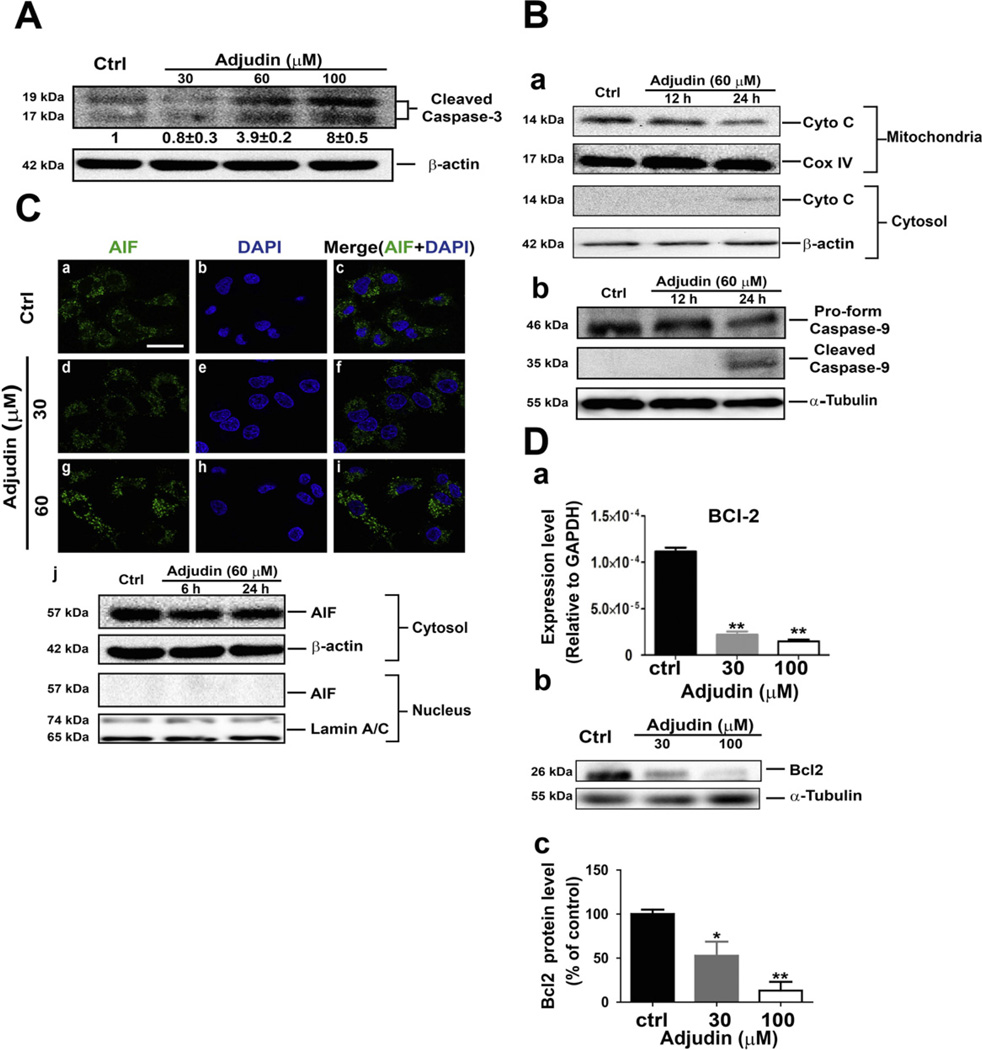
The effects of Adjudin on apoptosis induction were examined using the Annexin V and 7-AAD double staining method analyzed by flow cytometric measurement, which was used to quantify the extent of apoptosis in the total cell population. As shown in Fig. 4A, treatment of A549 cells with different concentrations of Adjudin (24 h treatment) induced the cell apoptosis. When A549 cells were treated with Adjudin at concentrations from 30 µM to 60 µM, the percentages of cells undergoing apoptosis as characterized by Annexin V staining increased from 18.7% to 24.6%. Similarly, treatment of PC3 cells with Adjudin also increased the ratio of apoptotic cells (Fig. 4B) from basal level of 1% for untreated cells to 31% for cells treated with 60 µM of Adjudin. However, apoptosis induced by Adjudin was not detected during the first 12 h treatment in A549 and PC3 cells using the flow cytometric method (Supplemental Fig. 1). It is known that dying cells can activate either one of two main cell death pathways: the Caspase-dependent pathway (in which Caspase-3 is the common downstream execution Caspase) or the Caspase-independent pathway (in which apoptosis-inducing factor, AIF, can be involved) [21]. We then evaluated whether apoptosis cells could be rescued by using the Caspase-3 inhibitor in A549 and PC3 cells. The Caspase-3 inhibitor Ac-DEVD-CHO significantly reduced the ratio of apoptotic cells both in A549 (Fig. 4C) and PC3 cells when they were treated with Adjudin (Fig. 4D). The involvement of Caspase-3 activation after Adjudin treatment (24 h) in A549 cells was also evidenced by the increasing the level of cleaved Caspase-3 in a concentration dependent manner as detected by Western blot (Fig.5A).The immunoblotting assay was also carried out to measure the changes in the level of proteins that regulate the apoptotic pathway, such as the cytochrome C release and the Caspase-9 activation. The cytochrome C released from mitochondria (Fig. 5B–a) and the activated/cleaved Caspase-9 (Fig. 5B–b) were detected after the 24-h treatment with Adjudin. The changes were not clearly detected at 12 h, which suggested that the actual activation occurred between 12 h and 24 h. This is in line with our findings in the flow cytometric measurement (Supplemental Fig. 1). We detected the location of AIF in A549 cells after treating with Adjudin for 24 h. AIF may mediate the Caspase-independent apoptosis and be translocated to the nucleus from the mitochondria upon death triggering [22]. However, when treated with 30 µM and 60 µM Adjudin over 24 h, AIF was not found to shuttle into the nucleus at the tested Adjudin concentrations (Fig. 5C–d and g). Using the same staining protocol, we could readily detect AIF translocation in PC-12 cells treated with Rotenone (positive control, Supplemental Fig. 2) [23]. To further validate this, we isolated the nuclei and cytosol fraction of the cells and measured the protein level of AIF in each fraction by immunoblotting, yet still we failed to detect any nuclear AIF at the early period (6 h) or the late period (24 h) (Fig. 5C–j). On the other hand, anti-apoptotic proteins such as BCL-2 reduced its mRNA expression (Fig. 5D–a) and protein level (Fig. 5D–b) in a concentration-dependent manner after Adjudin treatment for 24 h.
