Abstract
The response rate of non-M3 AML to all trans retinoic acid (ATRA) has been limited. Using Affymetrix expression arrays, we found that in diverse AML blasts RXRA was expressed at higher levels than RARA and that mouse Ctsg-PML-RARA leukemia responded to bexarotene, a ligand for RXRA. We therefore performed a phase I study of combination bexarotene and decitabine in elderly and relapsed AML patients. We found that this combination was well tolerated, although outcomes were modest (1 CRi, and 3 PR among 19 patients). Correlative studies found that patients with clinical response had increased differentiation to bexarotene both in vivo and ex vivo, suggesting that pre-treatment analysis might identify a more susceptible subgroup of patients.
Introduction
Current therapy for AML consists of cytotoxic chemotherapy followed by consolidation chemotherapy or stem cell transplantation. Two patient populations remain difficult to treat: patients with relapsed disease and patients who are over the age of 60. Salvage therapy successfully achieves a remission in less than 50% of relapsed patients and in the absence of consolidative allogeneic stem cell transplantation, remissions and survival are typically brief.(1) Compared with younger patients, AML patients who are older than 59 years have response rates that are lower, remissions that are briefer, and they are more likely to experience toxicities.(2–10) Therefore, more effective and less toxic therapies are needed.
Initial Affymetrix expression data of diverse AML samples demonstrated that RXRA was expressed at higher levels than RARA, suggesting that RXRA might be a better target for differentiation therapy than RARA, and might explain why clinical response of non-M3 AML to ATRA have been so limited.(11, 12)
Therefore, we investigated whether bexarotene can be combined with decitabine in elderly and relapsed acute myeloid leukemia (AML) patients. Both drugs have been well tolerated in AML patients as single agents,(13, 14) and have non-overlapping mechanisms and side-effects. Bexarotene activates transcriptional effects of RXRA through hetero- and homodimers, while decitabine induces DNA hypomethylation.(15–20)
We found that this combination was well tolerated, but lead to only modest responses in this population. We also observed that patients with clinical response tended to exhibit greater AML blast differentiation when exposed to bexarotene either in vivo or ex vivo, suggesting a possible future approach to identify patients more likely to gain benefit from therapy.
Methods
Clinical trial
We enrolled 19 subjects on a phase I, dose escalation study. The clinical trial was approved by the Washington University institutional review board, conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization/Good Clinical Practice, and listed on Clinicaltrials.gov (NCT01001143).
Enrollment
Patients with acute myeloid leukemia were either ≥60 years old or had relapsed disease. Major exclusion criterion were: white blood cell count (WBC) > 10,000/μl, bilirubin > 1.5 x upper limit of normal (ULN), AST/ALT > 2.5 x ULN, creatinine > 2 ULN, triglycerides > 1,000 mg/dl, active graft vs host disease, central nervous system involvement with leukemia, and performance status > 3. The exclusion of patients with WBC > 10,000/μl was because we have observed reduced response to decitabine in these patients.(13)
Treatment schedule
Patients were treated in 3+3 dose-escalating cohorts (Table 1). Patients received oral bexarotene in one of three cohorts: 100, 200, and 300 mg/m2/day, for all days of all cycles. All patients also were treated with decitabine 20 mg/m2 IV on days 1–5 of 28 day cycles. During cycle 1, decitabine was initiated after 3 days of bexarotene and following a repeat bone marrow collection for correlative studies.
Table 1.
| Dose level | Decitabine (mg/m2) IV Days 1–5 | Bexarotene (mg/m2) PO daily |
|---|---|---|
|
| ||
| 1 | 20 | 100 |
| 2 | 20 | 200 |
| 3 | 20 | 300 |
Supportive care
Supportive care and symptom management were provided according to institutional standards of care. In addition, all patients were monitored for hypertriglyceridemia, hypercholesterolemia, and hypothyroidism, and treated accordingly.(21)
Response and outcomes definitions
The maximum tolerated dose (MTD) was defined as the dose level immediately below the dose level at which 2 patients of a cohort (of 2 to 6 patients) experience dose-limiting toxicity during the first cycle. Myelosuppression, infection, differentiation syndrome, hypertriglyceridemia, hyperlipidemia, hypothyroidism, nausea, weight loss and reversible electrolyte abnormalities were not considered dose limiting. Toxicity grading during the first and all subsequent cycles was performed according to the revised National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, published May 29, 2009 and available at http://ctep.cancer.gov/reporting/ctc.html. Response was assessed according to the IWG criteria.(22)
Expression analysis
Affymetrix, Nanostring nCounter, and RNA-Seq methods have been described elsewhere.(23–25)
Methylcellulose analysis of mouse leukemia
Cryopreserved leukemic spleen cells were thawed, plated at 2 × 106/ml in RPMI with 15% FCS, 100 ng/ml SCF, 6 ng/ml IL-3, 10 ng/ml IL-6 (Peprotech, Rocky Hill, NJ) ± 1 μM ATRA (Sigma, St. Louis, MO) or 1 μM bexarotene (LC Laboratories, Woburn, MA) and maintained at 3% oxygen and 5% CO2 in a humidified chamber (Billups-Rothenberg, Del Mar, CA) for 48 hours. Cells were plated at 8.3 × 103/ml (MethoCult M3534 Stem Cell Technologies, Vancouver, Canada) and maintained in 3% oxygen and 5% CO2. After seven days, colonies were counted.
