According to the World Health Organization Global Burden of Disease 2004 Update, unipolar depressive disorder, which is twice as common in women, is the leading cause of disease burden for 15- to 44-year-old women residing in high-, middle-, and low-income countries (1). Anxiety is frequently comorbid with depression, and both panic disorder and posttraumatic stress disorder are 2-3 times more common in women than men. The sex bias for these affective disorders is not evident until after puberty, suggesting a possible role for ovarian hormones and their cyclicity in the manifestation of increased risk for these disorders in women. Of particular developmental importance is the predictable clustering of affective disturbances in a subgroup of women during hormonal shifts across the life span: the premenstrum, pregnancy, postpartum, and perimenopause. Specifically, premenstrual dysphoria appears to have a strong genetic component and is associated with an increased risk for affective disorders in the postpartum and perimenopause (2). The mechanism by which ovarian hormones and their neurosteroid metabolites contribute to affective disorders is likely to be multifactoral, given the widespread and diverse effects these hormones have on neurotransmitter systems, neurotrophins, and neuronal and structural morphology. To add complexity, environmental stressors can also influence the impact of ovarian hormones on brain systems and gene expression, making the study of these interactions challenging in the human setting.
In an elegant study published in this issue of Biological Psychiatry, Bath et al. (3) circumvent the environmental contributions and influence of other genetic variations on mood by using a transgenic mouse model that carries a common single-nucleotide polymorphism suspected to enhance risk for affective disturbance and other neuropsychiatric conditions in humans. The authors provide an insightful examination of developmental and estrous cycle stage effects on anxiety-related behaviors, comparisons that have been frequently lacking in animal models. Specifically, Bath et al. (3) utilized an established knock-in mouse model in which a single-nucleotide polymorphism was inserted into the prodomain of the brain-derived neurotrophic factor (BDNF) gene, resulting in an amino acid substitution of methionine (Met) for valine (Val) at position 66 (BDNF Val66Met). Rodents homozygous for the Met allele have normal concentrations of central BDNF but reduced stimulated neuronal BDNF release and are phenotypically anxious when placed in mildly stressful behavioral paradigms (4). Given the increased risk for affective disorders in women following puberty and with reproductive events, the investigators focused solely on female mice homozygous for the Val (wild-type) and Met (risk genotype), measuring anxiety- and depression-like behaviors in several standard behavioral paradigms across estrous cycle stages and at points across development. In Val mice, there was no estrous stage effect on behaviors in the open field, elevated plus maze, or forced swim tests. As predicted the Met mice showed greater anxiety- and depression-like behaviors during estrus, the point in the cycle when estrogen levels are rapidly declining compared with other stages. Likewise, although the investigators did not find a correlation between age and behaviors in the Val mice, there was a significant correlation between age and anxiety-like behaviors in Met mice, suggesting an impact of reproductive development on the manifestation of anxiety risk. A similar impact of the Val66Met polymorphism and estrous cycle stage on hippocampal memory function and molecular markers of dendritic spine formation has been shown previously (5).
The BDNF Val66Met polymorphism is common in humans, with approximately 30% and 4% of the Caucasian population being heterozygous and homozygous, respectively. BDNF, abundantly expressed in the hippocampus, prefrontal cortex, and amygdala, is critical for cell survival, neural plasticity, stress regulation, antide-pressant efficacy, and learning and memory. Stress experience, a common precipitating factor in affective disorder onset, significantly reduces BDNF levels in adults, and a recent meta-analysis confirmed the presence of substantially lower BDNF levels in depressed patients followed by a robust increase in BDNF with anti-depressant treatment (6). That peripheral BDNF levels differ by sex and with menstrual cycle phase in healthy individuals and show phase-specific alterations in women with severe premenstrual syndrome (7) provides an additional rationale for investigating the behavioral implications of BDNF genotype across development and different hormonal states. Unfortunately, many clinical studies in which peripheral BDNF levels have been measured did not characterize subjects according to the Val66Met polymorphism; thus, the mechanisms contributing to changes in BDNF expression levels cannot be determined. In addition, normal or elevated peripheral levels of BDNF may not actually reflect an optimal state if the individual is homozygous for the Met allele, resulting in diminished synaptic release of BDNF. The importance of such regulatory mechanisms remains to be explored (Figure 1).