Fig. 4.
Adjudin induced cancer cell apoptosis. Apoptosis triggered by Adjudin treatment for 24 hin A549 cells(A and C) and PC3 cells(B and D)was assayed by flow cytometry of Annexin V and 7-AAD double staining. The quantification of the apoptotic A549 cells (C) and PC3 cells (D) was achieved by averaging three independent flow cytometric analyses. As indicated, the cells were pre-incubated for 2 h with the Caspase-3 inhibitor Ac-DEVD-CHO (20 µM).
*P < 0.05, **P < 0.01 and ***P < 0.001 indicate significant differences from the respective control groups compared by one-way ANOVA to be followed Tukey’s Honest Significant Test.
Fig. 5.
Adjudin induced cancer cell apoptosis through the Caspase-3 dependent pathway. (A) Western blot analysis of cleaved Caspase-3 after treatment with different concentrations of Adjudin for 24 h in A549 cells. The blot was representative of three independent experiments, with the relative intensities of cleaved Caspase-3 (mean ± SEM) listed below the bands. (B) Western blot analysis of cytochrome C(Cyto C) release and the cleaved Caspase-9 after treatment with 60 µM Adjudin for indicated time periods in A549 cells. Cytochrome C that remained in mitochondria was determined using the extracts from mitochondrial fractions, and cytochrome C released to cytosol was determined using protein extracts from cytosolic fractions. The pro-form and cleaved Caspase-9 was assayed using total cellular extract in the same blots. The Cox IV, β-actin and α-tubulin were served as loading controls of the mitochondrial, cytosolic and total protein extracts respectively. (C) A549 cells were treated with 30 and 60 µM Adjudin for 24 h and stained for AIF. Green, AIF; blue, nuclei (DAPI). The scale bar shown in (a) represents 15 µM. (j) The protein levels of AIF in the cytosol and nuclei after treatment with 60 µM Adjudin for the indicated time were determined by Western blot using the extracts from the two isolated fractions of A549 cells. The β-actin and Lamin A/C were served as loading controls of the cytosolic and nuclear protein extracts. (D) The mRNA expression of BCL-2 (a) and its protein level (b) in A549 cells after treatment with Adjudin for 24 h. The quantification of its protein level is presented relative to the α-tubulin and normalized against the control (c). All experiments were performed in triplicate. Data are shown as mean ± SEM.
*P < 0.05 and **P < 0.01 indicate significant differences from the respective control groups compared by one-way ANOVA to be followed Tukey’s Honest Significant Test.