In vivo analysis of bexarotene response in mice
Leukemic sample arising in mouse 13341 was selected because this leukemic sample was the least immunophenotypically differentiated of the samples assessed at baseline based on CD117 and Gr1 expression.(26) 1 × 106 leukemia cells were injected by retro-orbit into recipient mice. Indicated mice received either 1 mg bexarotene dissolved in DMSO and corn oil, or placebo corn oil by gavage on days 8–10 (~300 mg/m2/day). Mice were sacrificed and analyzed on day 14. All of the mice were cared for in the experimental animal center of Washington University School of Medicine. The Washington University Animal Studies Committee approved all animal experiments.
Ex vivo analysis of human AML samples
Cryopreserved leukemia samples were thawed and co-cultured as previously described.(27) Bone marrow samples collected on day 0 and day 3 of bexarotene therapy were grown for 72 hours on MS5 murine stromal cells in media containing IL3, IL6, SCF, and TPO ± 1 μM bexarotene or ATRA and assayed by flow cytometry (anti-CD11b-APC, clone ICRF44, eBioscience, San Diego, CA) or EdU staining, performed per manufacturers recommendations, after culturing cells with EdU for 1 hour (Life Technologies, Grand Island, NY).
Results
Human expression of RARA and RXRA
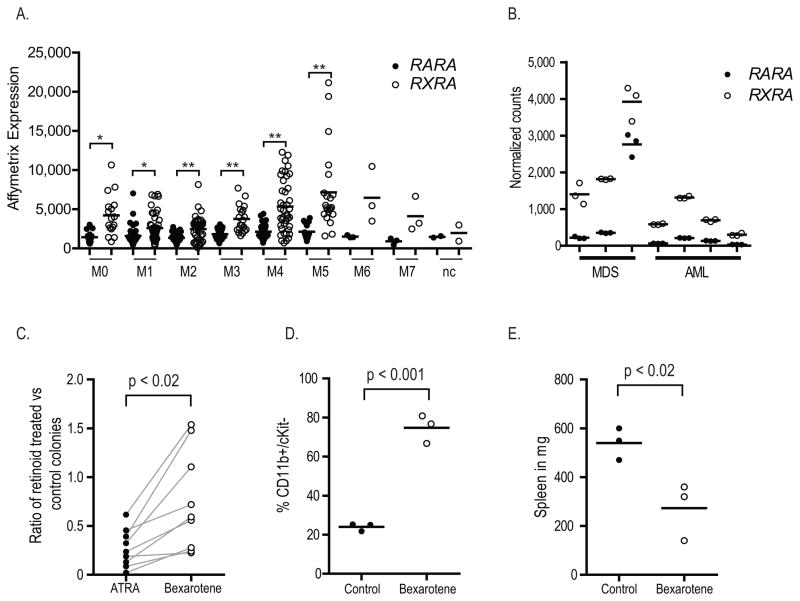
We used Affymetrix expression arrays to determine the relative level of retinoid receptors in diverse, unselected FAB AML cases. We observed elevated levels of RXRA relative to RARA in primary AML cells, regardless of FAB (Figure 1A). We observed no differences in either RARA or RXRA expression in AML cases with WBC > 10,000/μl vs. those with WBC < 10,000/μl, when controlled for FAB (data not shown). We validated these findings in a smaller set of AML and MDS cases using a different technology, Nanostring nCounter (Figure 1B). Each of these cases presented with WBC < 10,000/μl.
Figure 1.