Figure 1.
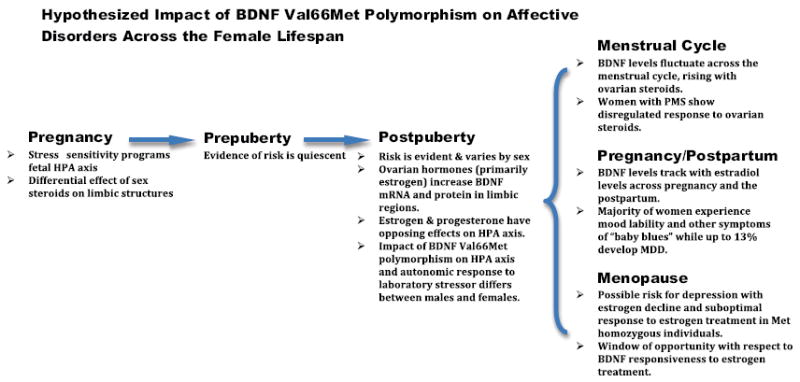
The figure highlights potential implications of BDNF Val66Met polymorphism for affective disturbances risk across the female life span. During pregnancy the offspring, which is homozygous for the Met allele, may be more sensitive to the HPA axis programming effects of enhanced glucocorticoid exposure because of maternal psychosocial stress or pregnancy factors such as hypoxia, placental insufficiency, and maternal diet, to name a few. Sexually dimorphic brain regions may be more or less sensitive to the organizing effects of androgens. Prior to puberty, the impact of Val66Met polymorphism is quiescent because ovarian hormones have not yet produced their effect on BNDF synthesis and function. Following puberty, females begin to have fluctuations in estradiol and progesterone on a monthly basis that reveal the propensity for affective disturbance through changes in BDNF synthesis and modulation of the HPA axis. Women who are homozygous for the Val allele demonstrate resilience in the face of dramatic hormonal change, whereas those homozygous for the Met allele experience a negative affect during the premenstrum, postpartum and perimenopause. BDNF, brain-derived neurotrophic factor; HPA, hypothalamic-pituitary-adrenal; MDD, major depressive disorder; Met, methionine; PMS, premenstrual syndrome; Val, valine.
The usual caution is warranted when generalizing findings from behavioral studies in rodents to complex human conditions such as depression and anxiety disorders. Nonetheless, the study by Bath et al. (3) suggests that the BDNF Val66Met polymorphism is important to the unmasking of the risk or resilience with respect to negative mood states after puberty and during periods of dynamic estrogen and progesterone fluctuation. Estradiol has been shown to induce BDNF messenger RNA and protein in the hippocampus, and in turn BDNF appears to mediate at least some of the effects of estradiol on neuronal plasticity (8). The effects of progesterone on BDNF levels may be more nuanced and depend on whether it is administered alone or with estradiol.
The issue of developmental stage with respect to BDNF genotype, BDNF levels, and their interaction is particularly important when the impact of environmental stressors is considered. In rodent and nonhuman primates, early adversity in the form of maternal separation results in an increase in BDNF expression in either both sexes or females only, depending on the model (9). In contrast, both acute and chronic stress during adulthood is typically associated with decreased peripheral BDNF levels and central neurogenesis. Again, combining a study of BDNF levels with genotype would provide a more thorough assessment of the individual’s potential neurotrophin status. Estradiol and progesterone modulate hypothalamic-pituitary-adrenal axis activity and glucocorticoid effects on BDNF, emphasizing the importance of controlling for early and enduring stress exposures in future studies. To their credit, Bath et al. (3) were careful to not overly stress the mice prior to testing, allowing the mice to be maternally reared and examining vaginal swabs for estrous cycle staging after the behavioral paradigms were completed.