3.4. Adjudin affected mitochondrial function
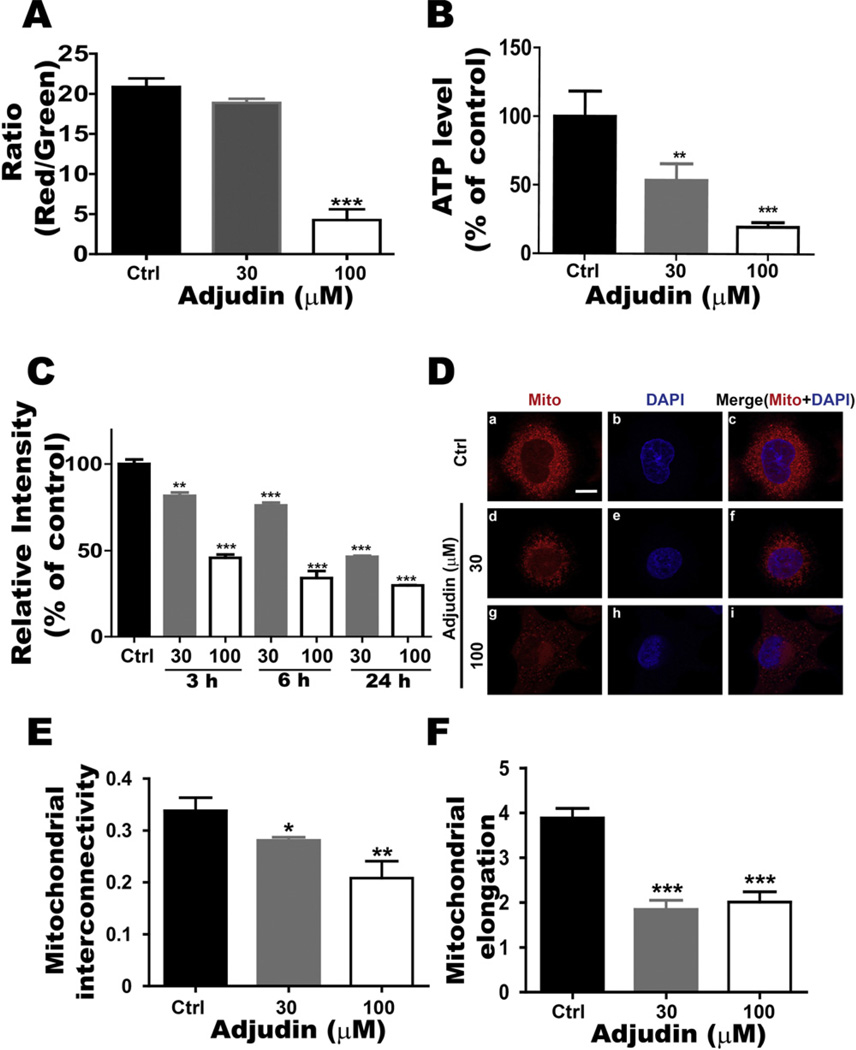
Previous work showed that LND could suppress the cell growth by impacting the mitochondria function [13,24–26]. Mitochondria play a key role in bioenergetics in most eukaryotic cells, which regulate metabolism and mediate acute cell death. Mitochondrial morphologic changes are tightly associated with their function [27,28]. The mitochondrial membrane potential is a key indicator of the cell mitochondrial function, as it reflects the pumping of hydrogen ions across the inner membrane during the process of electron transport and oxidative phosphorylation. Using the lipophilic mitochondrial probe JC-1, we investigated the changes in membrane potential (Δψm) after Adjudin treatment. Treatment of A549 cells with Adjudin decreased the mitochondrial membrane potential from control level of 20% to 18% and 5% for cells treated with 30 µM or 100 µM Adjudin respectively at 24 h (Fig. 6A). The trend toward decreased membrane potential with increasing levels of Adjudin suggested that the effect of Adjudin is associated with the lower membrane potential, the driving force behind ATP production. Subsequently, we assessed the effects of Adjudin on the intracellular ATP level, which is also a key factor mediating cell survival. We found that Adjudin led to a significant decrease in the intracellular ATP level at 24 h (Fig. 6B). Then we investigated whether Adjudin regulated mitochondrial mass (the marker of mitochondrial functions). MitoTracker green staining revealed that mitochondrial mass was reduced in A549 cells after Adjudin treatment in a concentration- and time-dependent manner (Fig. 6C). The change of mitochondrial mass was detected even at the early time points (3–6 h). Morphological analysis using immunofluorescence microscopy revealed that mitochondria, which exhibited normal morphology with distinct punctate structures in untreated A549 cells, became abnormal and diffused in cells treated with increasing concentration of Adjudin (Fig. 6D). The quantification of mitochondrial morphology was performed as described previously [19]. The mitochondrial interconnectivity (the area/perimeter ratio) and the elongation (the inverse circularity) were reduced after Adjudin treatment (Fig. 6E and F).
Fig. 6.