Expression profile of RARA (closed circles) and RXRA (open circles) in total bone marrow cells from 197 human AML cases using Affymetrix Human Genome U133 Plus 2.0 GeneChip Arrays (* p < 0.002; ** p < 0.001).(23) B. Expression profile of RARA (closed circles) and RXRA (open circles) in total bone marrow cells from 3 MDS patients and 4 AML patients using Nanostring nCounter (each sample analyzed in technical triplicates, two way ANOVA, p < 0.001). C. Reduction in methylcellulose colony forming by 9 unique leukemia samples derived from Ctsg-PML-RARA mice when treated for 48 hours with 1 μM of indicated retinoid and then assayed in myeloid methylcellulose. D. and E. In vivo differentiation of leukemia arising in mouse 13341. Following secondary transplantation of 1 x 106 leukemia cells, mice were treated with either placebo or bexarotene days 8–10, sacrificed and analyzed on day 14.
Response of murine AML to bexarotene
In order to determine whether an RXRA ligand (e.g. bexarotene) might be effective at differentiating AML blasts, we treated 9 unique mouse Ctsg-PML-RARA acute promyelocytic leukemia (APL) samples with either ATRA or bexarotene ex vivo. We found that bexarotene was able to decrease the number of colony forming units in Ctsg-PML-RARA leukemia cells, but not as effectively as ATRA (Figure 1C).
To determine whether bexarotene might be effective in vivo, secondary transplantation of the leukemia arising in mouse 13341 was performed and mice were treated with bexarotene or placebo. We observed an increase in the expression of CD11b and a decrease in the spleen weight, consistent with an in vivo response to bexarotene (Figure 1D – E). Based on these results, we established a phase I study of bexarotene and decitabine in patients with AML.
Clinical trial
The primary object of this trial was to determine the safety and tolerability of this combination. Secondary objectives were to determine the response rate and to correlate outcomes with in vivo and ex vivo differentiation response to bexarotene.
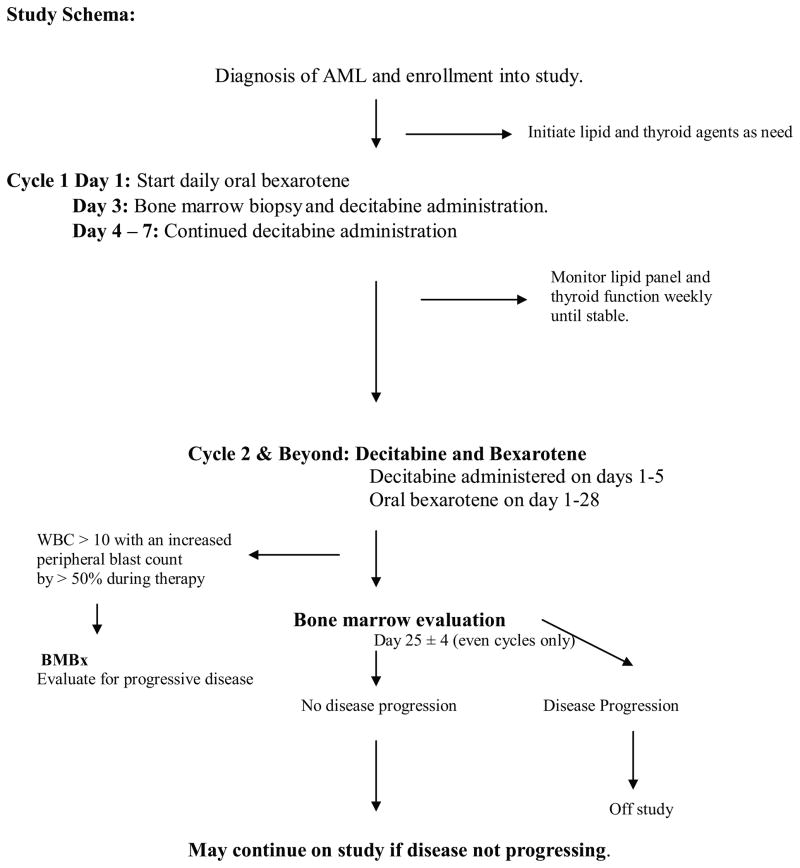
Patients were treated with oral bexarotene in three escalating dose cohorts, and with decitabine at the same dose in all cohorts (Table 1 and Figure 2).
Figure 2.
Study schema.
Patient characteristics are described in Table 2. The average age was 74. The median performance status was 1. An adverse karyotype was observed in 9 patients (48%). And 12 patients (63%) had relapsed after prior therapy.