Finally, exercise is a factor when considering the impact of the BDNF Val66Met polymorphism across development and reproductive stages. In rodents, physical activity increases hippocampal BDNF mRNA and protein levels in intact females, whereas estrogen loss diminishes this effect (8). Interestingly, there appears to be a proverbial window of opportunity during which the BNDF response to estradiol replacement remains intact, after which the effect is significantly diminished. These findings reflect the growing consensus regarding postmenopausal hormone therapy, namely that estrogen’s potential beneficial effects on target tissues diminishes with the duration of hypogonadism. Whether postmenopausal women lose the positive effects of exercise on BDNF, neurogenesis, mood, and cognition in the absence of estrogen proximal to their final menstrual period is not known and may depend on their Val66Met status. A recent study in young, healthy adults would support as much, given the effects reported for the exercise-mediated increases in BDNF blood levels and cognition was limited to individuals who were homozygous for the Val allele (10).
With the multifaceted interactions between sex, developmental stage, hormonal conditions, environmental stressors, and exercise, the individual contribution of the BDNF Val66Met polymorphism to the sex bias for affective disorders or their propensity to occur at reproductive stages can be difficult at best to differentiate. Future studies will need to include sufficient numbers of women and men to more closely examine sex differences, confirm menstrual cycle phase with ovarian steroid measurement, and assess timing and degree of physical activity in addition to exposure to stress when examining the role of BDNF Val66Met polymorphism effects on outcome(s) of interest. An interesting, and perhaps feasible, naturalistic approach would be to determine the prevalence of Met homozygosity in those with affective disorders related versus not related to reproductive events. Determining whether the Met allele confers risk for mood disorders at these discrete time points in a woman’s life would have great clinical as well as scientific value. Although mind-body interventions such as yoga and mindfulness therapy are thought to exert, albeit in part, their ameliorative effects on mood and cognition by dampening stress reactivity, whether these modalities reverse the adverse effects of stress on neurotrophins and neurogenesis is not known but is of great mechanistic interest. Likewise, relatively little is known about the impact of exercise on markers of neurogenesis in human subjects and whether the Val66Met polymorphism effects this relationship. In view of the preclinical and clinical literature, there are multiple lines of evidence implicating the Val66Met polymorphism as a fruitful line of investigation with respect to affective disorders in women, especially those related to reproductive function. Because premenstrual dysphoric disorder affects up to 5% of premenopausal women, postpartum depression occurs in approximately 13% of mothers, and perimenopausal women are at 2- and 5-fold increased risk for new-onset and recurrence of depression, respectively, having additional predictors of elevated risk would be of considerable public health value.
Acknowledgments
Dr. Epperson receives research support from Shire Pharmaceuticals for an Investigator Initiated Study and from Novartis in the form of product donation for research purposes. Dr. Epperson or a member of her family owns stock in Merck and Johnson and Johnson.
Footnotes
Dr. Bale reports no biomedical financial interest or potential conflicts of interest.
References
- 1.World Health Organization. [June 24, 2012];World Health Organization Global Burden of Disease Report: 2004 Update. 2008 Available at: www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 2.Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: Evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465–475. doi: 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (valine66methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neuroscience. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci U S A. 2010;107:4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression and antidepressant medications: a meta-analysis and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubeddu A, Bucci F, Giannini A, Russo M, Daino D, Russo N, et al. Brain-derived neurotrophic factor plasma variation during different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology. 2011;36:523–530. doi: 10.1016/j.psyneuen.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 9.Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins ME, Davis FC, Vantieghem MR, Whalen PJ, Bucci DJ. Differential effects of acute and regular exercise on cognition and affect. Neuroscience. 2012;215:59–68. doi: 10.1016/j.neuroscience.2012.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]