Adjudin affected mitochondrial function. (A) Flow cytometric assays of JC-1 staining were used to measure the mitochondrial membrane potential after 24 h treatment of Adjudin at different concentrations in A549 cells. Functional mitochondria containing J-aggregates (JC-1 aggregates) were stained fluorescent red (FL2 channel), whereas damaged mitochondria containing J-monomers (JC-1 monomers) were stained green (FL1 channel). The ratio of FL2/FL1 was plotted against the Adjudin concentrations. (B) After treatment with Adjudin for 24 h, the ATP levels of A549 cells were detected by the ATP assay as described in Section 2. The ATP concentration of each sample was calculated using an ATP standard, and normalized against the control. (C) A549 cells treated with Adjudin for 3 h, 6 h and 24 h were stained with MitoTracker Green and analyzed by flow cytometry to determine the mitochondrial mass. The MitoTracker Green intensity was plotted against the Adjudin concentrations and normalized against the control. (D) Mitochondrial (Mito) morphology was evaluated by using MitoTracker probe with Adjudin treatment at increasing concentrations for 6 h. Mitochondria and nuclei were stained by MitoTracker probe (red)or DAPI (blue), respectively. The scale bar shown in (a) is 10 µM. (E.F.) Morphometry quantification demonstrating the effects of Adjudin on the mitochondrial interconnectivity (mean area/perimeter ratio, E) and the mitochondrial elongation index (inverse circularity, F) of A549 cells at 6 h. The quantification of mitochondrial morphology was performed employing the custom macro for NIH ImageJ software as described previously (n = 30–50 cells analyzed per condition with at least three independent experiments) [19].
*P < 0.05, **P < 0.01 and ***P < 0.001 indicate significant differences from the respective control groups compared by one-way ANOVA to be followed Tukey’s Honest Significant Test.
3.5. Adjudin reduced lung and prostate carcinoma cells growth in vivo
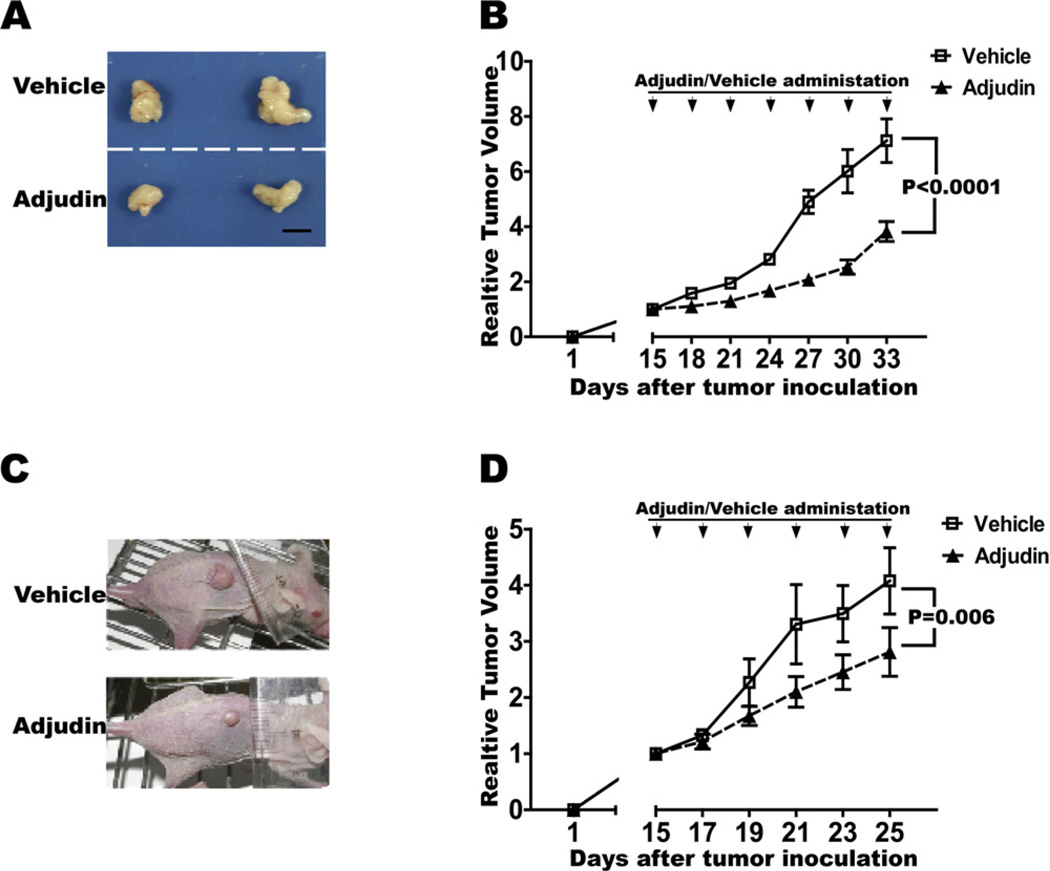
To determine whether Adjudin could inhibit lung and prostate cancer growth in vivo, we tested the effect of Adjudin in a subcutaneous model of lung and prostate cancer. Human lung carcinoma cells A549 (Fig. 7A and B) and prostate carcinoma cells PC3 (Fig. 7C and D) were injected into athymic nude mice subdermally at the lower back site respectively. Mice were then randomized into two treatment groups with similar mean tumor sizes: Adjudin and vehicle (control). Approximately 2 weeks after tumor inoculation Adjudin was injected intraperitoneally once every three days in lung carcinoma cells and every other day in prostate carcinoma cells at 100 mg/kg. As shown in previous studies, Adjudin treatment could be well tolerated in rodents [7,9,20]. In our study, we observed almost no changes on the body weights in the two models (data not shown). And Adjudin-treated mice showed significant tumor growth inhibition compared with the control group (P < 0.0001 in the human lung carcinoma cells A549 and P = 0.006 in the prostate carcinoma cells PC3) (Fig. 7B and D).