Table 2.
| ID | Age | Gender | Prior Tx History | WHO/FAB | Karyotype | ECOG @ Baseline |
Cohort | Dose Level | # Cycles Completed |
DLT | Best Response |
Survival (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A01 | 79 | M | No prior treatment | M1 | Complex | 1 | 1 | 1 | 1 | No | PD | 38 |
| A02 | 83 | M |
|
AML with MLD | Complex | 1 | 1 | 1 | 3 | No | PR | 125 |
| A03 | 81 | M |
|
M2 | Complex | 2 | 1 | 1 | 3 | No | SD | 118 |
| B04 | 76 | M |
|
AML with MLD | XY, +8 | 2 | 2 | 2 | 2 | No | PD | 299 |
| B05 | 79 | F | No prior treatment | M0 | XX, +8, +19 | 1 | 2 | 2 | 2 | Yes | PR | 240 |
| B06 | 64 | F | no prior treatment | AML, NOS | Complex | 1 | 2 | 2 | 5 | No | PR | 428 |
| B07 | 82 | F |
|
M2 | XX | 1 | 2 | 2 | < 6 | No | SD | 554 |
| B08 | 67 | M |
|
AML, NOS | Complex | 1 | 2 | 2 | 2 | No | CR | 543 |
| B09 | 68 | M |
|
M2 | XY, del(20)(q11.2q13.1) | 0 | 2 | 2 | < 1 | No | N/A | 10 |
| B10 | 79 | M |
|
AML with MLD | XY. NPM1 exon 12 insertion. | 1 | 2 | 2 | < 3 | No | SD | 84 |
| C11 | 72 | F |
|
M2 | Complex. JAK2 V617F. | 1 | 3 | 3 | 1 | No | PD | 27 |
| C12 | 80 | M |
|
M2 | XY | 1 | 3 | 3 | 1 | No | N/A | 38 |
| C13 | 39 | F |
|
AML | Complex | 1 | 3 | 3 | 1 | No | N/A | 17 |
| D14 | 69 | M |
|
AML | Complex. | 1 | Ex | 3 | 12 | No | SD | 427 |
| D15 | 82 | M |
|
AML | XY | 2 | Ex | 3 | <1 | No | PD | 84 |
| D16 | 77 | M |
|
M2 | XY. FLT3-ITD+. | 1 | Ex | 3 | <1 | No | PD | 19 |
| D17 | 71 | M |
|
AML with MLD | XY. | 2 | Ex | 3 | 3 | No | SD | 109 |
| D18 | 72 | M |
|
AML with MLD | XY, -7q, del(12)(q13q15) | 0 | Ex | 3 | 4 | No | SD | 673 |
| D19 | 81 | M | No prior treatment | AML | 92, XXYY[2]/46, XY[18] | 2 | Ex | 3 | 4 | No | SD | 303 |
The combination was well tolerated. Only one patient experienced a study-defined dose limiting toxicity (grade 3 fatigue, cohort 2) and 9 patients were treated with the maximum bexarotene dose, 300 mg/m2/day. Grade 3 – 5 toxicities noted during additional cycles are described in Table 3.
Table 3.
| Grade 3–4 Toxicity during any cycle | |
|---|---|
| Cardiac Arrest | 1 (5%) |
| Intracranial hemorrhage | 1 (5%) |
| Anemia | 17 (89%) |
| Neutropenia | 10 (53%) |
| Pneumonia | 9 (47%) |
| Purpura | 1 (5%) |
| Anorexia | 1 (5%) |
| Nausea/Vomiting | 2 (11%) |
| Hypertriglyceridemia | 7 (37%) |
| Hyperglyceridemia | 2 (11%) |
| Hypocalcemia | 3 (16%) |
| Hyponatremia | 1 (5%) |
| Pleural effusion | 1 (5% |
| Hypoalbuminemia | 1 (5% |
| Fatigue | 2 (11%) |
| Prolonged PTT/INR | 2 (11%) |
The overall response rate was 21%: 1 complete remission with incomplete count recovery (CRi), and 3 patients achieved blast reduction greater than 50% and less than 25% bone marrow blast cells (partial response, PR). Patients with CRi, PR, or SD completed an average of 4.2 cycles, while other patients completed an average of 1.2 cycles. The median survival was 118 days and 5 patients survived more than 1 year. One patient remains alive under continued follow-up (patient 18). Of note, 3 patients successfully transitioned to allogeneic transplant following therapy (patients 6, 8, and 18; average age 68). Unlike Tsai et al.,(14) we did not observe improved platelet counts during bexarotene treatment (data not shown), possibly due to decitabine co-treatment.