Fig. 7.
Adjudin reduced lung and prostate carcinoma cells growth in vivo. A549 cells (A and B) and PC3 cells (C and D) were inoculated subcutaneously in the adult nude mice respectively. After 2 weeks when the tumors reached ~6 µM in diameter, Adjudin (dissolved in DMSO and corn oil) and vehicle (only with DMSO and corn oil) were administered every three days for six times in A549 (n = 12 for each group) and every other day for five times in PC3 (n = 12 for each group). Tumor growth was measured every 2 or 3 days after treatment and calculated using the formula V= 1/2 × LW2, where L and W are the length and width of the tumor mass. The relative tumor volume was obtained by normalization to the initial tumor volume before Adjudin or vehicle treatment. Statistically significant differences on tumor volumes between the Adjudin and vehicle treatment groups (p < 0.0001 in A549, panel B; and ~0.006 in PC3, panel D) are determined by one-way ANOVA to be followed by Tukey’s Honest Significant Test. Representative images of subcutaneously grown tumors in nude mice after treatment of Adjudin or vehicle on Day 33 in A549 (A) and Day 25 in PC3(C) are also shown.
3.6. Adjudin enhances the sensitivity to cisplatin-induced cytotoxicity
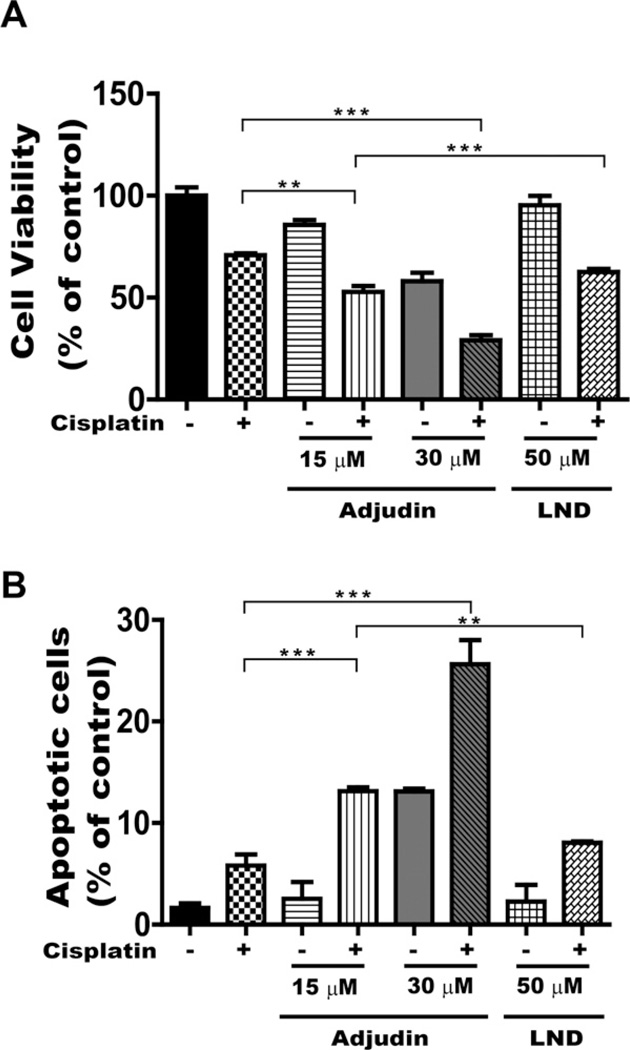
We showed that Adjudin alone is likely an effective anticancer drug both on cancer cell lines and nude mice models. It is possible, however, that Adjudin could better serve as an adjuvant chemotherapeutic, just like its analog LND. We then investigated the therapeutic effect by combining a fixed dose of cisplatin (a common chemotherapeutic for lung cancer) with Adjudin (15 µM and 30 µM) or LND (50 µM, equivalent to 16 µg/ml in plasma concentration in human obtained as described previously [13,29,30]) in A549 cells by cytotoxicity assay (measured by the modified MTT assays). After treatment with different concentrations of Adjudin and 25 µM cisplatin for 24 h, Adjudin enhanced the sensitivity of A549 cells to cisplatin, i.e., the growth inhibition by combination treatment was much more than by Adjudin or cisplatin alone. However, a significant reduction in cell proliferation was not achieved by combining cisplatin with LND in A549 cells (Fig. 8A). Similarly, the effect was also found in PC3 cells for the Adjudin and cisplatin pair (data not shown). As shown in Fig. 8B, apoptosis in A549 cells was greatly increased when Adjudin was combined with cisplatin. These data showed Adjudin enhances the sensitivity to cisplatin-induced cytotoxicity.
Fig. 8.
Combination of Adjudin and cisplatin enhanced the growth inhibition in A549 cells. (A) Inhibition of cancer cell proliferation by Adjudin or LND in combination with cisplatin was assessed using the modified MTT assay. Cells were exposed to the indicated regimen. Cisplatin was used at a fixed concentration (25 µM). Adjudin was used at two different concentrations (15 µM and 30 µM), and LND at 50 µM. The control cells were set as 100%. All experiments were performed in triplicate. Data are shown as mean ± SEM. (B) Apoptosis triggered by Ad alone, cisplatin alone, combination of Adjudin plus cisplatin and combination of LND plus cisplatin in A549 cells was assayed by flow cytometric measurement of Annexin V and 7-AAD double staining. The quantification of the apoptotic cells was achieved by averaging the results of three independent flow cytometric analyses.
**P < 0.01 and ***P < 0.0001 indicate significant differences from the respective control groups compared by one-way ANOVA to be followed Tukey’s Honest Significant Test.
4. Discussion
Adjudin is a potential non-hormonal male contraceptive that acts by inducing premature germ cell depletion and ultimately blocking the production of sperms from testes [7–11]. In this study, we found, for the first time, that Adjudin could also inhibit cancer cells both in vitro and in vivo. Our results reveal a new small molecule drug potentially useful for tumor treatment.
Mitochondrial-targeting drugs represent a promising approach to eradicate cancer cells in chemotherapy. LND is an energolytic agent for tumor treatment, which is characterized as a mitochondrial-targeting drug capable of binding to the adenine nucleotide translocator (ANT), causing mitochondrial permeability transition pore opening and apoptosis [13,24–26]. It has also been reported that LND inhibited the mitochondria-bound hexokinase, which increases the glycolytic activity and intracellular ATP levels. Adjudin was developed as a small molecular drug and as an analog of LND in screening for new non-hormonal male contraceptives. Recent studies have indicated the direct effects of Adjudin on sperm mitochondria [31]. Adjudin could cause the loss of sperm mitochondrial membrane potential and interfere with energy production [31], similar to the action of LND in tumor cells. In this study, we tested Adjudin’s effect on cancer cells and normal cells (Fig. 2). We demonstrated that Adjudin could induce cancer cell apoptosis through the Caspase-3 dependent pathway (Figs. 4 and 5). The mode of action might also be through affecting mitochondrial functions. We confirmed changes in the ATP level, mitochondrial membrane potential, mass, and mitochondrial morphology after treatment with Adjudin (Fig. 6). The changes of mitochondria occurred at the early time point (3 h), much sooner than the apoptotic induction by Adjudin that was detected at 24 h (at least later than 12 h) (Figs. 4 and 6, and Supplemental Fig. 1). Furthermore, the cytochrome C released from mitochondria and the cleaved Caspase-9 were only detected at 24 h after Adjudin treatment (Fig. 5B). The accurate time point of apoptosis that occurred after treatment with Adjudin was not captured in our study. However we hypothesized that the apoptosis induction by Adjudin occurred between 12 h and 24 h. Mechanistically, whether Adjudin could influence mitochondria-bound hexokinase as LND did and how Adjudin causes the mitochondrial dysfunction as the early response are still unknown and will require further investigation.
The tumor growth delayed action was found from 24 days after tumor inoculation (7 days after Adjudin administration) for A549 cells in vivo (Fig. 7B). From the tumor volume curve, the increase rate between the Adjudin group and vehicle group started to differentiate upon treatment, with Adjudin group increasing at a rather constant rate and a sudden leap in the vehicle group from 24 days. Adjudin slowed down the tumor volume increase. In the vehicle group, when the tumor volume reached a certain level, the tumor growth accelerated, suggesting that A549 cell-derived tumor grew more aggressively at this stage. PC3 cells are slightly more resistant to Adjudin than A549 cells in vitro (Fig. 2 and Table 1), which could possibly explain the fact that the tumors derived from PC3 cells were less sensitive than those from A549 cells in vivo (Fig. 7B and D).