Response of patient bone marrow cells to bexarotene in vivo and ex vivo
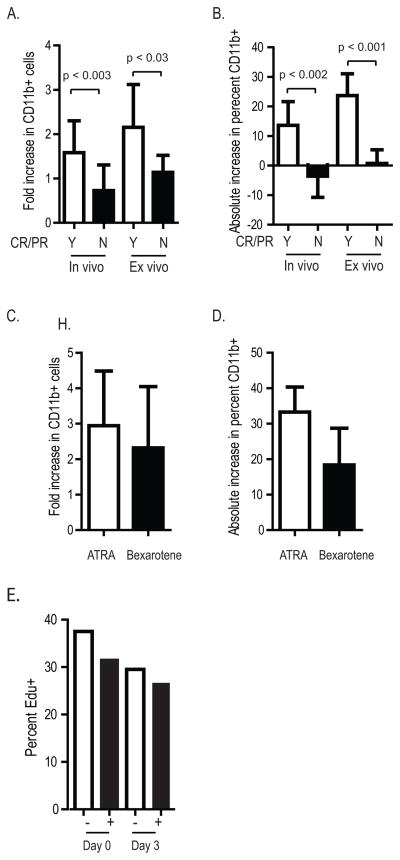
We correlated ex vivo bexarotene sensitivity with clinical response. Bone marrow cells were collected on day 0 and day 3 of bexarotene and co-cultured with irradiated MS5 stromal cells with or without further bexarotene treatment. We compared CD11b expression in cells treated ex vivo, and we compared expression between samples collected on day 0 vs day 3 (in vivo treatment). Bexarotene increased CD11b expression greater in the 4 responding patients vs non-responders (fold increase in CD11b: in vivo 1.6 ± 0.3 vs 0.7 ± 0.2, p < 0.03, and ex vivo 2.1 ± 0.3 vs 1.1 ± 0.1, p < 0.003; increase in absolute percentage of CD11b+ cells: in vivo 13.6% ± 4% vs −3.6% ± 2.2%, p < 0.002, and ex vivo 24% ± 2.6% vs 0.7% ± 1%, p < 0.001, Figure 3A – B). We also observed an equivalent or modestly increased induction of CD11b when cells were treated ex vivo with ATRA vs bexarotene (Figure 3C – D) (the fourth responding patient did not have adequate samples for this experiment).
Figure 3.
Ex vivo analysis of patient samples. A-B. Bone marrow cells collected on day 0 and day 3 of bexarotene therapy were grown for 72 hours on MS5 murine stromal cells and treated with and without 1 μM bexarotene and assayed by flow cytometry for CD11b expression. C-D. Bone marrow cells collected on day 0 from patients 2, 5, and 6 were cultured on MS5 cells without treatment or with 1 μM ATRA or 1 μM bexarotene and assayed for CD11b expression 72 hours later. E. Cell cycle analysis by EdU staining of patient 6 bone marrow cells grown ex vivo as in A.
One case with clinical response had sufficient bone marrow cells collected on day 0 and day 3 to also determine the extent of cell cycle kinetics by EdU staining. We observed a decrease in EdU staining following in vivo and ex vivo bexarotene (Figure 3E).
To determine transcriptional effects of in vivo bexarotene, we performed expression array profiling on 10 cases, 4 of which had clinical responses. Bone marrow samples were collected on day 0 and day 3 of oral bexarotene and total bone marrow cells subjected to Affymetrix expression array profiling. We observed no statistically significant differences in expression at baseline or in response to bexarotene in responders vs non-responders (expression difference >2 fold and FDR < 0.05, data not shown). We again compared the expression of RXRA vs RARA in this dataset and found no difference in expression levels, either between groups of patients, or within individual patients (Figure 4A). We reviewed the recently available RNA-Seq data from the TCGA AML project (Figure 4B),(28) which also was not consistent with increased absolute RXRA expression vs RARA expression across diverse FAB subtypes.
Figure 4.
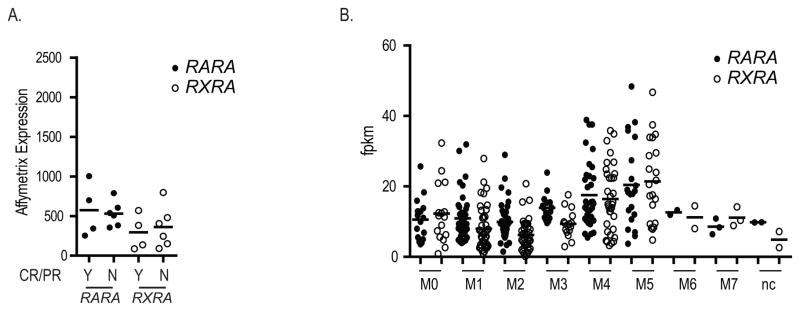
Expression analysis. A. Expression array analysis of RARA (closed circles) and RXRA (open circles) in total bone marrow cells collected on day 0 from 10 cases analyzed by Affymetrix Human Exon 1.0 ST arrays. B. Expression analysis of RARA (closed circles) and RXRA (open circles) in 179 human AML samples by RNA-Seq.(28) All arrays normalized to chip mean of 1,500.
Discussion
Initial expression analysis, using both Affymetrix and nCounter data, suggested that AML blasts express higher levels of RXRA compared with RARA (Figure 1A – B). We further found that bexarotene, a retinoid which selectively binds to and activates RXRs, but not RARs, lead to loss of self-renewal of mouse APL tumors ex vivo and increased differentiation and decreased growth of a selected mouse leukemia in vivo (Figure 1C – E). Consistent with these findings, other investigators have observed differentiation effects of bexarotene in leukemia cells lines.(15–17) These data suggested that a ligand specific for RXR may be more effective to induce retinoid-dependent differentiation in non-M3 AML than the RARA ligand ATRA.(12, 29) We therefore performed a phase I, dose-escalation study of combination decitabine and bexarotene, two drugs with non-overlapping mechanisms and non-overlapping side-effect profiles.
We found that bexarotene can be safely combined with decitabine in elderly and relapsed AML patients. This combination was well tolerated to a maximum bexarotene dose of 300 mg/m2/day, with toxicities similar to single agent decitabine.(13) The toxicities we observed, across all cohorts and cycles, were primarily cytopenias, infections, and hypertriglyceridemia, as expected (Table 3).
Combination bexarotene and decitabine lead to modest responses in a subset of patients, and could bridge elderly patients to allogeneic transplant. However, survival outcomes were limited, with a median survival in this cohort of only 118 days. These results are not significantly different from historical cases treated with single agent decitabine, which typically results in response rates of 22–30%,(13, 18, 30–38) or historical cases treated with single agent azacitidine, with response rates of 7–20%.(39–41) Therefore, from this small cohort of relapsed, pre-treated, and high risk AML, we cannot conclude that the combination of agents has improved clinical outcomes compared with decitabine alone.
We did find that in patients with clinical response, bexarotene lead to differentiation with increased CD11b expression and decreased cell proliferation, both following 3 days of oral bexarotene in vivo, and when bone marrow blasts were treated with bexarotene ex vivo (Figure 3).
However, expression analysis of bone marrow blasts from ten cases collected on day 0 and day 3 of bexarotene did not reveal statistically different expression, including differences in the expression of RARA vs RXRA (Figure 4A and data not shown). This is likely due to high variability in the bone marrow blast percentage and in the diversity of FAB classification present in the data set. Review of the recently published RNA-Seq data from TCGA also showed no difference in absolute expression of RARA vs RXRA. The differences we observed in our original dataset therefore may have been technical artifacts related to the Affymetrix U133 (Figure 1A) vs Exon arrays (Figure 4A) or probe selection.
Our original hypothesis that RXRA is more highly expressed in AML blasts than RARA and that RXRA may therefore be a better target for differentiation therapy is not supported by our observed modest response rates or by subsequent RNA-Seq and array analysis of expression levels.
Our data does suggest that a minority of AML patients respond to retinoid-based therapy ex vivo (e.g. ATRA or bexarotene) and that in this small cohort, this correlated with clinical response to the combination of bexarotene and decitabine. In light of the limited clinical success of retinoid therapy in non-M3 AML,(12, 29) these data suggest that cell intrinsic processes may facilitate retinoid-sensitivity in a subset of AML patients. This does not appear to correlate with RARA or RXRA expression levels. However, because these patients can be identified by ex vivo retinoid treatment, further studies of retinoids in non-M3 AML patients might benefit by selecting patients who display ex vivo sensitivity.
Acknowledgments
This work was supported as an IIS grant by Eisai pharmaceuticals and by NIH K99/R00 HL103975-03 (JSW) and P50 CA171963-01A1 (PI: DC Link; Project 1 Co-Leader: JS Welch). We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO., for the use of the Siteman Flow Cytometry Core, which provided flow cytometry service. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Conflicts of Interest.
The authors declare no relevant conflicts of interest.