Evasion of cell death is a hallmark of human cancers and a major cause of treatment failure [28]. Cancer cells can subsequently become more resistant to chemotherapeutics. Inevitably the side effects of chemotherapy drugs are one of the biggest drawbacks to their clinical use. One way to overcome drug resistance and improve drug efficacy is the combinatory therapy that use two or more chemotherapeutics in the treatment regimen. Drug(s) that target different hallmarks of cancer cells could lead to synergistic therapeutic effects. In this regard, the search for new drugs or better drugs to get over the hurdles in clinical treatment will always be of great significance. One approach, which is specially promising in the pursuit of anti-cancer agents, is to evaluate the ‘old drugs’ that could be used singly or in combination for cancer treatment. Chloroquine, the effective anti-malarial and anti-rheumatoid drug and metformin, the antidiabetic drug are the typical examples of ‘old drugs’ used in the clinical treatment of cancer [5,6]. The advantage of using ‘old drugs’ is that these agents have already been proved for their safety. In this study, we have provided another example of old drug’s new use for cancer therapy. Our work and the previous research have indicated that Adjudin is a safe drug that has little side effects on normal cells. Furthermore, our results implicated that Adjudin could be a useful chemotherapeutic for combination in cancer treatment. Combination therapy is considered more promising in clinical use. For example, in reported clinical trials, the combination treatment with bevacizumab (a VEGF inhibitor) and everolimus (an mTOR inhibitor) could be more beneficial to patients with metastatic renal cell carcinoma [32]. LND plus arsenic trioxide (ATO, Trisenox™) used as combination treatment for the human leukemia could increase cell apoptosis, thus eradicating the proliferation of lymphoid human leukemia cell lines more effectively [24]. Compared to LND, which efficacy was generally low when used in clinical treatment with tumors [16,24,33], Adjudin was shown to be a more potent drug to reduce cancer cells proliferation (Fig. 2B and Table 1). It is noted that the IC50 of Adjudin against cancer cells and the concentration of Adjudin used in lung and prostate carcinoma cells in vivo is still higher than the clinical anti-tumor drugs’, implicating it is less favorable to be used as a single anti-cancer drug.
Since Adjudin was found to be an anti-spermatogenic agent, the sperm activity would be affected by the continuous administration of Adjudin. It is known that a single dose of Adjudin treatment results in reversible infertility in rats followed with rebounded sperm production in about 2–3 months [7,8,34]. Whether multiple doses of Adjudin treatment would still yield reversible infertility in male mice in our study is yet to be determined. A recent study indicated that multiple doses of Adjudin could lead to blood–testis barrier disruption and impair spermatogenesis [35]. Actually for chemotherapy this type of side-effect is generally observed. Among a myriad of cancer cell lines tested, we chose PC3 and A549 cells that are not the most sensitive cancer cells to Adjudin (Fig. 2 and Table 1), and showed its effective drug efficacy. It is possible that for other cancer cell models, such as MDA-MB-231 cells, the treatment regimen would require a lower dose of Adjudin. And the use of Adjudin on breast cancer model in female mice would not be a problem regarding anti-sperm activity. Moreover, here we showed that when Adjudin was used in combination with cisplatin, both general toxicity and apoptosis were greatly increased, whereas the cisplatin-LND pair failed to perform these effective inhibitions (Fig. 8). We used the formula to calculate the combination index (CI) which determines the strength of interaction as described previously [36]. The average CI values of the 50% growth inhibition were 0.46 ± 0.02 for Adjudin-cisplatin pair (i.e. Adjudin 15 µM and cisplatin 25 µM in combination produced the effect 50%, CI = 15(Adjudin 15 µM)/IC50(Adjudin) + 25(cisplatin 25 µM)/IC50(Cisplatin)) in A549 cells, demonstrating the synergistic effect attributed to Adjudin. These results seem to suggest that Adjudin could be an adjuvant for combination cancer therapy, and, it might be a better adjuvant chemotherapeutics than LND. On the other hand, with the development of novel drug delivery vehicles, such as nanoparticles, it is possible to yield even better efficacy for Adjudin-based therapies [18,37]. We are now investigating the possibilities in this area.
In summary, our studies have demonstrated that the male contraceptive Adjudin exhibits effective anti-cancer activity against various cancer cells grown in vitro and in vivo, which showed better efficacy than its analog LND in the combination treatment regimen with cisplatin. We also showed that Adjudin induced cancer cell apoptosis through activating Caspase-3 dependent pathway and impairing the mitochondrial function, suggesting that mitochondria are the main target for the anticancer potency of Adjudin in vitro. Although more work needs to be done to dissect the mechanisms of Adjudin’s anti-cancer activity, we believe that these observations offer a good basis for its potential clinical applications in the future.