References
- 1.Craddock C, Tauro S, Moss P, Grimwade D. Biology and management of relapsed acute myeloid leukaemia. Br J Haematol. 2005;129:18–34. doi: 10.1111/j.1365-2141.2004.05318.x. [DOI] [PubMed] [Google Scholar]
- 2.Bow EJ, Sutherland JA, Kilpatrick MG, Williams GJ, Clinch JJ, Shore TB, Rubinger M, Schacter BA. Therapy of untreated acute myeloid leukemia in the elderly: remission-induction using a non-cytarabine-containing regimen of mitoxantrone plus etoposide. J Clin Oncol. 1996;14:1345–1352. doi: 10.1200/JCO.1996.14.4.1345. [DOI] [PubMed] [Google Scholar]
- 3.Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, Balcerzak SP, Appelbaum FR. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031) Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- 4.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 5.Lowenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, Cauchie C, Peetermans M, Solbu G, Suciu S, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 7.Martin MG, Abboud CN. Induction therapy for elderly patients with acute myeloid leukemia. Blood Rev. 2008;22:311–320. doi: 10.1016/j.blre.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Stone RM. The difficult problem of acute myeloid leukemia in the older adult. CA Cancer J Clin. 2002;52:363–371. doi: 10.3322/canjclin.52.6.363. [DOI] [PubMed] [Google Scholar]
- 9.Liu Yin JA, Johnson PR, Davies JM, Flanagan NG, Gorst DW, Lewis MJ. Mitozantrone and cytosine arabinoside as first-line therapy in elderly patients with acute myeloid leukaemia. Br J Haematol. 1991;79:415–420. doi: 10.1111/j.1365-2141.1991.tb08049.x. [DOI] [PubMed] [Google Scholar]
- 10.Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, Lee EJ, Moore JO, Powell BL, Schiffer CA. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 11.Bug G, Ritter M, Wassmann B, Schoch C, Heinzel T, Schwarz K, Romanski A, Kramer OH, Kampfmann M, Hoelzer D, Neubauer A, Ruthardt M, Ottmann OG. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer. 2005;104:2717–2725. doi: 10.1002/cncr.21589. [DOI] [PubMed] [Google Scholar]
- 12.Belhabri A, Thomas X, Wattel E, Chelghoum Y, Anglaret B, Vekhoff A, Reman O, Dombret H, Dhedin N, Michallet M, Fiere D, Archimbaud E. All trans retinoic acid in combination with intermediate-dose cytarabine and idarubicin in patients with relapsed or refractory non promyelocytic acute myeloid leukemia: a phase II randomized trial. Hematol J. 2002;3:49–55. doi: 10.1038/sj.thj.6200141. [DOI] [PubMed] [Google Scholar]
- 13.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 14.Tsai DE, Luger SM, Andreadis C, Vogl DT, Kemner A, Potuzak M, Goradia A, Loren AW, Perl AE, Schuster SJ, Porter DL, Stadtmauer EA, Goldstein SC, Thompson JE, Swider C, Bagg A, Mato AR, Carroll M. A phase I study of bexarotene, a retinoic X receptor agonist, in non-M3 acute myeloid leukemia. Clin Cancer Res. 2008;14:5619–5625. doi: 10.1158/1078-0432.CCR-07-5185. [DOI] [PubMed] [Google Scholar]
- 15.Tsai DE, Luger SM, Kemner A, Swider C, Goradia A, Tomczak E, DiPatri D, Bagg A, Nowell P, Loren AW, Perl A, Schuster S, Thompson JE, Porter D, Andreadis C, Stadtmauer EA, Goldsteini S, Ghalie R, Carroll M. Evidence of myeloid differentiation in non-M3 acute myeloid leukemia treated with the retinoid X receptor agonist bexarotene. Cancer Biol Ther. 2007;6:18–21. doi: 10.4161/cbt.6.1.3619. [DOI] [PubMed] [Google Scholar]
- 16.Kizaki M, Dawson MI, Heyman R, Elster E, Morosetti R, Pakkala S, Chen DL, Ueno H, Chao W, Morikawa M, Ikeda Y, Heber D, Pfahl M, Koeffler HP. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood. 1996;87:1977–1984. [PubMed] [Google Scholar]
- 17.Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, Schober WD, Wang RY, Chism D, Kornblau SM, Younes A, Collins SJ, Koeffler HP, Andreeff M. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther. 2004;3:1249–1262. [PubMed] [Google Scholar]
- 18.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, Liu S, Havelange V, Becker H, Schaaf L, Mickle J, Devine H, Kefauver C, Devine SM, Chan KK, Heerema NA, Bloomfield CD, Grever MR, Byrd JC, Villalona-Calero M, Croce CM, Marcucci G. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Kantarjian H, Guo Y, Lin E, Shan J, Huang X, Berry D, Ahmed S, Zhu W, Pierce S, Kondo Y, Oki Y, Jelinek J, Saba H, Estey E, Issa JP. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol. 2010;28:605–613. doi: 10.1200/JCO.2009.23.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubbert M. DNA methylation inhibitors in the treatment of leukemias, myelodysplastic syndromes and hemoglobinopathies: clinical results and possible mechanisms of action. Curr Top Microbiol Immunol. 2000;249:135–164. doi: 10.1007/978-3-642-59696-4_9. [DOI] [PubMed] [Google Scholar]