Supplementary Material
Acknowledgments
This study was supported by grants from NNSF China (30900756,31270032), Ministry of Science and Technology (2013CB945604), ‘‘Rising Star’’ Grant from Science and Technology, Commission of Shanghai (09QA1403400), a start-up grant from Ministry of Education China for returnees (K10MD06), and SJTU fundings (YG2011MS44, YG2012ZD05) and SJTU SMC Morning Star program. We are also grateful to Ms. Jin Xu for her assistance in flow cytometry and Mr. Xiang Gu for his assistance in confocal microscopy.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bcp.2012.11.008.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug. Mol Cancer Therapeut. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 5.Geng Y, Kohli L, Klocke BJ, Roth KA. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro-oncol. 2010;12:473–481. doi: 10.1093/neuonc/nop048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi A. Chloroquine in glioblastoma—new horizons for an old drug. Cancer. 2009;115:2380–2383. doi: 10.1002/cncr.24288. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- 9.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 10.Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood–testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA. 2007;104:3841–3846. doi: 10.1073/pnas.0610100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestrini B, Palazzo G, De Gregorio M. Lonidamine and related compounds. Prog Med Chem. 1984;21:110–135. [PubMed] [Google Scholar]
- 13.Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today (Barc) 2003;39:157–174. doi: 10.1358/dot.2003.39.3.799451. [DOI] [PubMed] [Google Scholar]
- 14.Ravagnan L, Marzo I, Costantini P, Susin SA, Zamzami N, Petit PX, et al. Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore. Oncogene. 1999;18:2537–2546. doi: 10.1038/sj.onc.1202625. [DOI] [PubMed] [Google Scholar]
- 15.Sordet O, Rebe C, Leroy I, Bruey JM, Garrido C, Miguet C, et al. Mitochondria-targeting drugs arsenic trioxide and lonidamine bypass the resistance of TPA-differentiated leukemic cells to apoptosis. Blood. 2001;97:3931–3940. doi: 10.1182/blood.v97.12.3931. [DOI] [PubMed] [Google Scholar]
- 16.Oudard S, Carpentier A, Banu E, Fauchon F, Celerier D, Poupon MF, et al. Phase II study of lonidamine and diazepam in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2003;63:81–86. doi: 10.1023/a:1023756707900. [DOI] [PubMed] [Google Scholar]
- 17.Miller-Kasprzak E, Jagodzinski PP. Endothelial progenitor cells as a new agent contributing to vascular repair. Arch Immunol Ther Exp (Warsz) 2007;55:247–259. doi: 10.1007/s00005-007-0027-5. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Xie QR, Zhang J, Xia W, Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 2011;32:9546–9556. doi: 10.1016/j.biomaterials.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 19.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su L, Cheng CY, Mruk DD. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:1864–1875. doi: 10.1016/j.biocel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 22.Ferri KF, Kroemer G. Control of apoptotic DNA degradation. Nat Cell Biol. 2000;2:E63–E64. doi: 10.1038/35008692. [DOI] [PubMed] [Google Scholar]
- 23.Marella M, Seo BB, Matsuno-Yagi A, Yagi T. Mechanism of cell death caused by complex I defects in a rat dopaminergic cell line. J Biol Chem. 2007;282:24146–24156. doi: 10.1074/jbc.M701819200. [DOI] [PubMed] [Google Scholar]
- 24.Calvino E, Estan MC, Simon GP, Sancho P, Boyano-Adanez Mdel C, de Blas E, et al. Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells reactive oxygen species generation and defensive protein kinase (MEK/ERK, Akt/mTOR) modulation. Biochem Pharmacol. 2011;82:1619–1629. doi: 10.1016/j.bcp.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Li YC, Fung KP, Kwok TT, Lee CY, Suen YK, Kong SK. Mitochondrial targeting drug lonidamine triggered apoptosis in doxorubicin-resistant HepG2 cells. Life Sci. 2002;71:2729–2740. doi: 10.1016/s0024-3205(02)02103-3. [DOI] [PubMed] [Google Scholar]
- 26.Belzacq AS, El Hamel C, Vieira HL, Cohen I, Haouzi D, Metivier D, et al. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene. 2001;20:7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- 27.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.DeAngelis LM, Currie VE, Kim JH, Krol G, O’Hehir MA, Farag FM, et al. The combined use of radiation therapy and lonidamine in the treatment of brain metastases. J Neurooncol. 1989;7:241–247. doi: 10.1007/BF00172917. [DOI] [PubMed] [Google Scholar]
- 30.Silvestrini B, Hahn GM, Cioli V, De Martino C. Effects of lonidamine alone or combined with hyperthermia in some experimental cell and tumour systems. Br J Cancer. 1983;47:221–231. doi: 10.1038/bjc.1983.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Chen XX, Wang LR, Mao YD, Zhou ZM, Sha JH. AF-2364 is a prospective spermicide candidate. Asian J Androl. 2010;12:322–335. doi: 10.1038/aja.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papaldo P, Lopez M, Cortesi E, Cammilluzzi E, Antimi M, Terzoli E, et al. Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol. 2003;21:3462–3468. doi: 10.1200/JCO.2003.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Mok KW, Mruk DD, Lie PP, Lui WY, Adjudin Cheng CY. a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011;141:571–580. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mok KW, Mruk DD, Lee WM, Cheng CY. A study to assess the assembly of a functional blood–testis barrier in developing rat testes. Spermatogenesis. 2011;1:270–280. doi: 10.4161/spmg.1.3.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naumova E, Ubezio P, Garofalo A, Borsotti P, Cassis L, Riccardi E, et al. The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res. 2006;12:1839–1849. doi: 10.1158/1078-0432.CCR-05-1615. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Zhang J, Sun W, Xie QR, Xia W, Gu H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int J Nanomed. 2012;7:999–1013. doi: 10.2147/IJN.S28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.