- 21.Talpur R, Ward S, Apisarnthanarax N, Breuer-Mcham J, Duvic M. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672–684. doi: 10.1067/mjd.2002.124607. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Payton JE, Grieselhuber NR, Chang LW, Murakami M, Geiss GK, Link DC, Nagarajan R, Watson MA, Ley TJ. High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J Clin Invest. 2009;119:1714–1726. doi: 10.1172/JCI38248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch JS, Klco JM, Gao F, Procknow E, Uy GL, Stockerl-Goldstein KE, Abboud CN, Westervelt P, DiPersio JF, Hassan A, Cashen AF, Vij R. Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a phase I study. Am J Hematol. 2011;86:796–800. doi: 10.1002/ajh.22092. [DOI] [PubMed] [Google Scholar]
- 26.Welch JS, Klco JM, Varghese N, Nagarajan R, Ley TJ. Rara haploinsufficiency modestly influences the phenotype of acute promyelocytic leukemia in mice. Blood. 2011;117:2460–2468. doi: 10.1182/blood-2010-08-300087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klco JM, Spencer DH, Lamprecht TL, Sarkaria SM, Wylie T, Magrini V, Hundal J, Walker J, Varghese N, Erdmann-Gilmore P, Lichti CF, Meyer MR, Townsend RR, Wilson RK, Mardis ER, Ley TJ. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood. 2013;121:1633–1643. doi: 10.1182/blood-2012-09-459313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Network TCGAR. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. New England Journal of Medicine. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlenk RF, Frohling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F, Grimminger W, Gotze K, Waterhouse C, Schoch R, Pralle H, Mergenthaler HG, Hensel M, Koller E, Kirchen H, Preiss J, Salwender H, Biedermann HG, Kremers S, Griesinger F, Benner A, Addamo B, Dohner K, Haas R, Dohner H, Ulm AMLSG. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–1803. doi: 10.1038/sj.leu.2403528. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian HM, O’Brien S, Huang X, Garcia-Manero G, Ravandi F, Cortes J, Shan J, Davisson J, Bueso-Ramos CE, Issa JP. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2007;109:1133–1137. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubbert M, Ruter B, Claus R, Schmid M, Germing U, Eimermacher H, Ganser A, Platzbecker U, Galm O, Brugger W, Heil G, Wijermans PW, Pfeifer D, Schmoor C, Dohner H. Continued Low-Dose Decitabine (DAC) Is an Active First-Line Treatment in All Cytogenetic Subgroups of Older AML Patients: Results of the FR00331 Multicenter Phase II Study. ASH Annual Meeting Abstracts. 2007;110:300. [Google Scholar]
- 32.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci G. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 33.Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS, Cortes J, Kantarjian HM. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Xiao L, Faderl S, Estrov Z, Cortes J, O’Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa J-P. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, Blum W, Marcucci G. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2011 doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubbert M, Ruter B, Claus R, Schmoor C, Schmid M, Germing U, Kundgen A, Rethwisch V, Ganser A, Platzbecker U, Galm O, Brugger W, Heil G, Hackanson B, Deschler B, Dohner K, Hagemeijer A, Wijermans P, Dohner H. A multicenter phase II trial of Decitabine as first-line treatment of older AML patients judged unfit for induction chemotherapy. Haematologica. 2011;97:393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petti MC, Mandelli F, Zagonel V, De Gregoris C, Merola MC, Latagliata R, Gattei V, Fazi P, Monfardini S, Pinto A. Pilot study of 5-aza-2′-deoxycytidine (Decitabine) in the treatment of poor prognosis acute myelogenous leukemia patients: preliminary results. Leukemia. 1993;7 (Suppl 1):36–41. [PubMed] [Google Scholar]
- 38.Wijermans PW, Krulder JW, Huijgens PC, Neve P. Continuous infusion of low-dose 5-Aza-2′-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia. 1997;11:1–5. doi: 10.1038/sj.leu.2400526. [DOI] [PubMed] [Google Scholar]
- 39.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 40.Sudan N, Rossetti JM, Shadduck RK, Latsko J, Lech JA, Kaplan RB, Kennedy M, Gryn JF, Faroun Y, Lister J. Treatment of acute myelogenous leukemia with outpatient azacitidine. Cancer. 2006 doi: 10.1002/cncr.22204. [DOI] [PubMed] [Google Scholar]
- 41.Edlin R, Connock M, Tubeuf S, Round J, Fry-Smith A, Hyde C, Greenheld W. Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia. Health Technol Assess. 2010;14 (Suppl 1):69–74. doi: 10.3310/hta14Suppl1/10. [DOI] [PubMed] [Google Scholar]